Meiotic Drivers: Cheaters divide and conquer
Cheaters who act selfishly to prosper at the expense of others are commonplace in the natural world, and genomes are no exception. Humans typically have two copies of each gene: we inherit one copy from our mother and the other from our father—and, if we have a child, we will pass on one of these copies essentially at random. However, there are genes or genetic elements that subvert the fairness of inheritance, often in creative and insidious ways, solely for their own benefit.
Some of these selfish genetic elements ensure that they get passed on to an individual's offspring more often than they should, by making their way into more than half of that individual’s gametes (e.g., sperm and egg cells in animals, or spores in fungi). Some of the best-studied examples of these genes are those that essentially commit a kind of fratricide to get ahead. Gamete killers directly cripple or kill any of their ‘sibling’ sperm or spores that did not inherit the killer gene, and these genes have been discovered in mice, flies and various fungi (Burt and Trivers, 2006).
Gamete killers are a subset of a broader class of 'meiotic drivers'. Meiotic drivers were originally defined as genes that could directly cheat when chromosomes are being segregated into the gametes (a process called meiosis). However, the term has now been more broadly applied to include any gene that subverts the fairness of inheritance by any means. Now, in eLife, Harmit Malik and colleagues at the Fred Hutchinson Cancer Research Center—including Sarah Zanders as first author—report that three meiotic drivers keep two yeast species reproductively isolated (Zanders et al., 2014).
By circumventing unbiased inheritance, meiotic drivers find shelter from being purged by natural selection acting on their hosts. Even meiotic drivers that cause a drop in fitness (in terms of survival or the number of offspring produced) can thus spread in populations. By eluding fitness-based selection, chromosomes carrying meiotic drivers can accumulate harmful mutations or structural rearrangements and this ‘baggage’ can be dragged along with the driver to higher frequency (Burt and Trivers, 2006). The negative effects of meiotic drivers select for other genes that suppress these effects, and this can initiate a molecular arms race between drivers and suppressors that is predicted to cause rapid evolutionary divergence of these genes. Because they can both rapidly diverge and compromise fertility, it has been suggested that meiotic drivers could cause related populations of organisms to become reproductively isolated (Hurst and Werren, 2001; McDermott and Noor, 2010)—something that might drive the generation of new species.
To date, evidence comes largely from studying the fruit fly Drosophila. But now, Zanders et al. have catalogued genetic elements that contribute to reproductive isolation between two closely related fission yeast species: S. pombe and S. kambucha. S. pombe has been studied as a model organism since the 1950s, while S. kambucha was isolated more recently from a fungus that has been used in China for centuries to make a drink called Che (Singh and Klar, 2002). These two species—which each have three chromosomes—can mate to produce hybrids, but these hybrids have very low fertility and often fail to produce viable spores.
Zanders et al. found that one region of the genome was the opposite way round (or inverted) in S. pombe (compared to S. kambucha), and that two essential genes had switched their positions in the genome of S. kambucha. Both of these rearrangements did affect fertility, but these differences were not sufficient to account for the extremely low spore production of hybrids of these two yeast species. After ruling out several alternatives, Zanders et al. discovered something remarkable: each chromosome in S. kambucha contains a spore killer gene. Spore killers are a type of gamete killer, and each encodes what is essentially a molecular poison. The spores that harbour a particular killer gene are immune to the respective poison, but the details of this immunity remain largely mysterious (though see Hammond et al., 2012). As such, a spore from the S. pombe/S. kambucha hybrid must receive all three killer genes by random segregation to be fully sheltered from all three poisons (Figure 1).
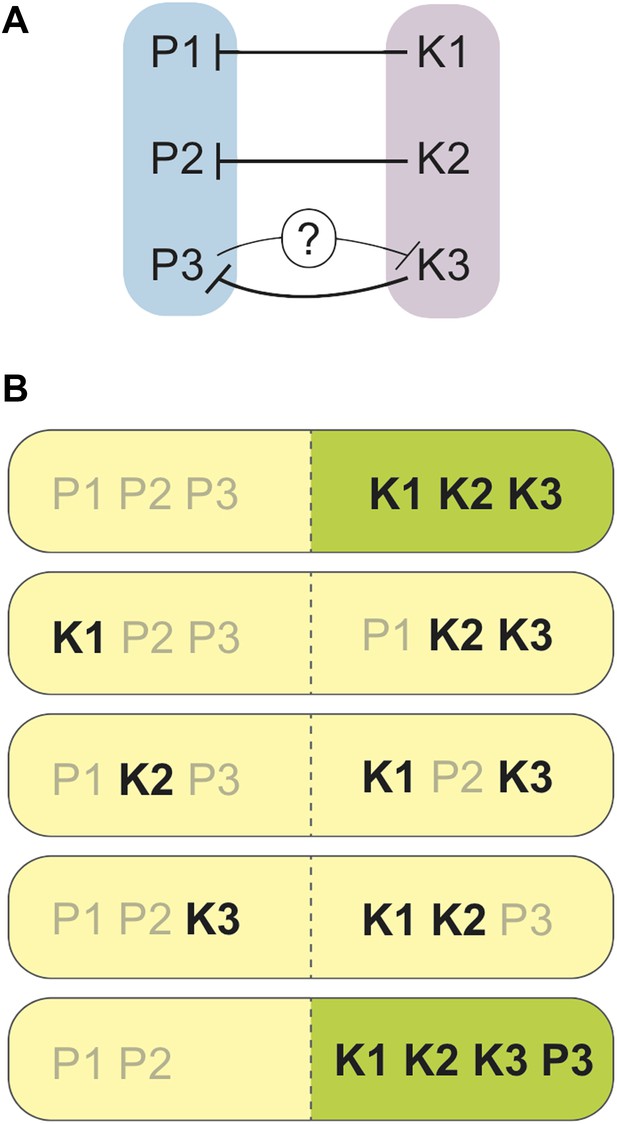
Spore killers interact and determine the survival of spores from S. pombe/S. kambucha hybrids.
(A) Each chromosome in S. kambucha (K1-3) harbours a spore killer gene that works against (black lines) the corresponding chromosome in S. pombe (P1-3). Zanders et al. propose that the P3 chromosome in S. pombe contains a killer gene that works against (black line with question mark) the K3 chromosome in S. kambucha. (B) Combinations of chromosomes generated by random segregation during spore-production by the S. pombe/S. kambucha hybrid, including one aneuploid that inherits copies of chromosome 3 from both parents (bottom). Chromosome combinations predicted to yield viable spores are shown in green, and those predicted to die in yellow. Spore killers are labelled in black.
To add to the complication, two of the killer genes interact: the killer gene on chromosome 2 is stronger when there is also a killer gene on chromosome 3; however, the killer gene on chromosome 3 is weakened by that on chromosome 2. The mechanism of this interaction, and whether it directly results from the killer genes themselves, remains unknown. Furthermore, only hybrid spores that inherit versions of these two chromosomes from the same yeast species (either both from S. pombe or both from S. kambucha) are viable. This is because two essential genes have been swapped between chromosomes 2 and 3 in one of the parent species, and thus a spore must inherit these two chromosomes together, or die because it ends up lacking one or the other of these genes.
There is yet another twist: spores from the hybrids often carried both copies of chromosome 3, one originally from S. pombe and the other from S. kambucha. Having ruled out that hybrids might simply produce more aneuploids (spores with extra or missing chromosomes), Zanders et al. propose that there may be a weaker killer gene on the S. pombe version of chromosome 3, such that aneuploids carrying both versions of chromosome 3 are more likely to survive than spores with only the S. kambucha variant. Whether this is caused by a different version of the same gene, or by a distinct driver that arose independently on S. pombe chromosome 3, will be a very interesting follow-up question.
In summary, Zanders et al. provide an exciting milestone for research on meiotic drive systems and their potential links to speciation. The finding that multiple independent meiotic drivers can differ between even closely related species, can change the structure of genomes, and can also act together to cause a very strong fertility barrier, is an important insight. This study highlights that meiotic drivers need not be rare, and that they can both directly and indirectly affect multiple chromosomes. The identification of the underlying genes, and any suppressors that may exist, will not only allow us to understand the molecular mechanisms of spore killing, but may also clarify how meiotic drivers can arise repeatedly. This study reminds us that much remains to be learned about the dynamics of drivers and possible piggy-back effects on genome architecture, speciation, and extinction.
References
-
BookGenes in conflict: the biology of selfish genetic elementsHarvard University Press.
-
Molecular dissection of Neurospora spore killer meiotic drive elementsProceedings of the National Academy of Sciences of the USA 109:12093–12098.https://doi.org/10.1073/pnas.120326710
-
The role of selfish genetic elements in eukaryotic evolutionNature Reviews Genetics 2:597–606.https://doi.org/10.1038/35084545
-
The role of meiotic drive in hybrid male sterilityPhilosophical Transactions of the Royal Society B 365:1265–1272.https://doi.org/10.1098/rstb.2009.0264
-
The 2.1-kb inverted repeat DNA sequences flank the mat2,3 silent region in two species of Schizosaccharomyces and are involved in epigenetic silencing in Schizosaccharomyces pombeGenetics 162:591–602.
Article and author information
Author details
Publication history
- Version of Record published: June 24, 2014 (version 1)
Copyright
© 2014, Bomblies
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,821
- Page views
-
- 61
- Downloads
-
- 3
- Citations
Article citation count generated by polling the highest count across the following sources: Crossref, Scopus, PubMed Central.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Chromosomes and Gene Expression
Splicing is the stepwise molecular process by which introns are removed from pre-mRNA and exons are joined together to form mature mRNA sequences. The ordering and spatial distribution of these steps remain controversial, with opposing models suggesting splicing occurs either during or after transcription. We used single-molecule RNA FISH, expansion microscopy, and live-cell imaging to reveal the spatiotemporal distribution of nascent transcripts in mammalian cells. At super-resolution levels, we found that pre-mRNA formed clouds around the transcription site. These clouds indicate the existence of a transcription-site-proximal zone through which RNA move more slowly than in the nucleoplasm. Full-length pre-mRNA undergo continuous splicing as they move through this zone following transcription, suggesting a model in which splicing can occur post-transcriptionally but still within the proximity of the transcription site, thus seeming co-transcriptional by most assays. These results may unify conflicting reports of co-transcriptional versus post-transcriptional splicing.
-
- Chromosomes and Gene Expression
- Genetics and Genomics
Heterogeneity in endothelial cell (EC) sub-phenotypes is becoming increasingly appreciated in atherosclerosis progression. Still, studies quantifying EC heterogeneity across whole transcriptomes and epigenomes in both in vitro and in vivo models are lacking. Multiomic profiling concurrently measuring transcriptomes and accessible chromatin in the same single cells was performed on six distinct primary cultures of human aortic ECs (HAECs) exposed to activating environments characteristic of the atherosclerotic microenvironment in vitro. Meta-analysis of single-cell transcriptomes across 17 human ex vivo arterial specimens was performed and two computational approaches quantitatively evaluated the similarity in molecular profiles between heterogeneous in vitro and ex vivo cell profiles. HAEC cultures were reproducibly populated by four major clusters with distinct pathway enrichment profiles and modest heterogeneous responses: EC1-angiogenic, EC2-proliferative, EC3-activated/mesenchymal-like, and EC4-mesenchymal. Quantitative comparisons between in vitro and ex vivo transcriptomes confirmed EC1 and EC2 as most canonically EC-like, and EC4 as most mesenchymal with minimal effects elicited by siERG and IL1B. Lastly, accessible chromatin regions unique to EC2 and EC4 were most enriched for coronary artery disease (CAD)-associated single-nucleotide polymorphisms from Genome Wide Association Studies (GWAS), suggesting that these cell phenotypes harbor CAD-modulating mechanisms. Primary EC cultures contain markedly heterogeneous cell subtypes defined by their molecular profiles. Surprisingly, the perturbations used here only modestly shifted cells between subpopulations, suggesting relatively stable molecular phenotypes in culture. Identifying consistently heterogeneous EC subpopulations between in vitro and ex vivo models should pave the way for improving in vitro systems while enabling the mechanisms governing heterogeneous cell state decisions.





