Membrane Protein Topology: The messy process of guiding proteins into membranes
One of the keys to predicting the three-dimensional structure of a membrane protein from its sequence of amino acid residues is to understand how structures called translocons guide the protein to its final folded state. Translocons are generally thought of as channels that allow proteins to cross cell membranes. In eukaryotes, it is thought that newly-formed secreted proteins pass through the Sec61 translocon as they emerge from the ribosome. New membrane proteins are thought to follow a similar path, except that the hydrophobic transmembrane helices in these proteins are diverted sideways so that they become embedded in the cell membrane. This ‘sequential-insertion’ scheme seems logical in the context of what we know about the structure of translocons (Rapoport et al., 2004; Cymer et al., 2015), but is it correct?
We cannot answer this question because we do not have experimental methods that can follow, residue-by-residue, the insertion and folding of the protein chains as they pass from the ribosome and into the membrane. The alternative is to simulate the process. However, a newly-formed protein chain elongates at a rate of about one residue every 50–100 milliseconds, which is orders of magnitude faster than can be modeled using standard molecular dynamics simulation methods. Now, in eLife, Reid van Lehn, Bin Zhang and Thomas Miller of the California Institute of Technology report a simplified approach that allows insertion and folding to be simulated on biological time scales (Van Lehn et al., 2015). Their results suggest that the membrane protein insertion/folding process is more complicated than commonly depicted in the sequential-insertion scheme.
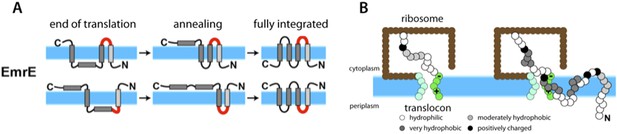
Van Lehn et al. modeled a protein called EmrE that sits in the inner membrane of Escherichia coli bacteria and is able to transport a wide range of antibiotic drugs out of the cell. This helps to make the bacteria resistant to these treatments. EmrE is a homodimer, and each monomer has four transmembrane helices (Chen et al., 2007). EmrE is unusual in that the two monomers are oriented in opposite directions (Figure 1A): this is known as dual topology.
Simulations suggest that membrane proteins take on their final structure after they have been inserted into the membrane.
(A) The topologies of the EmrE monomers first inserted into the cytoplasmic membrane (blue band) at the end of translation (left) do not necessarily reflect the final topologies, which are subsequently achieved through thermodynamics-driven annealing. The interhelical loops in red represent the loops that flip most slowly, and thereby have a major influence on the kinetics of folding. EmrE can take on two different, antiparallel topologies; each row in the figure shows how one of these topologies may develop. (B) Van Lehn et al. used a coarse-grained model to simulate the insertion and folding of the EmrE dual-topology membrane protein (Zhang and Miller, 2012). Coarse-grained beads are assigned approximate hydrophobicity values (indicated by the shadings of the beads). The ribosome (brown) and translocon (green) are also represented as coarse-grained beads. The translocon is negatively charged on the cytoplasmic end and positively charged at the periplasmic end to represent the known charge distribution of the Sec 61 translocon (Goder et al., 2004). The simulation proceeds by adding a bead at the C-terminus of the nascent chain every 125 milliseconds; the panel on the right shows the chain on the left at a later point in time. Figure adapted from Figures 1 and 4 of Van Lehn et al. (2015).
The topology (orientation) of membrane proteins is largely determined by the positive-inside rule (von Heijne, 1986). This rule suggests that if the connecting loops that join the transmembrane regions of the protein are rich in lysine and arginine residues, then these loops tend to orient inward, toward the cytoplasm of the cell. This is known as the K+R bias. EmrE, which is encoded in a single gene, has a weak K+R bias, and this means that the monomers can be inserted into the membrane in one of two opposite orientations (Rapp et al., 2006, 2007).
In 2010, researchers at Stockholm University reported, based on extensive mutation studies, that a single positively charged residue placed in different positions throughout the protein can control the topology of EmrE monomers and affect whether parallel or anti-parallel dimers form (Seppälä et al., 2010). Given the positive-inside rule and the sequential-insertion scheme, one would expect positive charges in the C-terminal region of a membrane protein to have a smaller influence on topology than charges in the N-terminal region. However, Seppälä et al. discovered that a single positive charge at the C-terminus itself could determine the orientation of EmrE!
Because the positive-inside rule was robustly verified in the Stockholm experiments, a logical conclusion is that the sequential-insertion scheme does not describe accurately how EmrE, and perhaps other membrane proteins, fold inside cells. The simulations now performed by Van Lehn et al. divulge the missing ingredients of membrane protein folding: stochastic insertion and post-insertion annealing. By stochastic insertion, I mean that protein chains can have various topologies after they have been made, creating what Van Lehn et al. refer to as an ‘end-of-translation ensemble’ (Figure 1A). After being inserted into the membrane, the members of the ensemble that are not initially in their lowest thermodynamic free energy state subsequently relax to their preferred topology through a process called annealing. In the case of EmrE, antiparallel dimers can form because there are two final topologies that have similar free energies.
Van Lehn et al. increased the speed of the simulations by treating the nascent protein chain as a sequence of coarse-grained beads, with each bead representing several amino acids (Figure 1B). Four beads were used to represent the transmembrane helices and five beads were to used represent the loops that connect these helices. Certain properties of the amino acid residues that are known to affect the topology of a protein were also incorporated into the simulation: for example, hydrophobicities were assigned to the beads using an experimentally-determined hydrophobicity scale (Wimley et al., 1996). Particularly important was the assignment of positive charges in the connecting loops between the transmembrane helices to mimic the mutation experiments of Seppälä et al. (2010). The ribosome and translocon were also represented by simple two-dimensional structures composed of coarse-grained beads (Zhang and Miller, 2012; Figure 1B). Crucially, the model translocon used in the simulations had two negative charges on its cytoplasmic side and two positive charges on its periplasmic side to mimic the known net charge distribution of the translocon (Goder et al., 2004).
The simulations were performed by adding a new bead at the C-terminal of the nascent chain every 125 milliseconds. In this way, van Lehn et al. simulated the insertion and folding of the many mutant EmrE proteins studied by Seppälä et al. (2010) and found remarkable agreement with the experimentally determined topologies.
The simulations of van Lehn et al. show that the stochastic insertion of newly-formed protein chains into the membrane, followed by thermodynamics-driven annealing, is a viable alternative to the current sequential-insertion view. What is needed now is direct experimental verification of how transmembrane proteins are inserted into the membrane. This will require new methods that can directly follow insertion and folding on the biological time scale.
References
-
X-ray structure of EmrE supports dual topology modelProceedings of the National Academy of Sciences of USA 104:18999–19004.https://doi.org/10.1073/pnas.0709387104
-
Mechanisms of integral membrane protein insertion and foldingJournal of Molecular Biology 427:999–1022.https://doi.org/10.1016/j.jmb.2014.09.014
-
Sec61p contributes to signal sequence orientation according to the positive-inside ruleMolecular Biology of the Cell 15:1470–1478.https://doi.org/10.1091/mbc.E03-08-0599
-
Membrane-protein integration and the role of the translocation channelTrends in Cell Biology 14:568–575.https://doi.org/10.1016/j.tcb.2004.09.002
-
Identification and evolution of dual-topology membrane proteinsNature Structural & Molecular Biology 13:112–116.https://doi.org/10.1038/nsmb1057
-
The distribution of positively charged residues in bacterial inner membrane proteins correllates with the trans-membrane topologyEMBO Journal 5:3021–3027.
Article and author information
Author details
Publication history
- Version of Record published: November 6, 2015 (version 1)
Copyright
© 2015, White
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 3,040
- views
-
- 387
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
- Cell Biology
Mediator of ERBB2-driven Cell Motility 1 (MEMO1) is an evolutionary conserved protein implicated in many biological processes; however, its primary molecular function remains unknown. Importantly, MEMO1 is overexpressed in many types of cancer and was shown to modulate breast cancer metastasis through altered cell motility. To better understand the function of MEMO1 in cancer cells, we analyzed genetic interactions of MEMO1 using gene essentiality data from 1028 cancer cell lines and found multiple iron-related genes exhibiting genetic relationships with MEMO1. We experimentally confirmed several interactions between MEMO1 and iron-related proteins in living cells, most notably, transferrin receptor 2 (TFR2), mitoferrin-2 (SLC25A28), and the global iron response regulator IRP1 (ACO1). These interactions indicate that cells with high MEMO1 expression levels are hypersensitive to the disruptions in iron distribution. Our data also indicate that MEMO1 is involved in ferroptosis and is linked to iron supply to mitochondria. We have found that purified MEMO1 binds iron with high affinity under redox conditions mimicking intracellular environment and solved MEMO1 structures in complex with iron and copper. Our work reveals that the iron coordination mode in MEMO1 is very similar to that of iron-containing extradiol dioxygenases, which also display a similar structural fold. We conclude that MEMO1 is an iron-binding protein that modulates iron homeostasis in cancer cells.
-
- Biochemistry and Chemical Biology
- Structural Biology and Molecular Biophysics
NADPH oxidases (NOX) are transmembrane proteins, widely spread in eukaryotes and prokaryotes, that produce reactive oxygen species (ROS). Eukaryotes use the ROS products for innate immune defense and signaling in critical (patho)physiological processes. Despite the recent structures of human NOX isoforms, the activation of electron transfer remains incompletely understood. SpNOX, a homolog from Streptococcus pneumoniae, can serves as a robust model for exploring electron transfers in the NOX family thanks to its constitutive activity. Crystal structures of SpNOX full-length and dehydrogenase (DH) domain constructs are revealed here. The isolated DH domain acts as a flavin reductase, and both constructs use either NADPH or NADH as substrate. Our findings suggest that hydride transfer from NAD(P)H to FAD is the rate-limiting step in electron transfer. We identify significance of F397 in nicotinamide access to flavin isoalloxazine and confirm flavin binding contributions from both DH and Transmembrane (TM) domains. Comparison with related enzymes suggests that distal access to heme may influence the final electron acceptor, while the relative position of DH and TM does not necessarily correlate with activity, contrary to previous suggestions. It rather suggests requirement of an internal rearrangement, within the DH domain, to switch from a resting to an active state. Thus, SpNOX appears to be a good model of active NOX2, which allows us to propose an explanation for NOX2’s requirement for activation.





