Coordination of planar cell polarity pathways through Spiny-legs
Figures
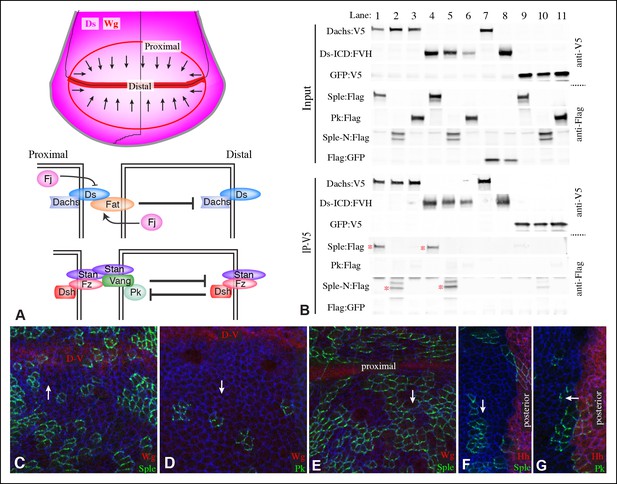
Localization of Pk and Sple in wing discs, and their interaction with Dachs and Ds.
(A) Schematic diagram illustrating the general direction of PCP protein polarity (arrows), expression gradient of Ds (magenta) and organization of Ds-Fat and Fz PCP pathway components in the Drosophila wing disc. (B) Western blots, using antibodies indicated on the right, showing the results of co-immunoprecipitation experiments between V5-tagged Dachs (lanes 1–3,7), Ds-ICD (lanes 4–6,8) or GFP (lanes 9–11) of Flag-tagged Sple (lanes 1,4,9), Sple-N (lanes 2,5,10), Pk (lanes 3,6,11) or GFP (lanes 7,8). Upper panels (Input) show blots on lysates of S2 cells, lower panels (IP-V5) show blots on proteins precipitated from these lysates by anti-V5 beads. Similar results were obtained in three independent biological replicates of this experiment. (C–G) Portions of wing imaginal discs with clones of cells expressing GFP:Sple (C,E,F) or GFP:Pk (D,G) (green), stained for expression of E-cadherin (blue), and showing either anti-Wg (C–E) or hh-Gal4 UAS-mCD8-RFP (F,G) (red). White arrows indicate direction of polarization of Sple or Pk.
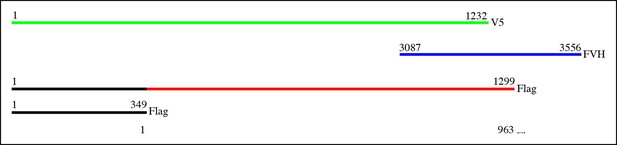
Proteins used in co-immunoprecipitation assays.
Schematics of tagged isoforms of full length Dachs, Ds intracellular domain, full length Sple, Sple N-terminal domain and full length Pk.
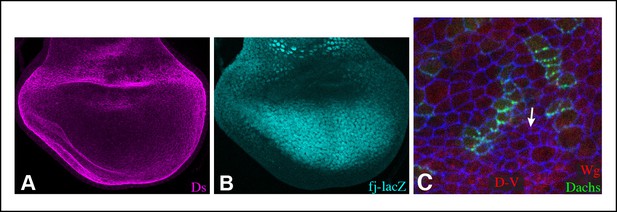
Ds and Fj gradients in wing discs.
(A,B) Expression of Ds (magenta) and fj-lacZ (cyan) in a wild-type wing disc. (C) Portion of a wing disc with clones of cells expressing Dachs:Cit (green), stained for Wg (red) and E-cadherin (blue). White arrow indicates the direction of Dachs polarization.
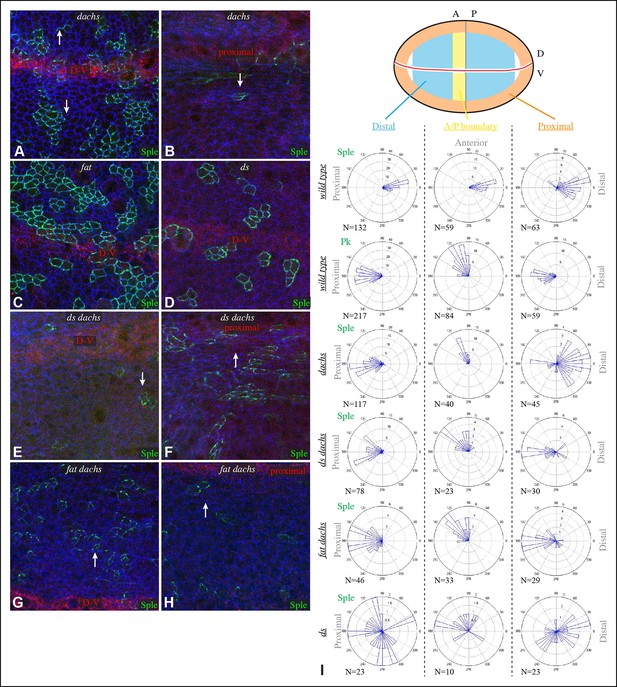
Localization of Sple in Ds-Fat pathway mutants.
(A–H) Portions of wing imaginal discs with clones of cells expressing GFP:Sple (green) in dGC13/ d210 (A and B), ft8/ ftG-rv (C), ds36D/ dsUA071 (D), dGC13 ds36D/ dGC13 dsUA071 (E and F) and dGC13 ft8/ dGC13 ftG-rv (G and H) mutants. Discs were stained for E-cad (blue) and Wg (red). Wg is expressed along the D-V boundary and in proximal rings, the locations of Wg expression shown are indicated. White arrows indicate general direction of Sple polarization. (I) Rose plots summarizing orientation of Sple or Pk in the indicated genotypes, with proximal at left, distal at right, and in the central row, anterior at top. Orientations were scored separately in the three regions depicted in the cartoon, the number of cells scored is indicated by N.
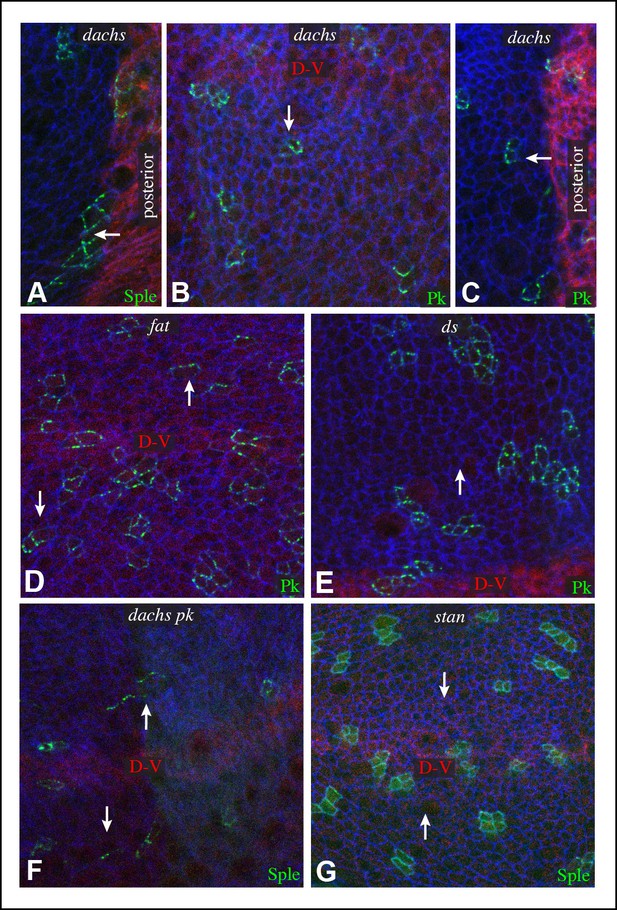
Additional characterization of Pk and Sple localization in mutants.
Portions of wing discs with clones of cells expressing GFP:Sple (A,F,G) or GFP:Pk (B–E) (green) in dGC13/ d210 (A–C), ft8/ ftG-rv (D), ds36D/ dsUA071 (E), dGC13 pk30 (F), and vangstbm6(G) mutants. Discs were stained for E-cad (blue) and Wg (red) (B,D,E,F and G) or hh-Gal4 UAS-mCD8-RFP (red) (A,C). The white arrows indicate direction of polarization.
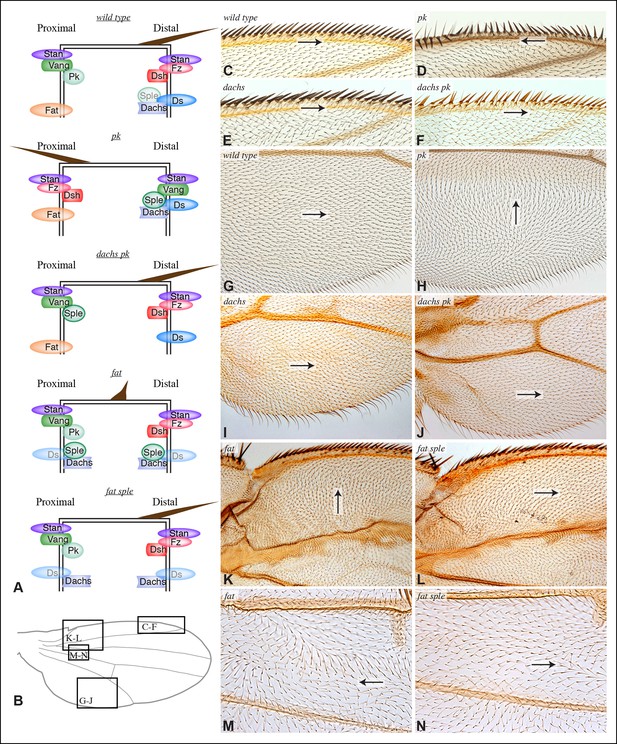
Contribution of Dachs and Sple to PCP mutant wing phenotypes.
(A) Cartoons depicting inferred protein localization and hair orientation (brown) in wing cells of the indicated genotypes to explain rescue of pk by dachs, and rescue of fat by sple. Faint Sple and Ds indicate lower levels. (B) Schematic adult wing to show approximate location of panels shown in close-up, as indicate by letters. (C–N) Close-ups of portions of wings (as indicated in panel B) to show hair and bristle orientation in the indicated genotypes. Arrows indicate general direction of polarity. (C–F) Show wing margin bristles, (G–N) show wing hairs, in wild type (C,G), pk30 (D,H), dGC13/ d210 (E,I), dGC13 pk30 (F,J), UAS-RNAi-fat/+; C765-Gal4/UAS-dcr2 (K,M) and sple1 UAS-RNAi-fat/ sple1; C765-Gal4/UAS-dcr2 (L,N). Suppression of pk polarity phenotypes by dachs was 100% penetrant. For suppression of fat phenotypes by sple, near the proximal anterior wing margin (region K,L) in 10/10 fat RNAi wings scored hairs point predominantly towards the wing margin, whereas in 7/8 fat sple wings scored hairs point predominantly distally, and in 1/8 wings scored a substantial fraction of hairs (∼1/4) point towards the wing margin. Near the anterior cross-vein (region M-N), in 8/9 fat RNAi wings scored hairs point predominantly proximally, and in 1/9 wings scored hairs point predominantly towards the L3 vein, whereas in 5/8 fat sple wings scored hairs point distally, in 2/8 they point towards the L3 vein, and in 1/8 they point proximally.
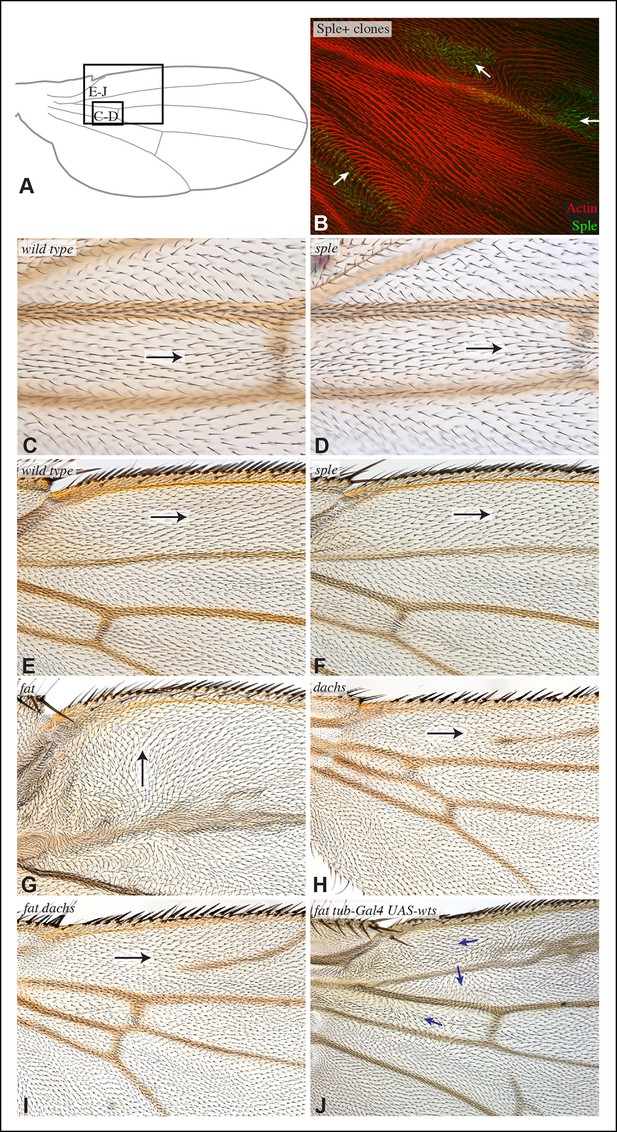
Hair polarity in wing is not affected by loss of sple or dachs.
(A) Schematic adult wing to show approximate location of panels shown in close-up, as indicate by letters. (B) Pupal wing with clones of cells expressing GFP:Sple (green) and F-actin in hairs stained by phalloidin (red). (C–J) Close-ups of portions of wings (as indicated in panel A) to show hair orientation in the indicated genotypes. Arrows indicate general direction of polarity. Hair polarity in wild type (C,E), sple1 mutant (D,F), UAS-dcr2; UAS-RNAi-fat/nub-Gal4 (G), UAS-dcr2; nub-Gal4; UAS-RNAi-dachs (H), UAS-dcr2; UAS-RNAi-fat/nub-Gal4; UAS-RNAi-dachs (I), and fat8/fatG-rv; UAS-wts/tub-Gal4 (J).
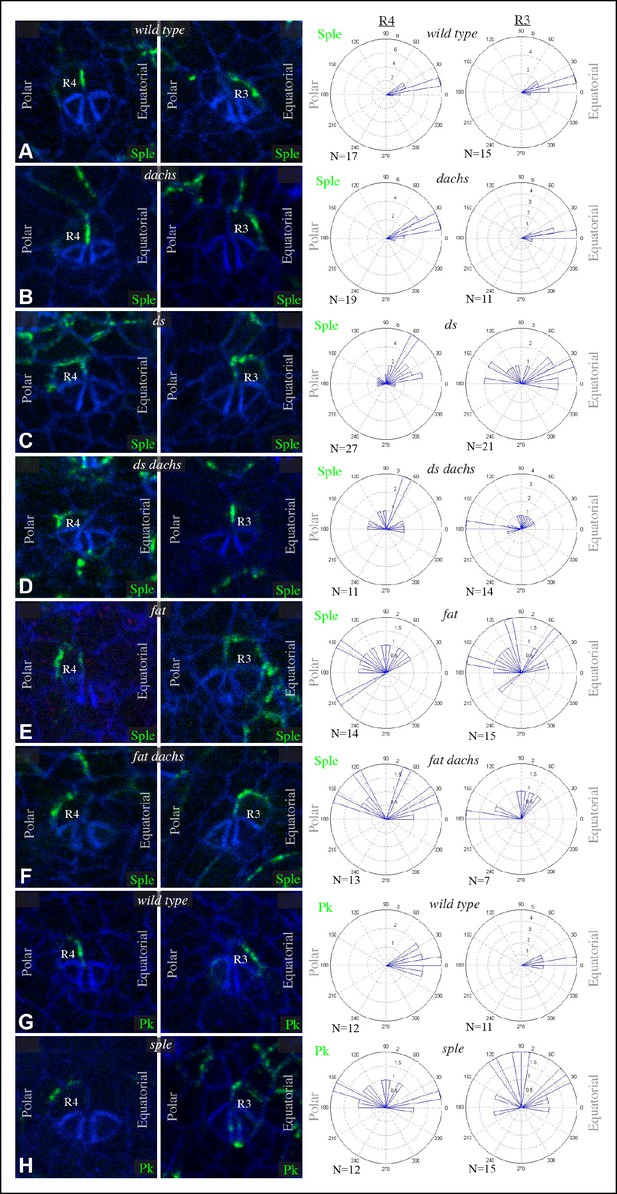
Sple and Pk localization in photoreceptor cells.
Localization of GFP:Sple (A–F) or GFP:Pk (G,H) in cells with expression in R4 or R3 photoreceptor cells in wild type (A,G), dGC13/ d210 (B), ds36D/ dsUA071 (C), dGC13 ds36D/ dGC13 dsUA071 (D), ft8/ ftG-rv (E), dGC13 ft8/ dGC13 ftG-rv (F), and sple1 (H) mutants. Rose plots summarize localization based on the indicated number (N) of examples, with equatorial to the right, polar to the left, and anterior (towards the morphogenetic furrow) at top.
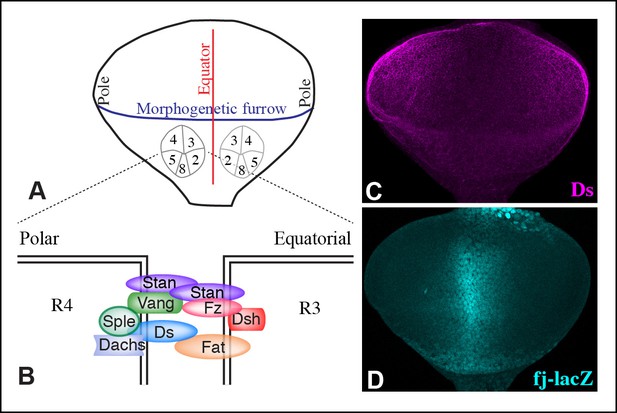
Polarity and gradients in eye discs.
(A) Cartoon illustrating the arrangement and orientation of photoreceptor cells in eye discs at the 5-cell pre-cluster stage. (B) Schematic illustrating the subcellular localization of Ds-Fat and Fz PCP pathway components at the R4 and R3 interface. (C,D) Expression of Ds (magenta) and fj-lacZ (cyan) in a wild-type eye disc.
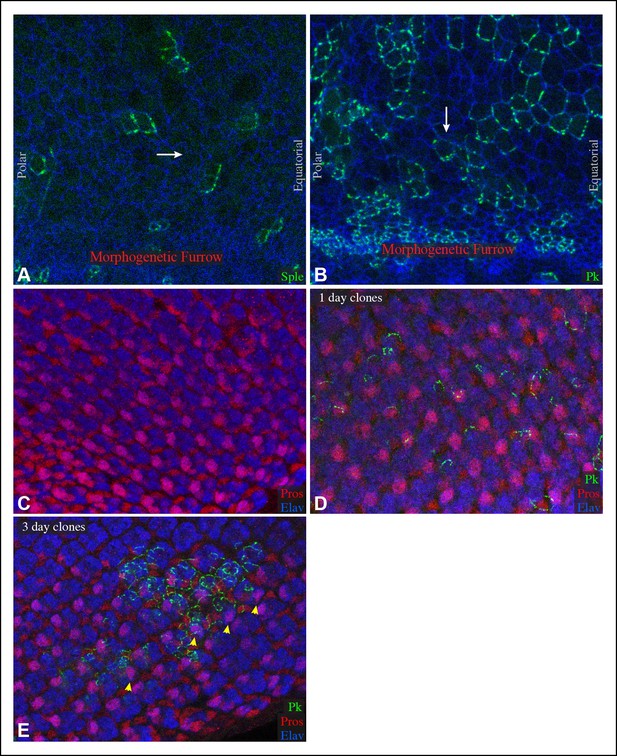
Sple and Pk polarity in front of the morphogenetic furrow, and influence of GFP:Pk on PCP.
(A,B) Portions of eye imaginal discs in front of the morphogenetic furrow with clones of cells expressing GFP:Sple (A) or GFP:Pk (B) (green), stained for expression of E-cadherin (blue). The white arrows indicates general direction of Sple or Pk polarization. (C,D) Portions of eye imaginal discs stained for Elav (marks photoreceptor cells) and Prospero (Pros, overlap with Elav marks R7) and expressing GFP:Pk (green). (C) control disc with no GFP:Pk clones. (D) Disc with 1-day-old GFP:Pk clones. (E) Disc with 3-day-old GFP:Pk clones, yellow arrowheads highlight ommatidia with abnormal polarity.
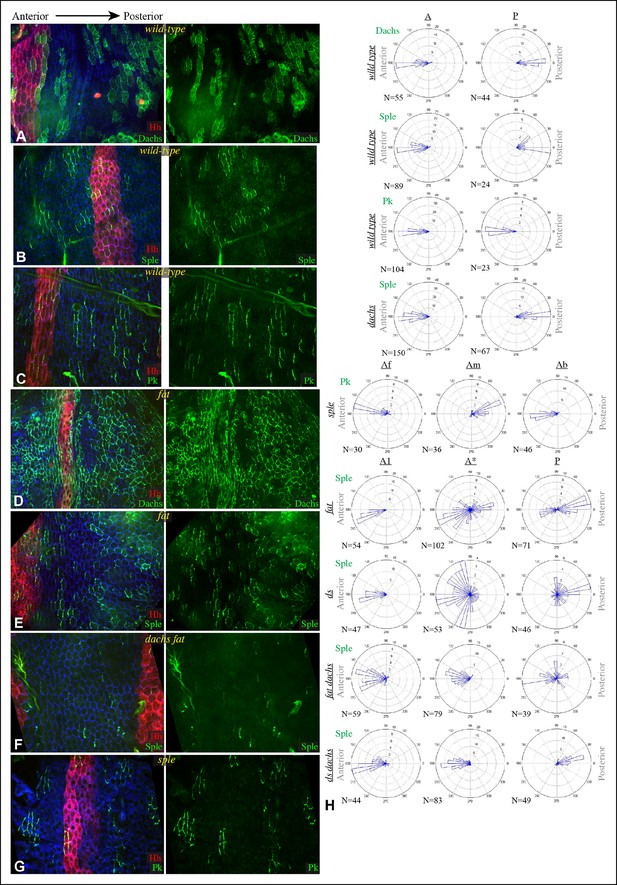
Localization of Dachs, Sple and Pk in abdominal pleura.
(A–G) Pleura of wildtype (A–C), ft8/ ftG-rv (D,E), dGC13 ft8/ dGC13 ftG-rv (F) and sple1/sple1 (G) pupae with clones of cells expressing GFP:Dachs (A,D), GFP:Sple (B,E,F) and GFP:Pk (C,G) (green). Posterior compartments are marked by hh-Gal4 UAS-mCD8-RFP (red). Anterior-posterior body axis is indicated at top. (H) Rose plots depicting polarization of GFP:Dachs, GFP:Sple or GFP:Pk in pleural cells of the indicated genotypes; anterior polarization is to the left and posterior polarization is to the right. For wild type and dachs mutants cells were scored separately in A and P compartments. For fat, ds, fat dachs, and ds dachs the anterior compartment was further subdivided into a front region (A1, anterior-most 8 cells), and the remainder of the A compartment (A*). For sple, the A compartment was subdivided into a front region of 5 cells (Af), a back region of 10 cells (Ab), and a middle region comprising the rest of the compartment (Am); P compartment localization is summarized in Figure 6—figure supplement 1. Scoring of sub-regions of the A compartment in wild-type is shown in Figure 6—figure supplement 1. Apparent variations in cell size are mostly due to the flexibility of the pleura, which is easily stretched or compressed.
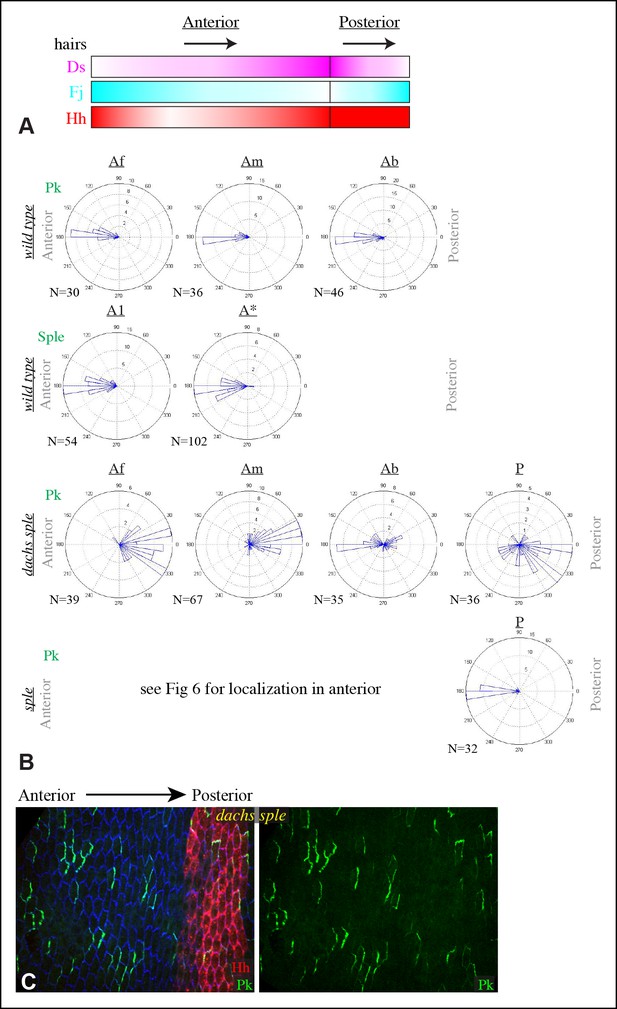
Gradients influencing PCP in the abdomen.
(A) Schematic illustrating the orientation of hairs and approximate gradients of Ds, Fj and Hh expression in the abdomen (Casal et al., 2002; Struhl et al., 1997). (B) Rose plots depicting polarization of GFP:Sple or GFP:Pk in pleural cells of the indicated genotypes. Additional wild-type analysis is for comparison to mutants shown in Figure 6; anterior polarization is to the left and posterior polarization is to the right. For GFP:Sple the anterior compartment was subdivided into a front region (A1, anterior-most 8 cells), and the remainder of the A compartment (A*). For GFP:Pk the A compartment was subdivided into a front region of 5 cells (Af), a back region of 10 cells (Ab), and a middle region comprising the rest of the compartment (Am). (C) Pleura of dGC13 sple1/ dGC13 sple1 pupa with clones of cells expressing GFP:Pk (green). Posterior compartments are marked by hh-Gal4 UAS-mCD8-RFP (red). Anterior-posterior body axis is indicated at top.
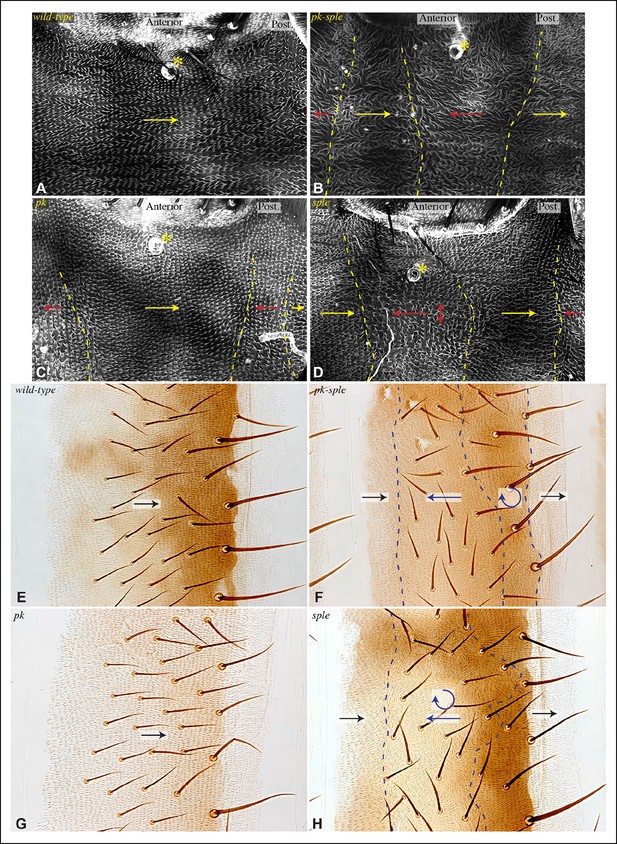
Influence of Pk and Sple on hair polarity in the abdomen.
(A–D) Hair polarity in pleura revealed by F-actin (phalloidin staining) in wild type (A), and in pk-sple14 (B), pk30 (C) and sple1 (D) mutants. Yellow asterisk indicates the position of the spiracle, which forms near the center of the anterior compartment. Yellow arrows indicate the region where hair orientation is normal, and red arrows indicate the region where hair orientation is disrupted. Dashed yellow lines mark approximate boundaries between regions with normal and abnormal polarity. (E–H) Hair polarity in tergites of wild-type (E), pk-sple14 (F), pk30 (G) and sple1 (H) mutant animals. Black arrows indicate regions where hair orientation is normal, and blue arrows indicate the region where hair orientation is abnormal. Dashed blue line mark approximate boundaries between regions with normal and abnormal polarity.
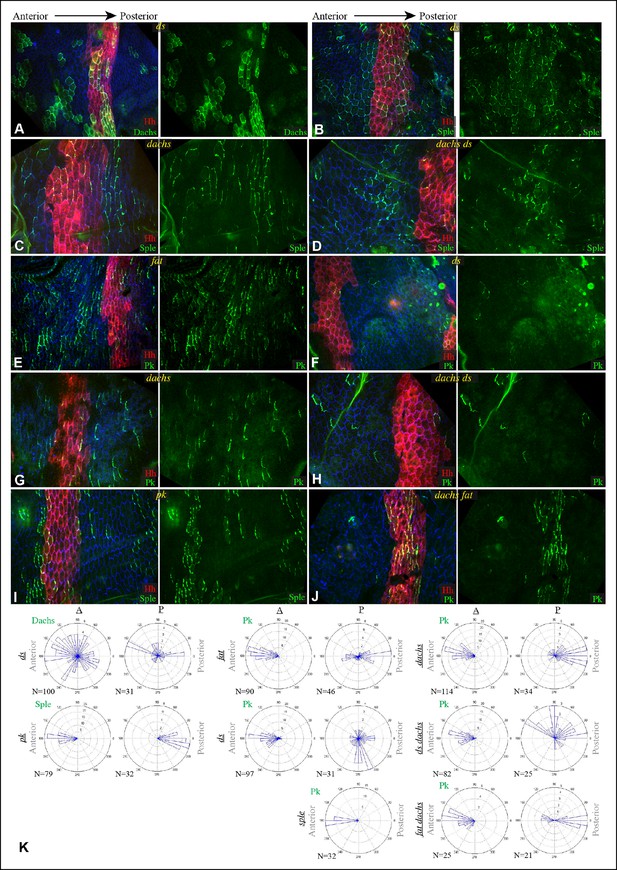
Localization of Dachs, Sple and Pk in abdominal pleura of additional genotypes.
(A–J) Pleura of ds36D/dsUA071 (A,B,F), dachsGC13/dachs210 (C,G), ds36D dachsGC13/dsUA071 dachsGC13 (D,H), ft8/ftG-rv (E), pk30 (I) and ft8 dachsGC13/ftG-rv dGC13 (D,J) mutant pupae with clones of cells expressing of GFP:Dachs (A), GFP:Sple (B,C,D,I) and GFP:Pk (E-H,J) (green). Posterior compartments are marked by hh-Gal4 UAS-mCD8-RFP (red). (K) Rose plots depicting polarization of GFP:Dachs, GFP:Sple or GFP:Pk in pleural clones of the indicated genotypes; anterior polarization is to the left and posterior polarization is to the right. Clones were scored separately in A and P compartments
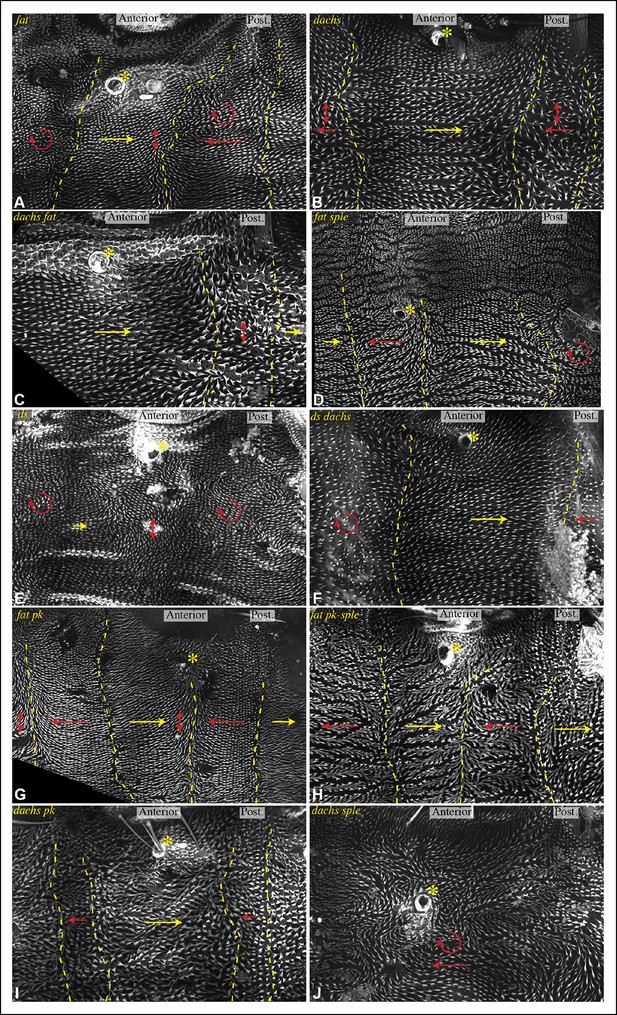
Influence of Ds-Fat PCP on hair polarity in abdominal pleura.
Hair polarity in pleura revealed by F-actin (phalloidin staining) in ft8/ftG-rv (A), dGC13/d210 (B), dGC13 ft8/dGC13 ftG-rv (C), ft8 sple1/ftG-rv sple1 (D), ds36D/dsUA071 (E), dGC13 ds36D/dGC13 dsUA071 (F), ft8 pk30/ftG-rv pk30 (G), ft8 pk-sple14/ftG-rv pk-sple14 (H), dGC13 pk30 (I) and dGC13 sple1 (J) mutant animals. Yellow asterisk indicates the position of the spiracle. Yellow arrows indicate the region where hair orientation is normal, and red arrows indicate the region where hair orientation is disrupted. Dashed yellow lines mark approximate boundaries between regions with normal and abnormal polarity.
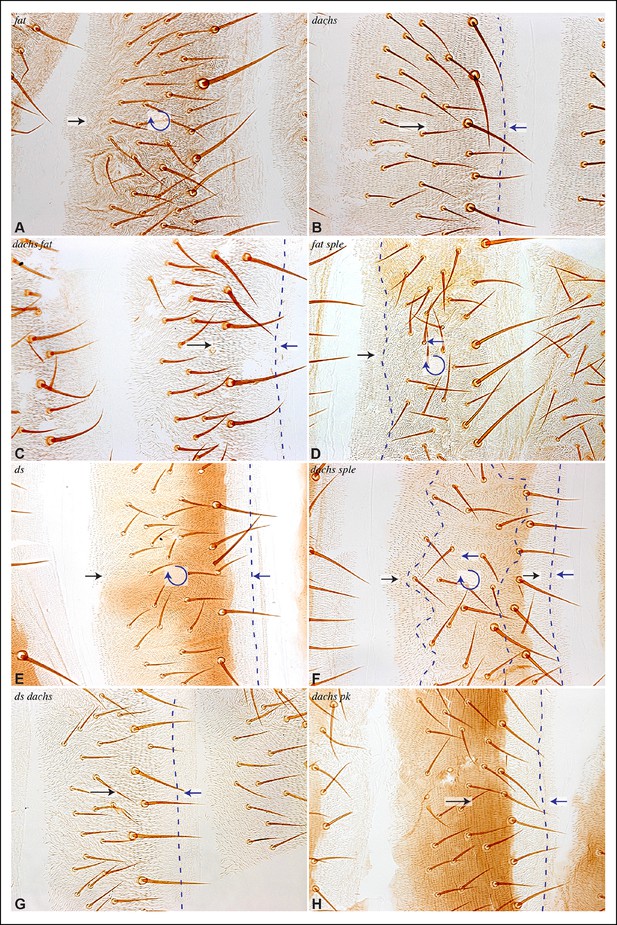
Influence of Ds-Fat PCP on hair polarity in abdominal tergites.
Hair polarity in tergites of ft8/ftG-rv (A), dachsGC13/dachs210 (B), ft8 dachsGC13/ftG-rv dachsGC13 (C), ft8 sple1/ftG-rv sple1 (D), ds36D/dsUA071 (E), dachsGC13 sple1/dachsGC13 sple1 (F), ds36D dachsGC13/dsUA071 dachsGC13 (G), and dachsGC13 pk30/dachsGC13 pk30 (H) mutant animals. Black arrows indicate the region where hair orientation is normal, and blue arrows indicate regions where hair orientation is disrupted. Dashed blue line mark approximate boundaries between regions with normal and abnormal polarity.





