Proteomics: Dissecting apoptosis the omics way
Most anticancer drugs work by triggering tumour cells to undergo programmed cell death, or apoptosis. However, many tumour cells activate a stress response in an attempt to combat the effects of the drugs. To date, most studies that have examined this response have focused on how the drugs affect the production of messenger RNA (mRNA) via transcription in the tumour cells, and much less is known about the effects of these drugs on the translation of the mRNA, or on proteins. Now, in eLife, James Wells of the University of California, San Francisco and co-workers—including Arun Wiita as first author—use a new approach to study changes in transcription, translation and protein degradation in tumour cells exposed to an anticancer drug. They show that by targeting key regulators of the stress response, they can lower the threshold for triggering apoptosis in the tumour cells (Wiita et al., 2013).
To study apoptosis in detail, Wiita et al. used a systems-level approach to examine transcription, translation and protein degradation (proteolysis) in a myeloma cell line—a type of cancer of white blood cells—exposed to an anticancer drug called bortezomib. They paired mRNA sequencing with a technique called ribosome profiling, in which the presence of a ribosome bound to mRNA (a ‘footprint’) is used to reveal which mRNAs are being translated. This technique has proven to be indispensable for the genome-wide study of translation (Ingolia et al., 2009). Wiita et al. used an additional suite of tools to study the abundance of proteins and the rate at which they were broken down by apoptotic proteases (Mahrus et al., 2008; Agard et al., 2012).
By comparing the number of ribosome footprints with the number of mRNA sequencing reads, it was possible to deduce the efficiency of translation within the tumour cells (Figure 1). In line with previous reports, they found that bortezomib—which inhibits a complex called the proteasome—caused misfolded proteins to accumulate inside cells. As a result, the endoplasmic reticulum (which is involved in protein trafficking) became overwhelmed, triggering the unfolded protein response (Obeng et al., 2006). This leads to a series of cellular processes to counteract the accumulation of misfolded proteins, including increased protein folding, inhibition of translation, and, as a last resort, the induction of apoptosis. Witta et al. showed that bortezomib increased both transcription and translation of a number of genes involved in the folding or degradation of proteins, and downregulated the expression of genes involved in cellular proliferation.
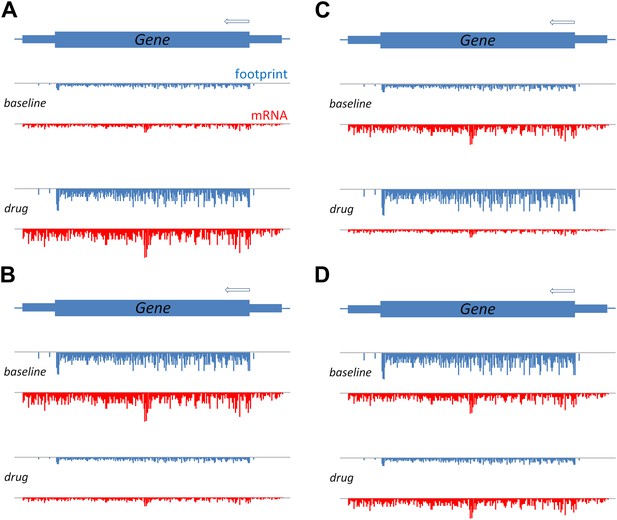
A schematic to show how translation efficiency can be assessed by means of ribosome profiling.
The production of a protein from a gene involves transcription, which can be measured in terms of mRNA sequence reads (red), and translation, which can be measured in terms of ribosome footprints (blue). (A) Relative to a baseline condition (top), bortezomib increases both the transcription and the translation of certain genes, as can be seen from the mRNA levels and the ribosome footprints (bottom). (B) Bortezomib can also decrease both transcription and translation. For other genes, bortezomib had differing effects on these two processes: an increase in protein levels relative to baseline, despite a reduction in mRNA, indicates that the efficiency of translation is increased (C); and a reduction in protein levels relative to baseline, despite no change in mRNA, indicates that the efficiency of translation is reduced (D).
The coding sequence of genes is preceded by an upstream regulatory region called the 5′-leader. Ribosomes have frequently been shown to initiate translation from within this 5′-leader when cells are exposed to oxidative stress (Gerashchenko et al., 2012) or undergo differentiation (Ingolia et al., 2011), thereby providing another level of translational control. Ribosome occupancy of the 5′-leader sequence may indicate the translation of short regulatory polypeptides encoded by upstream open reading frames, or point to the expression of alternative isoforms of the protein extended at the N-terminus (Ingolia et al., 2011). Wiita et al. found that genes whose expression was regulated by bortezomib showed altered translation from the 5′-leader compared to non-regulated genes. Nevertheless, despite the relatively large changes in mRNA and footprint reads that were seen across the tumour cell genes, the vast majority of proteins showed no changes in their relative abundance, as inferred from quantitative proteomics data.
Based on estimates of the absolute levels of transcripts and proteins, Wiita et al. then devised a model to account for the limited changes in protein levels relative to those of mRNA and mRNA-translation events. Their proposed ‘mass-action model of translation’ describes changes in the number of copies of a protein per cell as a function of the number of copies of a transcript per cell, as well as the rates of mRNA translation and protein degradation. The model demonstrates that increases in the abundance of proteins tend to be coupled to increases in absolute transcript levels, and implies that for the same increase in relative transcript levels, low abundance proteins will undergo a larger increase in relative protein abundance than will high abundance proteins. Finally, the results of Wiita et al. revealed that proteins that were upregulated in response to bortezomib were not preferentially processed by proteases relative to other proteins, suggesting that the changes in transcription and translation do not affect the dynamics of protein processing once apoptosis ensues.
Myeloma cells produce large quantities of antibodies and have therefore adapted to cope with an increased burden of secretory protein synthesis and folding, but these changes have also made them particularly sensitive to the effects of proteasome inhibition. Although cells can adapt to protein folding insults following chronic exposure (Jager et al., 2012), the acute nature of bortezomib treatment is likely to prevent such adaptation, despite the translational re-programming observed in bortezomib treated myeloma cells. Adaptation has also been linked to low levels of activation of the stress response and a distinct pattern of protein expression (Rutkowski et al., 2006). Since adaptation could underlie the ability of cells to become resistant to chemotherapy, it would be interesting to compare the responses of resistant and sensitive cells. It also remains to be explored how the actions of bortezomib relate to those of other chemotherapeutic drugs and other apoptosis inducing agents. Overall, it is likely that efforts to integrate the transcriptome, translatome, proteome and degradome responses will typify future systems biology research and lead to new molecular insights into cancer and the drugs used to treat it.
References
-
Global kinetic analysis of proteolysis via quantitative targeted proteomicsProc Natl Acad Sci USA 109:1913–1918.https://doi.org/10.1073/pnas.1117158109
-
Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stressProc Natl Acad Sci USA 109:17394–17399.https://doi.org/10.1073/pnas.1120799109
Article and author information
Author details
Publication history
- Version of Record published: November 12, 2013 (version 1)
Copyright
© 2013, Van Damme
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 378
- views
-
- 29
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
Transporter research primarily relies on the canonical substrates of well-established transporters. This approach has limitations when studying transporters for the low-abundant micromolecules, such as micronutrients, and may not reveal physiological functions of the transporters. While d-serine, a trace enantiomer of serine in the circulation, was discovered as an emerging biomarker of kidney function, its transport mechanisms in the periphery remain unknown. Here, using a multi-hierarchical approach from body fluids to molecules, combining multi-omics, cell-free synthetic biochemistry, and ex vivo transport analyses, we have identified two types of renal d-serine transport systems. We revealed that the small amino acid transporter ASCT2 serves as a d-serine transporter previously uncharacterized in the kidney and discovered d-serine as a non-canonical substrate of the sodium-coupled monocarboxylate transporters (SMCTs). These two systems are physiologically complementary, but ASCT2 dominates the role in the pathological condition. Our findings not only shed light on renal d-serine transport, but also clarify the importance of non-canonical substrate transport. This study provides a framework for investigating multiple transport systems of various trace micromolecules under physiological conditions and in multifactorial diseases.
-
- Biochemistry and Chemical Biology
- Cell Biology
Mediator of ERBB2-driven Cell Motility 1 (MEMO1) is an evolutionary conserved protein implicated in many biological processes; however, its primary molecular function remains unknown. Importantly, MEMO1 is overexpressed in many types of cancer and was shown to modulate breast cancer metastasis through altered cell motility. To better understand the function of MEMO1 in cancer cells, we analyzed genetic interactions of MEMO1 using gene essentiality data from 1028 cancer cell lines and found multiple iron-related genes exhibiting genetic relationships with MEMO1. We experimentally confirmed several interactions between MEMO1 and iron-related proteins in living cells, most notably, transferrin receptor 2 (TFR2), mitoferrin-2 (SLC25A28), and the global iron response regulator IRP1 (ACO1). These interactions indicate that cells with high MEMO1 expression levels are hypersensitive to the disruptions in iron distribution. Our data also indicate that MEMO1 is involved in ferroptosis and is linked to iron supply to mitochondria. We have found that purified MEMO1 binds iron with high affinity under redox conditions mimicking intracellular environment and solved MEMO1 structures in complex with iron and copper. Our work reveals that the iron coordination mode in MEMO1 is very similar to that of iron-containing extradiol dioxygenases, which also display a similar structural fold. We conclude that MEMO1 is an iron-binding protein that modulates iron homeostasis in cancer cells.





