A genetically attenuated malaria vaccine candidate based on P. falciparum b9/slarp gene-deficient sporozoites
Abstract
A highly efficacious pre-erythrocytic stage vaccine would be an important tool for the control and elimination of malaria but is currently unavailable. High-level protection in humans can be achieved by experimental immunization with Plasmodium falciparum sporozoites attenuated by radiation or under anti-malarial drug coverage. Immunization with genetically attenuated parasites (GAP) would be an attractive alternative approach. In this study, we present data on safety and protective efficacy using sporozoites with deletions of two genes, that is the newly identified b9 and slarp, which govern independent and critical processes for successful liver-stage development. In the rodent malaria model, PbΔb9ΔslarpGAP was completely attenuated showing no breakthrough infections while efficiently inducing high-level protection. The human PfΔb9ΔslarpGAP generated without drug resistance markers were infective to human hepatocytes in vitro and to humanized mice engrafted with human hepatocytes in vivo but completely aborted development after infection. These findings support the clinical development of a PfΔb9ΔslarpSPZ vaccine.
https://doi.org/10.7554/eLife.03582.001eLife digest
Vaccines commonly contain a weakened or dead version of a disease-causing microorganism, or its toxins, or surface proteins. These prime the immune system to rapidly recognize, respond to, and eliminate the actual infectious pathogen if later encountered.
While vaccines are currently available to help prevent a large number of diseases, vaccines for many deadly diseases, including malaria, do not yet exist. Malaria is caused by a group of parasites called Plasmodium, which are transferred to humans by mosquitoes. While measures to control mosquito populations and prevent mosquito bites have helped to reduce the incidence of malaria in some countries, the number of people—and especially children—that die of malaria every year remains very high.
When a mosquito carrying Plasmodium in its salivary glands bites a human, the parasite is injected into the human's bloodstream and travels to the liver. The parasite reproduces in the liver cells until there are so many of them that the cells rupture, and the parasites are released back into the bloodstream. Any mosquito that then feeds on the blood of the infected individual may also suck up the parasite. The parasite then goes through a further stage of development in the mosquito, eventually migrating to the salivary glands, from where the parasite can be transmitted into a new human host.
Recent work in rodents suggests that genetically altered or weakened Plasmodium falciparum sporozoites—the form of the parasite found in mosquito saliva—could be used to vaccinate humans against malaria caused by this parasite species. Now, van Schaijk, Ploemen et al. evaluate whether a safe and effective vaccine could be made from sporozoites that lack two genes, called b9 and slarp, which are critical for the parasites to develop inside liver cells. When mice were injected with the modified sporozoites, their immune cells were able to detect the parasites and respond against them. The mice subsequently did not develop malaria when they were infected with normal, unmodified parasites. Furthermore, none of the mice contracted malaria from the modified sporozoites.
The modified sporozoites behaved similarly in human liver cells: after invading these cells, the parasites were unable to develop. Clinical testing and further development are now needed to see if a successful malaria vaccine can be made from these sporozoites.
https://doi.org/10.7554/eLife.03582.002Introduction
A vaccine that induces high-level (>90%) sterile protection by inducing immunity that attacks the non-pathologic, asymptomatic pre-erythrocytic stages of Plasmodium falciparum (Pf) will prevent infection, disease, and transmission and could be a powerful instrument to eliminate Pf malaria from geographically defined areas (Plowe et al., 2009; malERA Consultative Group on Vaccines, 2011). In rodent models, sterile protection can be induced by immunization with live Plasmodium sporozoites attenuated by either irradiation, genetic modification (GAP), or by concomitant anti-parasitic drug treatment (For reviews see Hoffman et al., 2010; Butler et al., 2012; Khan et al., 2012; Nganou-Makamdop and Sauerwein, 2013). In humans, induction of complete sustained protective immunity against a challenge infection has been achieved by previous exposure to the bites of mosquitoes infected with i) live radiation-attenuated Plasmodium sporozoites that invade but then completely arrest in the liver (Clyde et al., 1973; Hoffman et al., 2002) and ii) live sporozoites in volunteers taking chloroquine chemoprophylaxis (CPS) with full parasite liver-stage development; once released into the circulation asexual blood stages are killed by chloroquine (Roestenberg et al., 2009, 2011). More recently it has been demonstrated for the first time that sterile immunity can be achieved by intravenous immunization with radiation-attenuated aseptic, purified, cryopreserved Pf sporozoites (SPZ) called PfSPZ Vaccine (Seder et al., 2013).
From a product manufacturing perspective, GAPs have the clear advantage of representing a homogeneous parasite population with a defined genetic identity. The genetic attenuation is an irreversible, intrinsic characteristic of the parasite that does not require additional manufacturing steps like irradiation. Furthermore, in the manufacturing process of GAP-infected mosquitoes, operators are never exposed to Pf parasites that can cause disease. However, clinical development of GAPs has suffered from safety problems related to breakthrough infections during immunization leading to pathological blood stage infections responsible for clinical symptoms and complications. Strains of mice showed differential susceptibility to breakthrough infections after injection of sporozoites of rodent malaria GAPs, demonstrating the need for extensive preclinical rodent screening (Annoura et al., 2012). The P. falciparum GAP PfΔp52Δp36 is the only GAP so far that has been assessed in humans but the trial in which the Pf sporozoites were administered by mosquito bite had to be terminated, because of breakthrough infections in one volunteer during immunization (Spring et al., 2013). Our in vitro experiments with PfΔp52Δp36 confirm that this double gene deletion GAP (i.e. two genes removed from the genome) is not fully attenuated similar to the equivalent rodent GAP PbΔp52Δp36 in the Plasmodium berghei/C57BL/6 model (Annoura et al., 2012). Therefore, identification of additional genes critical and uniquely selective for liver-stage development has become a major challenge for GAP vaccine development (Annoura et al., 2012; Khan et al., 2012; Ploemen et al., 2012). Furthermore, single gene deletion GAPs will most likely not be adequate (Ploemen et al., 2012).
This prompted us to generate and test a GAP with deletions of two independent genes critical for liver-stage development. We recently identified a novel P. berghei (Pb) gene deletion mutant, PbΔb9, lacking the expression of the B9 protein (Pf ortholog: PFC_0750w; PF3D7_0317100) (Annoura et al., 2014). This protein is a newly identified member of the Plasmodium 6-Cys family of proteins. Initial safety evaluation in rodents demonstrated that PbΔb9 mutants have a stronger attenuation phenotype than mutants lacking the 6-Cys proteins P52 and P36 (van Dijk et al., 2005; van Schaijk et al., 2008; VanBuskirk et al., 2009; Annoura et al., 2014). As second target gene for liver-stage attenuation, we selected the slarp and sap1 orthologs reported in Pb and Plasmodium yoelii (Py), respectively (Pf ortholog: PF11_0480; PF3D7_1147000; hereafter termed slarp). These slarp mutants show an excellent safety profile by full arrest in the liver in mice (Aly et al., 2008; Silvie et al., 2008). The SLARP protein is expressed in sporozoites and in early liver-stages and is involved in the regulation of transcription (Silvie et al., 2008; Aly et al., 2011).
In this study, we report the generation and evaluation of a rodent GAP lacking the genes encoding for B9 and SLARP (PbΔb9Δslarp) and the generation and evaluation of the equivalent human Pf GAP lacking the Pf ortholog genes. PfΔb9Δslarp was generated using constructs that allowed for the removal of the drug selectable marker from the genome by FRT/FLPe recombinase methodology (van Schaijk et al., 2010). The safety and efficacy of PbΔb9Δslarp and the lack of development of PfΔb9Δslarp in human hepatocytes, in vitro, and, in vivo, in chimeric mice provide strong support for clinical development of a PfΔb9Δslarp PfSPZ vaccine.
Results
Arrest of liver-stage development and induced protection after P. berghei Δb9Δslarp GAP
Previously, we generated a Pb mutant with disruption of the b9 locus (PbΔb9) by standard genetic modification using a double cross-over integration event, followed by removal of the drug-selectable marker cassette by negative selection (Lin et al., 2011). Characterization of the PbΔb9 phenotype showed that liver-stage development was fully abrogated in BALB/c mice and severely compromised in the more stringent C57BL/6 murine model for P. berghei (Annoura et al., 2014). Immunization of a single dose of 10k (i.e. 10,000 sporozoites) or 5k PbΔb9 protected BALB/c mice against a 10k WT-sporozoite challenge, while 80% of mice were still protected after a single 1k immunizing dose (Table 1). In C57BL/6 mice, immunization with 50K/20K/20K of PbΔb9 resulted in complete protection lasting up to 180 days, reducing to 45% protection when challenged at 1 year post-immunization. However, sporozoite administration occasionally resulted in blood stage infections after administration of high doses, thereby compromising the safety profile (Annoura et al., 2014).
Protection of mice after immunization with P. berghei PbΔb9 or PbΔb9Δslarp sporozoites
| Mouse strain | Pb mutant | Day of challenge* | Immunization regimes no. protected/no challenged | ||
|---|---|---|---|---|---|
| BALB/c | 10k† | 5k | 1k | ||
| PbΔb9 | 10 | 10/10‡ | 18/20 | 8/10 | |
| PbΔb9Δslarp | 10 | 20/20 | 10/10 | 20/20 | |
| C57Bl6 | 50/20/20k§ | 10/10/10k | 1/1/1k | ||
| PbΔb9 | 10 | 4/4 | nd | nd | |
| 90 | 5/5 | ||||
| 180 | 9/9# | ||||
| 365 | 5/11 | ||||
| PbΔb9Δslarp | 10 | Nd | 10/10 | 6/10 | |
| 180 | 6/6 | nd | nd | ||
-
*
Number of days post last immunization; 104 wild-type sporozoites were injected by IV route.
-
†
Immunization dose: number of sporozoites x1000.
-
‡
Protected/total # of immunized mice (%); protection was 0/15 in naive control BALB/c and 0/10 in C57BL/6 mice.
-
§
Immunization dose with 7 day intervals between immunizations.
-
#
Immunization dose 50/10/20k with 7 day intervals between immunizations. nd = not done.
Previously, it has been shown by others that PbΔslarp parasites are completely arrested in liver-stage development with a complete lack of breakthrough blood-stage infections (Aly et al., 2008; Silvie et al., 2008). Therefore, we generated a new single gene deletion mutant PbΔslarp in a parasite line that constitutively expressed a fusion of the reporter proteins GFP and luciferase, using a slarp-targeting DNA-construct for deletion by double cross-over homologous integration (Figure 1—figure supplement 1). The PbΔslarp mutant showed blood stage growth and mosquito infections with functional sporozoites similar to wild-type (Supplementary file 1). However, intravenous injection of up to 500k PbΔslarp sporozoites never led to full development of parasites in the liver as assayed by in vivo imaging (Figure 1—figure supplement 1) or analysis of blood stage infection (Table 2). PbΔslarp sporozoites arrested very soon after invasion of cultured Huh7 hepatocytes corroborating the excellent safety findings by Silvie et al. (2008).
Breakthrough blood-stage infections after intravenous injection of PbΔslarp and PbΔb9Δslarp sporozoites
| Mouse strain | Mutant | Infection* Spz x 103 | Breakthrough blood infection/total # mice | Pre-patent period‡ (days) |
|---|---|---|---|---|
| BALB/c | WT† | 10 | 5/5 | 4–5 |
| PbΔslarp | 50 | 0/5 | ||
| PbΔslarp | 25 | 0/10 | ||
| PbΔb9Δslarp | 25 | 0/10 | ||
| C57BL/6 | WT† | 10 | 5/5 | 4–5 |
| PbΔslarp | 500 | 0/5 | ||
| PbΔslarp | 200 | 0/10 | ||
| PbΔb9Δslarp | 200 | 0/10 | ||
| PbΔb9Δslarp | 150 | 0/5 |
-
*
Inoculation dose of sporozoites administered IV.
-
†
P. berghei ANKA strain: line cl15cy1.
-
‡
Day with parasitemia of 0.5–2%.
Therefore, in order to create a completely attenuated and safe rodent GAP, we additionally disrupted the slarp gene in the PbΔb9 genome by double cross-over integration (Figure 1). Asexual growth and sporogonic development/function equaled wild-type (Supplementary file 1). However, PbΔb9Δslarp sporozoites arrested soon after invasion of cultured Huh7 hepatocytes (Figure 1) and intravenous injection of 150–200K PbΔb9Δslarp sporozoites never resulted in breakthrough blood-stage infections in mice (Table 2). Finally, protective efficacy induced by PbΔb9Δslarp was studied in both BALB/c and C57BL/6 mice. A single immunization dose of 10K, 5K, or even 1K of PbΔb9Δslarp sporozoites in BALB/c mice induced full protection against a 10K wild-type sporozoite challenge (Table 1). C57BL/6 mice were 100% protected after 3 × 10K immunization with PbΔb9Δslarp sporozoites, and the protective efficacy reduced to 60% after a 3 × 1K immunization dose. A challenge at day 180 post-immunization of a 50/20/20K dose still resulted in complete protection. The combined data showed that PbΔb9Δslarp completely arrest during liver-stage development and induce a highly efficient protective immunity in two different strains of mice.
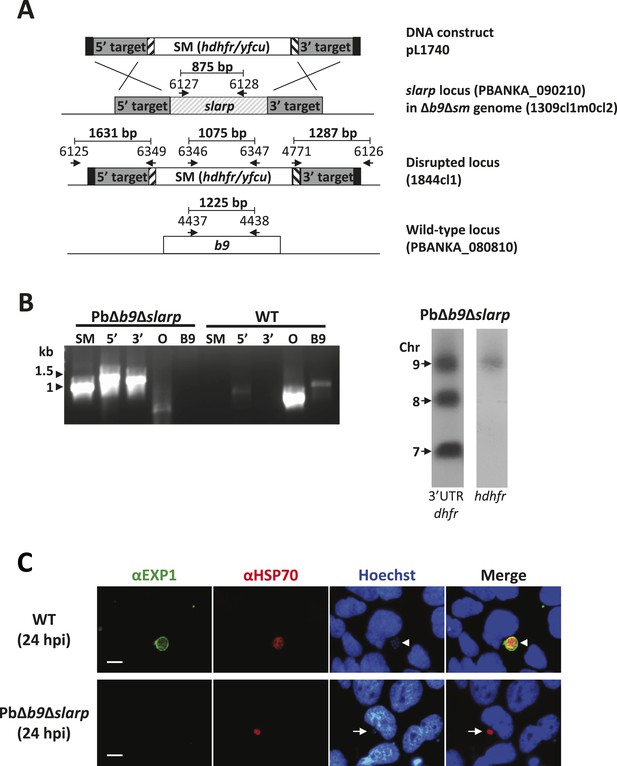
Generation and genotype analyses of P. berghei mutant PbΔb9Δslarp.
(A) Generation of mutant PbΔb9Δslarp. For PbΔb9Δslarp, the DNA-construct pL1740 was generated containing the positive/negative selectable marker cassette hdhfr/yfcy. This construct was subsequently used to generate the mutant PbΔb9Δslarp in the PbΔb9Δsm mutant. See Supplementary file 2A for the sequence of the primers. (B) Diagnostic PCR and Southern analysis of Pulse Field Gel (PFG)-separated chromosomes of mutant PbΔb9Δslarp confirming correct disruption of the slarp and the b9 locus. See Supplementary file 2A for the sequence of the primers used for the selectable marker gene (SM); 5′-integration event (5′); 3′-integration event (3′); and the slarp and the b9 ORF. For Southern analysis, PFG-separated chromosomes were hybridized using a 3′UTR pbdhfr probe that recognizes the construct integrated into P. berghei slarp locus on chromosome 9, the endogenous locus of dhfr/ts on chromosome 7, and a 3′UTR pbdhfr probe that recognizes the construct integrated into P. berghei b9 locus on chromosome 8. In addition, the chromosomes were hybridized with the hdhfr probe recognizing the integrated construct into the slarp locus on chromosome 9. (C) Development of liver-stages in cultured hepatocytes as visualized by staining with antibodies recognizing the parasitophorous vacuole membrane (anti-EXP1; green) and the parasite cytoplasm (anti-HSP70; red). Nuclei are stained with Hoechst-33342. Hpi: hours post-infection. Scale bar represents 10 µm.
Generation of a P. falciparum Δb9Δslarp GAP
Considering the desired phenotype as observed in P. berghei, we generated a Pf mutant lacking expression of both B9 (PF3D7_0317100) and SLARP (PF3D7_1147000; sporozoite asparagine-rich protein). These genes are conserved between rodent and human species, both at the level of syntenic location in their respective genomes on chromosomes 3 and 11 respectively, and at the sequence level. Pfb9 shows 37% amino acid sequence identity and 54% sequence similarity with Pbb9 ((Annoura et al., 2014)); Pfslarp shows 28% amino acid sequence identity and 46% sequence similarity with Pbslarp.
First, we generated two independent Pf mutants lacking slarp by standard double cross-over integration of a DNA construct and analyzed their phenotype throughout the parasite life cycle (Figure 2—figure supplement 1,2). Blood-stage development of two independently derived PfΔslarp (i.e. PfΔslarp-a and–b) parasites was comparable to WT parasites. PfΔslarp mutants produced WT numbers of gametocytes, oocysts and sporozoites (Figure 2). The intra-cellular PfΔslarp-a and -b parasite development in primary human hepatocytes was not significantly different in number and morphologically identical to WT parasites at 3 and 24 hours post-infection (hpi) (Figure 3). However, their number was more than 10-fold reduced at 48 hpi and not detectable from day 3 onwards to day 7 post-infection. Parasites lacking b9 in P. falciparum arrested before day 2 post-infection of primary human hepatocytes with the exception of one observed liver schizont at a later timepoint (Annoura et al., 2014). PfΔslarp-a and -b parasites still showed positive HSP70 staining and morphologically normal parasites at 48 hpi in primary human hepatocytes, indicating time point of arrest later compared to PfΔb9 parasites.
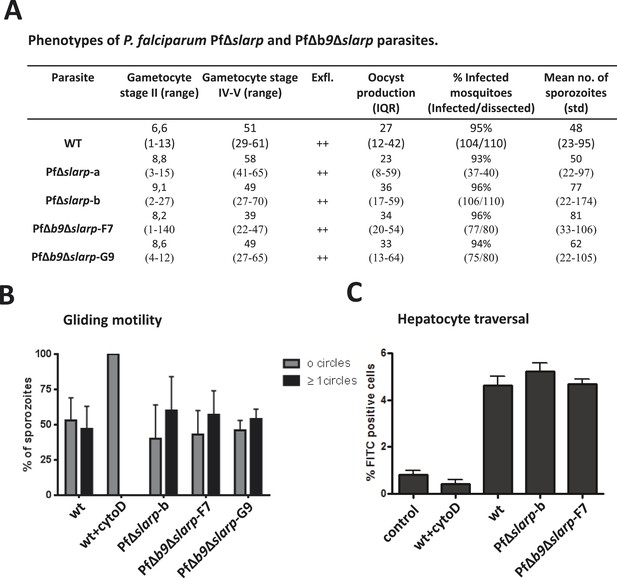
Phenotypes of P. falciparum PfΔslarp and PfΔb9Δslarp parasites.
(A) Gametocyte, oocyst, and sporozoite production. Gametocyte numbers (stage II and IV–V) per 1000 erythrocytes at day 8 and day 14 after the start of gametocyte cultures. Exflagellation (Exfl) of male gametocytes in stimulated samples from day 14 cultures (++ score = >10 exflagellation centers per microscope field at 400× magnification). Median number of oocysts at day 7, IQR is the inter quartile range and sporozoite (day 21) production (×1000) in A. stephensi mosquitoes. (B) Gliding motility of P. falciparum WT (cytochalasin D treated and untreated), PfΔslarp-b, PfΔb9Δslarp-F7, and PfΔb9Δslarp-G9 parasites. Gliding motility was quantified by determining the mean percentage ± standard deviation of parasites that exhibited gliding motility by producing characteristic CSP trails (≥1 circles) or parasites that did not produce CSP trails (0 circles). (C) Cell traversal ability of P. falciparum NF54, PfΔslarp-b and PfΔb9Δslarp-F7 sporozoites as determined by FACS counting of Dextran positive Huh7 cells. Shown is the mean percentage ±standard deviation of FITC positive cells. Dextran control (control): hepatocytes cultured in the presence of Dextran but without the addition of sporozoites.
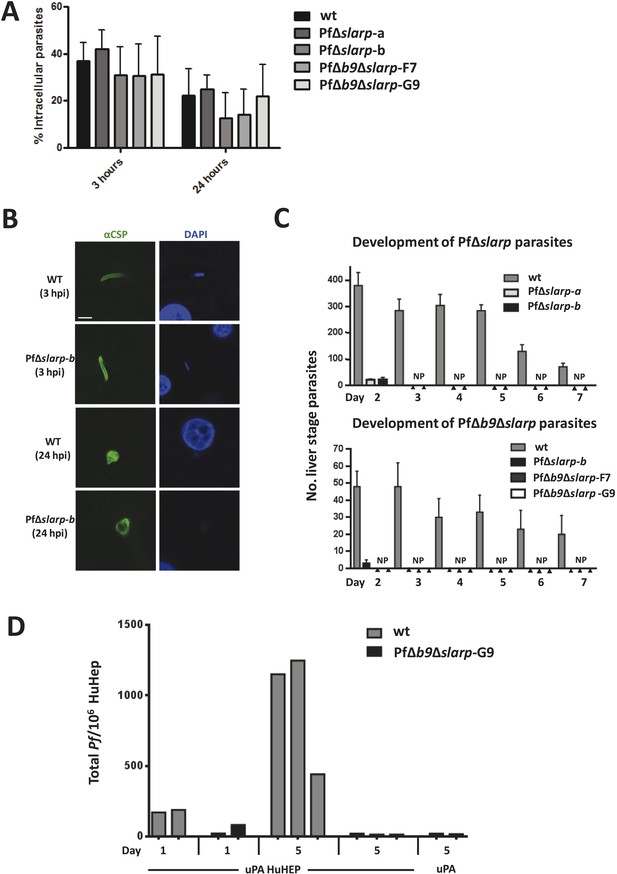
Development of P. falciparum PfΔslarp and PfΔb9Δslarp parasites in human primary hepatocytes.
(A) In vitro invasion of P. falciparum wt, PfΔslarp-a, PfΔslarp-b, PfΔb9Δslarp-F7, and PfΔb9Δslarp-G9 sporozoites in primary human hepatocytes. Invasion is represented as the mean ratio ± standard deviation of extra- and intra-cellular sporozoites by double staining at 3 and 24 hr post-infection, determined after three wash steps to remove sporozoites in suspension. (B) Immunofluorescence assay of PfΔslarp-b parasites in human primary hepatocytes at 3 and 24 hr post-infection. Parasites are visualized by staining with anti-PfCSP antibodies (green; Alexa-488) and parasite, and hepatocyte nuclei are stained with DAPI (blue). Images were photographed on an Olympus FV1000 confocal microscope. Scale bar represents 5 µm. (C) Development of P. falciparum wt, PfΔslarp-a, PfΔslarp-b (top panel), PfΔb9Δslarp-F7, and PfΔb9Δslarp-G9 (bottom panel) liver-stages in primary human hepatocytes following inoculation with 40,000 sporozoites. From day 2 to 7, the mean number ± standard deviation of parasites per 96-well was determined by counting parasites stained with anti-P. falciparum HSP70 antibodies. The bottom panel represents experiments performed in primary human hepatocytes from 2 different donors. No parasites present (NP). (D) Development of liver-stages of PfΔb9Δslarp GAP in chimeric mice engrafted with human hepatocytes. Mice were infected with 106 wt or PfΔb9Δslarp-G9 sporozoites by intravenous inoculation. At 24 hr or at 5 days after sporozoite infection, livers were collected from the mice and the presence of parasites determined by qPCR of the parasite-specific 18S DNA. uPA HuHEP; chimeric homozygous uPA+/+-SCID mice engrafted with human hepatocytes. As controls, uPA mice; heterozygous uPA+/−-SCID mice not engrafted with human hepatocytes were used.
Next, we generated double gene-deletion PfΔb9Δslarp mutants using the FRT/FLPe recombinase methodology (van Schaijk et al., 2010). This methodology employs FLPe recombinase to remove a FRT-site flanked drug resistance marker cassette introduced into the Pf genome when the target gene has been removed by double cross-over homologous recombination as shown for PfΔslarp-b parasites in Figure 2—figure supplement 1,2. After cloning, this ‘marker-free’ line was subsequently transfected with the Pfb9 gene-targeting construct pHHT-FRT-GFP-b9 (Annoura et al., 2014) to delete the b9 locus from the PfΔslarp-b genome (Figure 2—figure supplement 1,2). Subsequently two ‘marker-free’ clones, PfΔb9Δslarp-F7 and PfΔb9Δslarp-G9, were obtained containing the correct genotype that is removal of the slarp and b9 gene loci as well as both respective drug selection cassettes (Figure 2—figure supplement 2). In addition, we confirmed the loss of expression of both slarp and b9 by RT-PCR analysis by demonstrating the absence of transcripts in mRNA collected from PfΔb9Δslarp-F7 and PfΔb9Δslarp-G9 salivary gland sporozoites (Figure 2—figure supplement 2). We then examined the phenotype of PfΔb9Δslarp-F7 and PfΔb9Δslarp-G9 mutants during blood stage and mosquito development. Asexual blood stage growth of PfΔb9Δslarp parasites was normal as both clones reached an asexual parasitemia between 0.5 and 5% during cloning within 21 days and PfΔb9Δslarp clones produced WT-like numbers of gametocytes, oocysts, and sporozoites (Figure 2).
Developmental arrest of PfΔb9Δslarp GAPs in human hepatocytes
We next analyzed the development of PfΔb9Δslarp in human hepatocytes using cultured primary hepatocytes and uPA+/+-SCID mice engrafted with human hepatocytes (human liver-uPA-SCID mice) (Meuleman et al., 2005). PfΔb9Δslarp sporozoites showed normal gliding motility, hepatic cell traversal (Figure 2), as well as invasion of primary human hepatocytes, but parasites were completely absent in two independent experiments at day 2 up to day 7 post-infection, following inoculation of primary human hepatocytes with 40,000 PfΔb9Δslarp F7 or G9 sporozoites (Figure 3). Detailed analyses of 80 individual wells at day 4 post-infection did not result in identification of a single developing parasite. The combined day 2 and day 4 data of PfΔb9Δslarp indicated that the timing of arrest is similar to PfΔb9 (Annoura et al., 2014) and there had been complete arrest of liver-stage development, similar to PfΔslarp parasites.
In addition, human liver-uPA-SCID mice were intravenously inoculated with 1 × 106 WT or PfΔb9Δslarp sporozoites. Two heterozygous uPA+/−-SCID mice, not engrafted with human hepatocytes, served as controls and were also challenged with P. falciparum sporozoites. Livers were collected either at 24 hpi or 5 days post-infection for detection of P. falciparum 18S DNA by quantitative real-time PCR (Foquet et al., 2013). Both mice infected with WT Pf and 1 of the 2 mice infected with PfΔb9Δslarp were positive for Pf 18S DNA at 24 hr post-infection, demonstrating successful sporozoite infection in human hepatocytes (Figure 3). A lower signal was observed in PfΔb9Δslarp-infected mice at day 1 after infection compared to WT parasites, likely reflecting the early time point of arrest of this GAP. All mice infected with Pf WT (3/3) showed a strong increase in parasite 18S DNA at day 5 post-infection, representing successful liver-stage development. In contrast, none of the human liver-uPA-SCID mice infected with PfΔb9Δslarp sporozoites showed 18S DNA higher than heterozygous uPA+/−-SCID mice, not engrafted with human hepatocytes that had been infected with PfΔb9Δslarp sporozoites (Figure 3). Although these studies were performed with a limited number of mice, these findings indicate that PfΔb9Δslarp parasites can invade but do not develop in livers of humanized mice. Our combined results demonstrate abrogation of development of PfΔb9Δslarp inside human hepatocytes.
Discussion
The Pf GAP PfΔb9Δslarp containing two gene deletions is proposed as a whole-parasite malaria vaccine candidate. Rationale and arguments are based on in vitro and in vivo experiments and supported by safety and protection data with rodent Pb GAP with deletions of the orthologous genes. The rodent GAP PbΔb9Δslarp completely arrested early in liver-stage development in two different mouse strains after injection of very high number of sporozoites. In addition, immunizations with PbΔb9Δslarp efficiently induced sterile and long-lasting protective immunity in both BALB/c and C57BL/6 mice. Similarly, the Pf GAP PfΔslarpΔb9 completely aborted development in human hepatocytes 1 day after invasion, while sporozoites were fully motile and invasive with infectivity comparable to Pf WT sporozoites. Importantly, asexual parasite growth and production of salivary gland sporozoites in the mosquito were unaffected ensuring normal GAP production. PbΔb9Δslarp is to our knowledge the first completely attenuated rodent mutant in which multiple genes have been deleted that are critical for two independent biological processes during liver-stage development, that is regulation of parasite genes/transcripts that play a role in early liver-stage development stages (Silvie et al., 2008; Aly et al., 2011) and the establishment of the PV within the infected hepatocyte (Annoura et al., 2014).
A number of Pb and Py GAPs have previously been reported to arrest at different time points during development in the liver (Khan et al., 2012; Nganou-Makamdop and Sauerwein, 2013). These include GAPs based on genes essential for i) the formation and maintenance of a parasitophorous vacuole (PV) (b9, p52, p36, uis3, and uis4; (van Dijk et al., 2005; Kumar et al., 2009; Annoura et al., 2014) and ii) type II fatty acid synthesis (i.e. fabb/f, fabz, pdh e1α; (Vaughan et al., 2009; Annoura et al., 2012)), and iii) the regulation of gene expression in the liver-stages (sap1/slarp (Aly et al., 2008; Silvie et al., 2008; Aly et al., 2011)). A critical safety requirement for GAPs in order to qualify as vaccine candidate is the total absence of blood infections during immunization and therefore the complete abrogation of liver-stage development. Unfortunately many of the above mentioned target genes including p52, p36, and those involved in type II fatty acid synthesis show a leaky phenotype, resulting in blood stage infections after administration of high number of sporozoites. Incomplete liver-stage arrest obviously disqualifies GAPs for further clinical development for safety reasons.
In P. falciparum, GAPs have been generated that lack both the p52 and p36 genes (van Schaijk et al., 2008; VanBuskirk et al., 2009). In the Pb rodent model, this GAP was not completely attenuated (Annoura et al., 2012). Similarly, this Pf GAP while severely attenuated by the lack of both genes, a low percentage of parasites of this GAP are able to develop into mature liver-stage (Annoura et al., 2012). These observations indicate a partially redundant function for these proteins; indeed, a breakthrough blood infection was observed in one out of the six volunteers after exposure to the bite of mosquitoes infected with sporozoites of a PfΔp52Δp36 GAP (Spring et al., 2013).
Since functional redundancy of related genes has been reported more often in Plasmodium (Liu et al., 2006; Heiss et al., 2008; van Dijk et al., 2010; Lin et al., 2013), we pursued the generation of GAPs from which multiple genes were removed from the genome, each governing a critical yet independent cellular process. The selection of those target genes excluded type II fatty acid synthesis (FAS II) because P. falciparum mutants lacking FAS II enzymes fail to generate sporozoites inside the oocyst, indicating that the FAS II pathway is essential for sporogony (van Schaijk et al., 2013). The gene encoding liver-stage antigen 1 (LSA-1) may be an attractive candidate, but no orthologues are present in rodent or non-human primate Plasmodium species precluding sufficient pre-clinical testing (Mikolajczak et al., 2011). The reverse is true for two published rodent GAPs with deletions of the genes uis3 or uis4 of which unequivocal orthologues are absent in the P. falciparum genome. Alternatively, genes encoding proteins with a role in the late stage parasite liver development could be an attractive target, since induction of protection by late arresting GAPs may be superior to early arresting GAPs (Butler et al., 2011; Nganou-Makamdop and Sauerwein, 2013) However, late arresting GAPs are likely more risky and prone to breakthrough infection as shown for GAPs lacking the genes palm or lisp (Khan et al., 2012).
Therefore, we decided to focus on early liver-stage arrest and selected the newly identified b9 as a prime candidate. PbΔb9 elicits long-lived protective immune responses in mice and only few breakthrough blood infections occur in mice, albeit less than were observed with PbΔp52Δp36 GAP sporozoites (Annoura et al., 2012). The genes p52, p36, and b9, all belong to the recently expanded 6-Cys family of Plasmodium proteins and may share a similar function in formation or maintenance of the PV membrane at the interface of parasite and host cell. Indeed, a triple gene-deletion mutant lacking p52, p36, and b9 is no more attenuated than a mutant lacking b9, suggesting that these genes do not drive independent biological pathways (van Dijk et al., 2005; Ploemen et al., 2012; Annoura et al., 2014). To date, the early arresting slarp mutant is the only rodent GAP with a Pf ortholog without a record of breakthrough blood infections in mice. Indeed, our data confirm that rodent sporozoites lacking slarp are fully capable of hepatocyte invasion and formation of a PV but completely abort development soon after invasion as previously reported (Aly et al., 2008; Silvie et al., 2008; Aly et al., 2011). In this study, we report for the first time that P. falciparum mutants lacking slarp, that is PfΔslarp, completely arrest at day 3 post-infection of primary human hepatocytes, while morphologically normal liver-stage parasites are still observed at 48 hpi. PfΔb9 parasites arrest at a point in time before day 2 after hepatocyte invasion, with the exception of a single liver schizont observed at a later time point (Annoura et al., 2014). The multiple attenuated PbΔb9Δslarp indeed passed our stringent pre-clinical safety screen and no breakthrough blood infections were observed in all conditions tested. In addition, we showed that immunization with PbΔb9Δslarp sporozoites induced strong and sustained protective immunity in BALB/c and C57BL/6 mice, with similar efficacy as reported for mutant sporozoites lacking P52 (or P52 and P36) or γ-radiated sporozoites (Nussenzweig et al., 1967; van Dijk et al., 2005; Douradinha et al., 2007; Labaied et al., 2007).
Live vaccine strains (attenuated by natural selection or genetic engineering) may be potentially released into the environment. Therefore, safety issues concerning the medical as well as environmental aspects must be considered including the absence of heterologous DNA sequences (in particular drug resistance genes) from the genome of GAPs (Committee for Medical Products for Human Use, 2006; Frey, 2007). Thus, a PfΔb9Δslarp GAP was generated free of a drug resistance marker using FRT/FLPe-recombinase methodology. This approach permits the removal of drug resistance markers that were introduced to generate the mutant and results in an altered genome that retains only two 34 nucleotide FRT sequences. The removal of the drug resistance marker has the additional advantage that these parasites are easily amenable to further genetic modification (van Schaijk et al., 2010).
The PfΔb9Δslarp GAP aborted early development in cultured primary human hepatocytes, with a phenotype and timing similar to PfΔb9, and studies performed in a limited number of chimeric mice engrafted with human hepatocytes confirm this arrest phenotype. From the combined Pb and Pf data, one can conclude that Δb9 attenuation phenotype induces highly effective protection, although it may at a low frequency produce a breakthrough blood infection. Therefore, the additional deletion of slarp in these mutants provides these parasites with complete attenuation that is essential in order to proceed with human trials.
An important prerequisite for further downstream clinical development and manufacturing (Seder et al., 2013) is to show that production of PfΔb9Δslarp sporozoites is unabated and similar to WT parasites. We have shown that the PfΔb9Δslarp GAP produces WT numbers of sporozoites that are fully capable of infecting hepatocytes. In addition, we have produced aseptic, purified, cryopreserved PfΔb9Δslarp sporozoites (data not shown). Preliminary data from a 6-day attenuation assay in HC-04 cells showed that like irradiated PfSPZ (Hoffman et al., 2010; Epstein et al., 2011), none of the PfΔb9Δslarp sporozoites developed to mature liver-stage parasites expressing PfMSP-1 (data not shown), as aseptic, purified, cryopreserved WT sporozoites (Roestenberg et al., 2013).
In conclusion, we have generated a multiply attenuated PfΔb9Δslarp GAP, free of any drug resistance gene, and demonstrated that PfΔb9Δslarp sporozoites invade hepatocytes comparably to WT sporozoites and are completely attenuated. These findings provide a solid foundation for clinical development and testing of a PfSPZΔb9Δslarp vaccine.
Note added at proof
While this manuscript was in preparation an article was published that also describes a multiple-gene deletion P. falciparum parasite that has undergone pre-clinical evaluation (Mikolajczak et al., 2014). In that study, the authors describe a P. falciparum mutant that, like our work, also lacks the gene slarp (sap1) as well as the paralogous pair of genes, p52 and p36.
Materials and methods
P. berghei reference parasite lines
Request a detailed protocolThe following reference lines of the ANKA strain of P. berghei were used: line cl15cy1 (Janse et al., 2006a, 2006b) and line 676m1cl1 (PbGFP-Luccon; see RMgm-29 in www.pberghei.eu). PbGFP-Luccon expresses a fusion protein of GFP and luciferase from the eef1a promoter (Franke-Fayard et al., 2004; Janse et al., 2006a).
P. falciparum parasites and culture
Request a detailed protocolFor transfections, the parasite used was directly from a characterized good manufacturing process (GMP) and produced working cell bank (WCB) of the P. falciparum NF54 wild-type strain (Ponnudurai et al., 1981), produced by Sanaria Inc, identical to that described previously (Hoffman et al., 2010; Epstein et al., 2011; Roestenberg et al., 2013). Blood stages of wt, PfΔslarp-a, PfΔslarp-b, PfΔb9Δslarp-F7, and PfΔb9Δslarp-G9 were cultured in a semi-automated culture system using standard in vitro culture conditions for P. falciparum and induction of gametocyte production in these cultures was performed as previously described (Ifediba and Vanderberg, 1981; Ponnudurai et al., 1982, 1989). Fresh human red blood cells and serum were obtained from Dutch National blood bank (Sanquin Nijmegen, NL; permission granted from donors for the use of blood products for malaria research). Cloning of transgenic parasites was performed by the method of limiting dilution in 96-well plates as described (Thaithong, 1985). Parasites of the positive wells were transferred to the semi-automated culture system and cultured for further phenotype and genotype analyses (See below).
Experimental animals
Request a detailed protocolFor P. berghei infections, female C57BL/6J and BALB/c (12-week old; Janvier France) and Swiss OF1 (8 weeks old Charles River) were used. All animal experiments with rodent parasites performed at the LUMC (Netherlands) were approved by the Animal Experiments Committee of the Leiden University Medical Center (DEC 07171; DEC 10099) and at the RUNMC (Netherlands) by the Radboud University Experimental Animal Ethical Committee (RUDEC 2008-123, RUDEC 2008-148, RUDEC 2010-250, RUDEC 2011-022, RUDEC 2011-208). The Dutch Experiments on Animal Act is established under European guidelines (EU directive 86/609/CEE) regarding the Protection of Animals used for Experimental and Other Scientific Purposes.
Human liver-uPA-SCID mice (chimeric mice) were produced as described before (Meuleman et al., 2005). The study protocol for infecting these mice with P. falciparum sporozoites was approved by the animal ethics committee of the Faculty of Medicine and Health Sciences of the Ghent University.
Generation and genotyping of P. berghei mutants
Request a detailed protocolTo disrupt the P. berghei slarp gene (PBANKA_090210), a construct was generated using the adapted ‘Anchor-tagging’ PCR-based method as described (Annoura et al., 2012) (Figure 1—figure supplement 1). The two targeting fragments (1195 bp and 823 bp) of slarp were amplified using genomic DNA (parasite line cl15cy1) as template with the primer pairs 5960/5961 (5′target sequence) and 5962/5963 (3′target sequence). See Supplementary file 2A for the sequence of the primers. Using this PCR-based targeting construct (pL1740), the mutant PbΔslarp-a (1839cl3) was generated in the PbGFP-Luccon reference line using standard methods of transfection and positive selection with pyrimethamine (Figure 1—figure supplement 1). The generation of the drug-selectable marker-free mutant PbΔb9Δsm (1309cl1m0cl2; RMgmDB no. 934) has been described by Annoura et al. (2014). This mutant, which contains a disrupted b9 gene and is drug-selectable marker free, was used for deleting the slarp gene (PBANKA_090210). To delete the slarp gene, the gene-deletion construct pL1740 was used as described above. Using this construct the mutant PbΔb9Δslarp (line 1844cl1) was generated in the PbΔb9Δsm line using standard methods of transfection and positive selection with pyrimethamine (Figure 1).
Correct integration of the constructs into the genome of mutant parasites was analyzed by diagnostic PCR-analysis and Southern analysis of PFG-separated chromosomes as shown in Figure 1 and Figure 1—figure supplement 1. PFG-separated chromosomes were hybridized with a probe recognizing hdhfr or the 3′-UTR dhfr/ts of P. berghei (Janse et al., 2006b).
Generation and genotyping of P. falciparum mutants
Request a detailed protocolThe slarp gene (PF3D7_1147000) in P. falciparum WT parasites (NF54wcb) was deleted using a modified construct based on plasmid pHHT-FRT-(GFP)-Pf52 (van Schaijk et al., 2010) (Figure 2—figure supplement 1). Targeting regions were generated by PCR using primers BVS179 and BVS180 for the 5′ target region and primers BVS182 and BVS184 for the 3′ target region (see Supplementary file 2B for primer sequences). The 5′and 3′ target regions were cloned into pHHT-FRT-(GFP)-Pf52 digested with BsiWI, BssHII and NcoI, XmaI, respectively, resulting in the plasmid pHHT-FRT-GFP-slarp. The b9 gene (PF3D7_0317100) of PfΔslarp-b P. falciparum parasites was deleted using a modified construct based on plasmid pHHT-FRT-(GFP)-Pf52 (van Schaijk et al., 2010) (Figure 2—figure supplement 1). Targeting regions were generated by PCR using primers BVS84 and BVS85 for the 5′ target region and primers BVS88 and BVS89 for the 3′ target region. The 5′and 3′ target regions were cloned into pHHT-FRT-(GFP)-Pf52 digested with NcoI, XmaI and MluI, BssHII resulting in the plasmid pHHT-FRT-GFP-b9. All DNA fragments were amplified by PCR amplification (Phusion, Finnzymes) from genomic P. falciparum DNA (NF54 strain) and all PCR fragments were sequenced after TOPO TA (Invitrogen, Leek, The Netherlands) sub-cloning. Transfection of WT (NF54wcb) parasites with the plasmid pHHT-FRT-GFP-slarp and selection of mutant parasites were performed, as described (van Schaijk et al., 2010), resulting in the selection of the parasite line PfΔslarp-a. The second PfΔslarp parasite line, originating from an independent transfection, was subsequently transfected with pMV-FLPe to remove the drug-selectable marker cassette using FLPe as described (van Schaijk et al., 2010) and cloned resulting in the parasite clone PfΔslarp-b. Subsequent transfection of PfΔslarp-b parasites with the plasmid pHHT-FRT-GFP-b9 and selection were performed, as described above, resulting in the parasite line PfΔb9Δslarp. The parasite line PfΔb9Δslarp was subsequently transfected with pMV-FLPe to remove the drug-selectable marker cassette using FLPe and cloned, as described above, resulting in the cloned parasite lines PfΔb9Δslarp-F7 and PfΔb9Δslarp-G9 that are free of drug resistance markers.
Genotype analysis of PfΔslarp and PfΔb9Δslarp parasites was performed by Expand Long range dNTPack (Roche) diagnostic, long-range, PCR (LR-PCR) and Southern blot analysis (Figure 2—figure supplement 2). Genomic DNA of blood stages of WT or mutant parasites was isolated and analyzed by LR-PCR using primer pair p1, p2 (slarp) and p3, p4 (b9) (See Supplementary file 2B for primer sequences) for correct integration of the constructs in the respective slarp and b9 loci by double cross-over homologous recombination. The LR-PCR program has an annealing step of 48°C for 30 s and an elongation step of 62°C for 10–15 min. All other PCR settings were according to manufacturer's instructions. PCR products were directly analyzed by standard agarose gel electrophoresis or first digested with restriction enzymes for further confirmation of the genotype and removal of resistance markers was confirmed by sequencing. For Southern blot analysis, genomic DNA was digested with TaqI or RcaI restriction enzymes for analysis of integration into the slarp and b9 loci, respectively. Southern blot was generated by capillary transfer as described (Sambrook and Russel, 2001) and DNA was hybridized to radioactive probes specific for the targeting regions used for the generation of the mutants and generated by PCR (See above).
The presence or absence of slarp and b9 transcripts in WT and mutant sporozoites was analyzed by reverse transcriptase-PCR (Figure 2—figure supplement 2). Total RNA was isolated using the RNeasy mini Kit (Qiagen) from 106 salivary gland sporozoites collected by dissection of mosquitoes 16 days after feeding with WT, PfΔslarp-a, PfΔslarp-b, PfΔb9Δslarp-F7, and PfΔb9Δslarp-G9 parasites. Remaining DNA was degraded using DNAseI (Invitrogen). cDNA was synthesized using the First Strand cDNA synthesis Kit for RT-PCR AMV (Roche). As a negative control for the presence of genomic DNA, reactions were performed without reverse transcriptase (RT−). PCR amplification was performed for regions of slarp using primers BVS290, BVS292 and for regions of b9 using primers BVS286 and BVS288. Positive control was performed by PCR of 18S rRNA using primers 18Sf and 18Sr.
Phenotype analyses of blood stages of P. berghei and P. falciparum mutants
Request a detailed protocolAsexual multiplication rate and gametocyte production of P. berghei blood stages were determined as described (Annoura et al., 2012). The P. berghei mutants were maintained in Swiss mice. The multiplication rate of blood stages and gametocyte production were determined during the cloning procedure (Janse et al., 2006b) and were not different from parasites of the reference ANKA lines. P. falciparum blood stage development and gametocyte production were analyzed as described (van Schaijk et al., 2010).
Analysis of P. berghei and P. falciparum sporozoite production and in vitro motility, hepatocyte traversal, and infectivity of sporozoites
Request a detailed protocolFeeding of A. stephensi mosquitoes with P. berghei and P. falciparum, determination of oocyst production and sporozoite collection, as well as P. berghei gliding motility were performed as described (Annoura et al., 2012). P. falciparum gliding motility of sporozoites was determined as described (Stewart and Vanderberg, 1988; van Schaijk et al., 2008). P. falciparum cell traversal and invasion of hepatocytes were determined in Huh7 cells and primary human hepatocytes respectively as described (van Schaijk et al., 2008). Infectivity of P. berghei sporozoites and development was determined in cultures of Huh7 cells as described (van Schaijk et al., 2008). For analysis of liver-stage development by immunofluorescence, parasites were stained with the following primary antibodies: anti-PbEXP1 (PBANKA_092670; raised in chicken (Sturm and Heussler, 2007)) and anti-PbHSP70 (PBANKA_081890; raised in mouse (Mueller et al., 2005)). Infectivity of P. falciparum sporozoites and development was analyzed in primary human hepatocytes as described (van Schaijk et al., 2008). Briefly for analysis of development by immunofluorescence, parasites were stained with the following primary antibodies: anti-HSP70 (PF3D7_0930300 (Renia et al., 1990)) and anti-CSP (PF3D7_0304600; 3SP2) using double labeling. Anti-mouse secondary antibodies, conjugated to Alexa-488 or Alexa-594 (Invitrogen), were used for visualization. Primary human hepatocytes were isolated from healthy parts of human liver fragments, which were collected during unrelated surgery in agreement with French national ethical regulations (Gouagna et al., 2007) and after oral informed consent from adult patients undergoing partial hepatectomy as part of their medical treatment (Service de Chirurgie Digestive, Hépato-Bilio-Pancréatique et Transplantation Hépatique, Hôpital Pitié-Salpêtrière, Paris, France). The collection and use of this material for the purposes of the study presented here were undertaken in accordance with French national ethical guidelines under Article L. 1121-1 of the ‘Code de la Santé Publique’. Given that the tissue samples are classed as surgical waste, that they were used anonymously (the patient's identity is inaccessible to the researchers), and that they were not in any way genetically manipulated, article L. 1211-2 stipulates that their use for research purposes is allowed provided that the patient does not express any opposition to the surgeon prior to surgery and after being informed of the nature of the research in which they might be potentially employed. Within this framework, the collection and use of this material was furthermore approved by the Institutional Review Board (Comité de Protection des Personnes) of the Centre Hospitalo-Universitaire Pitié-Salpêtrière, Assistance Publique-Hôpitaux de Paris, France.
Analysis of P. berghei sporozoite infectivity in mice and in vivo imaging of liver-stage development
Request a detailed protocolC57BL/6 or BALB/c mice were inoculated with sporozoites by intravenous injection of different sporozoite numbers, ranging from 1 × 104 to 5 × 105. Blood stage infections were monitored by analysis of Giemsa-stained thin smears of tail blood collected on day 4–14 after inoculation of sporozoites. The prepatent period (measured in days post sporozoite infection) is defined as the day when a blood stage infection with a parasitemia of 0.5–2% is observed. Liver-stage development in live mice was monitored by real time in vivo imaging of liver-stages as described (Ploemen et al., 2012). Liver-stages were visualized by measuring luciferase activity of parasites (expressing luciferase under the eef1a promoter) in whole bodies of mice (Ploemen et al., 2009).
Immunizations of mice with P. berghei sporozoites
Request a detailed protocolPrior to immunization, P. berghei sporozoites were collected at day 21–27 after mosquito infection by hand-dissection. Salivary glands were collected in DMEM (Dulbecco's Modified Eagle Medium from GIBCO) and homogenized in a homemade glass grinder. The number of sporozoites was determined by counting in triplicate in a Bürker-Türk counting chamber using phase-contrast microscopy. BALB/c and C57BL/6 mice were immunized by intravenous injection using different numbers of mutant sporozoites. BALB/c mice received one immunization and C57BL/6 mice received three immunizations with two 7 day intervals. Immunized mice were monitored for blood infections by analysis of Giemsa stained films of tail blood at day 4–16 after immunization. Immunized mice were challenged at different time points after immunization by intravenous injection of 1 × 104 sporozoites from the P. berghei ANKA reference line cl15cy1. In each experiment, age matched naive mice were included to verify infectivity of the sporozoites used for challenge. After challenge, mice were monitored for blood infections by analysis of Giemsa stained films of tail blood at day 4–21.
Development of Pf Δb9Δslarp GAP in chimeric mice engrafted with human hepatocytes
Request a detailed protocolHuman liver-uPA-SCID mice were produced as described before (Meuleman et al., 2005). Briefly, within two weeks after birth homozygous uPA+/+-SCID mice (Foquet et al., 2013) were transplanted with approximately 106 cryopreserved primary human hepatocytes obtained from a single donor (BD Biosciences, Erembodegem, Belgium). To evaluate successful engraftment, human albumin was quantified in mouse plasma with an in-house ELISA (Bethyl Laboratories Inc., Montgomery, TX). The study protocol was approved by the animal ethics committee of the Faculty of Medicine and Health Sciences of the Ghent University. Human liver-uPA-SCID mice (n = 10) and non-chimeric heterozygous uPA+/−-SCID mice (control, n = 2) were intravenously injected with 106 fresh isolated PfΔb9Δslarp-G9 or as a control WT sporozoites. One and 5 days post-infection livers were removed and each liver was cut into 12 standardized sections and stored in RNAlater (Sigma) at 4°C until analysis as described (Foquet et al., 2013). From each part DNA was extracted to assess the parasite load by Pf18S qPCR and to assess the number of human and mouse hepatocytes by Multiplex qPCR PTGER2 analysis (Foquet et al., 2013).
While this manuscript was in preparation an article was published that also describes a multiple-gene deletion P. falciparum parasite that has undergone pre-clinical evaluation (Mikolajczak et al, 2014). In that study, the authors describe a P. falciparum mutant that, like our work, also lacks the gene slarp (sap1) as well as the paralogous pair of genes, p52 and p36.
Data and Materials availability
Request a detailed protocolThe materials described in this study must be acquired through a material transfer agreement.
References
-
Whole parasite vaccination approaches for prevention of malaria infectionTrends in Immunology 33:247–254.https://doi.org/10.1016/j.it.2012.02.001
-
Immunization of man against sporozite-induced falciparum malariaThe American Journal of the Medical Sciences 266:169–177.https://doi.org/10.1097/00000441-197309000-00002
-
Guideline on environmental risk assessments for medicinal products consisting of, or containing, genetically modified organisms (gmos)European Medicines Agency, Available at http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500003805.
-
Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protectionInternational Journal for Parasitology 37:1511–1519.https://doi.org/10.1016/j.ijpara.2007.05.005
-
A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycleMolecular and Biochemical Parasitology 137:23–33.https://doi.org/10.1016/j.molbiopara.2004.04.007
-
Role of heat-labile serum factor or host complement in the inhibition of Plasmodium falciparum sporogonic stages in Anopheles stephensi by gametocyte carriers' serological factorsParasitology 134:1315–1327.
-
Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoitesThe Journal of Infectious Diseases 185:1155–1164.https://doi.org/10.1086/339409
-
High efficiency transfection of Plasmodium berghei facilitates novel selection proceduresMolecular and Biochemical Parasitology 145:60–70.https://doi.org/10.1016/j.molbiopara.2005.09.007
-
Genetic engineering of attenuated malaria parasites for vaccinationCurrent Opinion in Biotechnology 23:908–916.https://doi.org/10.1016/j.copbio.2012.04.003
-
Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systemsProceedings of the National Academy of Sciences of USA 103:8840–8845.https://doi.org/10.1073/pnas.0601876103
-
A research agenda for malaria eradication: vaccinese1000398, PLoS Medicine, 8, 10.1371/journal.pmed.1000398.
-
Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interfaceProceedings of the National Academy of Sciences of USA 102:3022–3027.https://doi.org/10.1073/pnas.0408442102
-
Liver or blood-stage arrest during malaria sporozoite immunization: the later the better?Trends in Parasitology 29:304–310.https://doi.org/10.1016/j.pt.2013.03.008
-
The potential role of vaccines in the elimination of falciparum malaria and the eventual eradication of malariaThe Journal of Infectious Diseases 200:1646–1649.https://doi.org/10.1086/646613
-
Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro cultureTropical and Geographical Medicine 33:50–54.
-
Cultivation of fertile Plasmodium falciparum gametocytes in semi-automated systems. 1. Static culturesTransactions of the Royal Society of Tropical Medicine and Hygiene 76:812–818.https://doi.org/10.1016/0035-9203(82)90116-X
-
Protection against a malaria challenge by sporozoite inoculationThe New England Journal of Medicine 361:468–477.https://doi.org/10.1056/NEJMoa0805832
-
Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoitesThe American Journal of Tropical Medicine and Hygiene 88:5–13.https://doi.org/10.4269/ajtmh.2012.12-0613
-
BookMolecular Cloning: A Laboratory Manual (3rd edition)Cold Spring Harbor Laboratory press, Cold Spring Harbor.
-
Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motilityThe Journal of Protozoology 35:389–393.https://doi.org/10.1111/j.1550-7408.1988.tb04115.x
-
Live and let die: manipulation of host hepatocytes by exoerythrocytic Plasmodium parasitesMedical Microbiology and Immunology 196:127–133.https://doi.org/10.1007/s00430-007-0044-3
-
Application of genetic engineering to research on tropical disease pathogens with special reference to Plasmodia379–387, Application of genetic engineering to research on tropical disease pathogens with special reference to Plasmodia, Bangkok.
-
Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cellsProceedings of the National Academy of Sciences of USA 102:12194–12199.https://doi.org/10.1073/pnas.0500925102
-
Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by designProceedings of the National Academy of Sciences of USA 106:13004–13009.https://doi.org/10.1073/pnas.0906387106
Article and author information
Author details
Funding
Top Institute Pharma (TI Pharma) (T4-102)
- Ben C L van Schaijk
- Ivo H J Ploemen
- Takeshi Annoura
- Martijn W Vos
- Geert-Jan van Gemert
- Severine Chevalley-Maurel
- Marga van de Vegte-Bolmer
- Mohammed Sajid
- Cornelius C Hermsen
- Stephen L Hoffman
- Chris J Janse
- Robert W Sauerwein
The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We would like to thank the following people from RUMC (Nijmegen) for technical support: Claudia Lagarde, Alex Ignacio, Daniëlle Janssen, Rianne Siebelink-Stoter, Wouter Graumans, Jolanda Klaassen, Laura Pelser-Posthumus, Astrid Pouwelsen, and Jacqueline Kuhnen; and the following people from LUMC (Leiden) for technical support: Jai Ramesar, Jing-wen Lin, and Hans Kroeze. We acknowledge the Sanaria Manufacturing Team for the GMP produced working cell bank of PfNF54. Sanaria’s development efforts supported in part by NIAID Small Business Innovation Research grants, 5R44AI069631 and 5R44AI058375.
Ethics
Human subjects: Primary human hepatocytes were isolated from healthy parts of human liver fragments which were collected during unrelated surgery in agreement with French national ethical regulations and after oral informed consent from adult patients undergoing partial hepatectomy as part of their medical treatment (Service de Chirurgie Digestive, Hépato-Bilio-Pancréatique et Transplantation Hépatique, Hôpital Pitié-Salpêtrière, Paris, France). The collection and use of this material for the purposes of the study presented here were undertaken in accordance with French national ethical guidelines under Article L. 1121-1 and article L. 1211-2.
Animal experimentation: All animal experiments with rodent parasites performed at the LUMC (Netherlands) were approved by the Animal Experiments Committee of the Leiden University Medical Center (DEC 07171; DEC 10099) and at the RUNMC (Netherlands) by the Radboud University Experimental Animal Ethical Committee (RUDEC 2008-123, RUDEC 2008-148, RUDEC 2010-250, RUDEC 2011-022, RUDEC 2011-208). The Dutch Experiments on Animal Act is established under European guidelines (EU directive 86/609/CEE) regarding the Protection of Animals used for Experimental and Other Scientific Purposes. Human liver-uPA-SCID mice (chimeric mice) were produced as described before. The study protocol for infecting these mice with P. falciparum sporozoites was approved by the animal ethics committee of the Faculty of Medicine and Health Sciences of the Ghent University. The study protocol was approved by the animal ethics committee of the Faculty of Medicine and Health Sciences of the Ghent University.
Version history
- Received: June 4, 2014
- Accepted: November 19, 2014
- Accepted Manuscript published: November 19, 2014 (version 1)
- Accepted Manuscript updated: November 21, 2014 (version 2)
- Version of Record published: December 23, 2014 (version 3)
Copyright
© 2014, van Schaijk et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,797
- Page views
-
- 316
- Downloads
-
- 66
- Citations
Article citation count generated by polling the highest count across the following sources: Scopus, Crossref, PubMed Central.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Microbiology and Infectious Disease
For most retroviruses, including HIV, association with the plasma membrane (PM) promotes the assembly of immature particles, which occurs simultaneously with budding and maturation. In these viruses, maturation is initiated by oligomerization of polyprotein precursors. In contrast, several retroviruses, such as Mason-Pfizer monkey virus (M-PMV), assemble in the cytoplasm into immature particles that are transported across the PM. Therefore, protease activation and specific cleavage must not occur until the pre-assembled particle interacts with the PM. This interaction is triggered by a bipartite signal consisting of a cluster of basic residues in the matrix (MA) domain of Gag polyprotein and a myristoyl moiety N-terminally attached to MA. Here, we provide evidence that myristoyl exposure from the MA core and its insertion into the PM occurs in M-PMV. By a combination of experimental methods, we show that this results in a structural change at the C-terminus of MA allowing efficient cleavage of MA from the downstream region of Gag. This suggests that, in addition to the known effect of the myristoyl switch of HIV-1 MA on the multimerization state of Gag and particle assembly, the myristoyl switch may have a regulatory role in initiating sequential cleavage of M-PMV Gag in immature particles.
-
- Microbiology and Infectious Disease
- Physics of Living Systems
Natural environments of living organisms are often dynamic and multifactorial, with multiple parameters fluctuating over time. To better understand how cells respond to dynamically interacting factors, we quantified the effects of dual fluctuations of osmotic stress and glucose deprivation on yeast cells using microfluidics and time-lapse microscopy. Strikingly, we observed that cell proliferation, survival, and signaling depend on the phasing of the two periodic stresses. Cells divided faster, survived longer, and showed decreased transcriptional response when fluctuations of hyperosmotic stress and glucose deprivation occurred in phase than when the two stresses occurred alternatively. Therefore, glucose availability regulates yeast responses to dynamic osmotic stress, showcasing the key role of metabolic fluctuations in cellular responses to dynamic stress. We also found that mutants with impaired osmotic stress response were better adapted to alternating stresses than wild-type cells, showing that genetic mechanisms of adaptation to a persistent stress factor can be detrimental under dynamically interacting conditions.





