A tight relationship between BOLD fMRI activation/deactivation and increase/decrease in single neuron responses in human association cortex
Figures
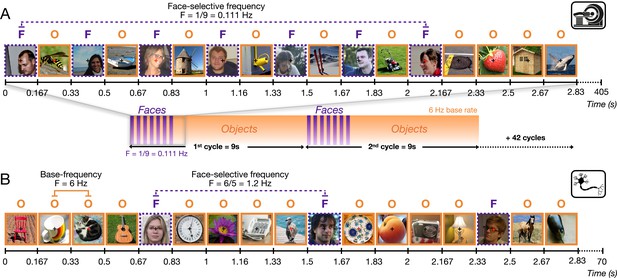
The frequency-tagging Face Localizer paradigm in fMRI (Gao et al., 2018) and intracerebral electrophysiological recordings (Jonas et al., 2016).
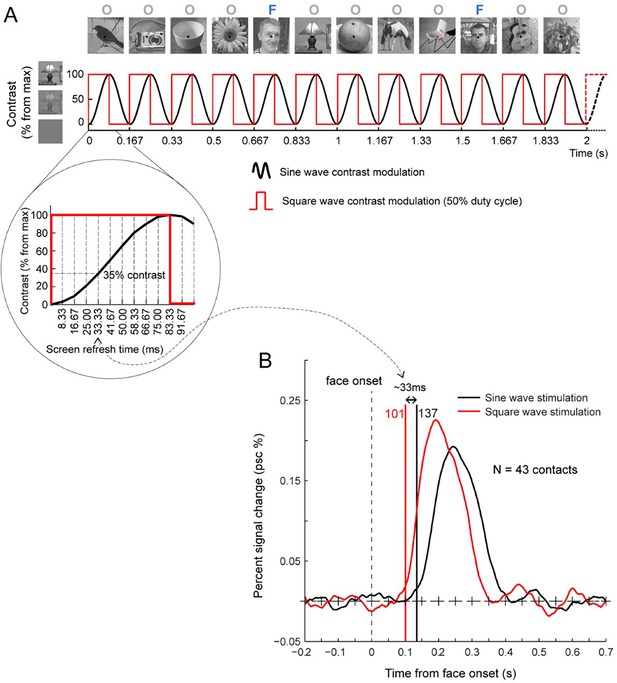
In both cases, the same variable natural non-face images alternate at a 6 Hz rate (1 fixation/image). (A) During a 405 s fMRI run, a ‘mini-burst’ of 7 images of variable faces (purple: ‘F’) alternating with 6 non-face object images (orange: ‘O’) is presented every 9 s (0.111 Hz). A full run is composed of 44 cycles (3 s shown here). (B) During intracerebral recordings, variable face images are inserted periodically every fifth image (1.2 Hz). Each recording includes two 70 s stimulation sequences (3 s shown here). With both recording methods (and EEG; Rossion et al., 2015), this paradigm provides robust population-level face-selective activity devoid of low-level sensory confounds at the tagged frequencies. Here it is applied to single and multi-unit recording activity in the human fusiform gyrus.
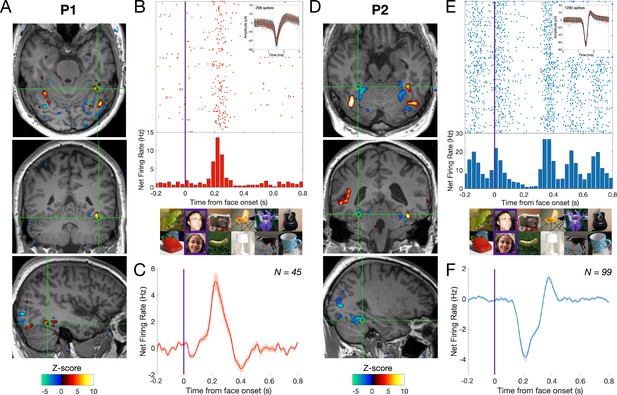
Relationship between BOLD signal and neuronal activity in the human MidFG.
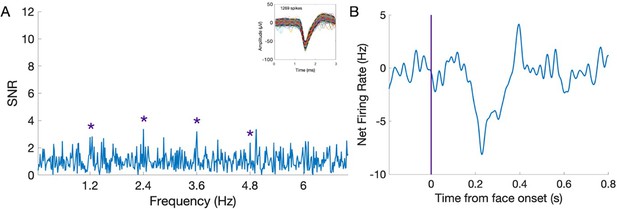
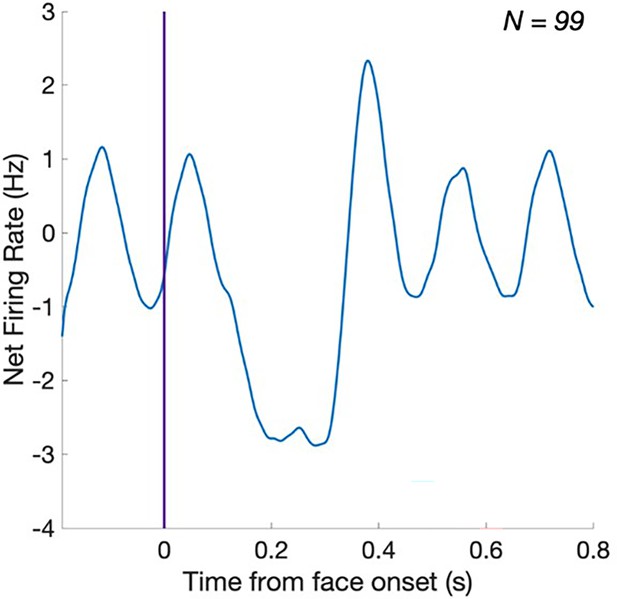
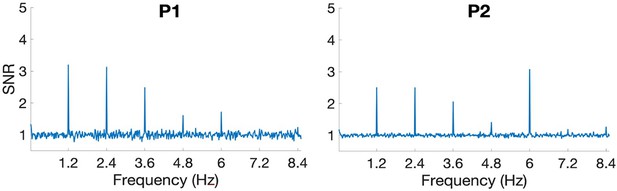
(A), (D) Significant face-selective activations (hot colors) and deactivations (cold colors) on axial, coronal, and sagittal slices (Z > 3.1; p < 0.001). The estimated location of the microelectrode (green circle) falls in left MidFG activation in P1 and right MidFG deactivation in P2. (B), (E) Representative raster plots of two face-selective single-units (SU) showing activity increase in P1 and decrease in P2 to faces, respectively. The representative face-selective SU in panel E also exhibits a high increase in firing rate to the general visual stimulation at 6 Hz. Each line corresponds to a 1 s epoch time-locked to the onset of a face (at 0 s), from a 140 s of recording. SU waveforms are shown in the upper-right corners. (C), (F) Average time courses of all face-selective SU identified (N = 45 across 4 sessions in P1, N = 99 across 7 sessions in P2; Z > 2.32, p < 0.01), showing response increase to faces in P1 and decrease to faces in P2. Note that the average time courses are computed on notch-filtered data to remove the general visual response at 6 Hz and harmonics (see Materials and methods).