Biofilms: Flipping the switch
As single-celled organisms bacteria are exposed to a variety of stresses, but their ability to form multicellular structures called biofilms helps them to grow and survive in challenging environments. In addition to offering protection against predators, antimicrobial treatments and other forms of stress, being in a biofilm also makes bacteria more effective in spreading diseases (Costerton et al., 1995).
The cells in a biofilm are embedded in an elastic matrix made of polysaccharides, DNA and proteins that have been secreted by the bacteria, and, like bricks in a wall, they are precisely organized (Drescher et al., 2016). However, in spite of its toughness, the matrix must remain permeable to nutrients and flexible so that the biofilm can continue to grow.
To achieve this, bacteria use a set of matrix proteins that are patterned in space and time (Berk et al., 2012). However, it is still unclear how these matrix proteins interact to simultaneously remain a connected and flexible multicellular structure. Now, in eLife, Carrie Partch, Fitnat Yildiz and co-workers – including Jiunn Fong of the University of California, Santa Cruz as first author – report new insights into how the architecture of the matrix of the bacterium Vibrio cholerae is regulated (Fong et al., 2017).
Despite improved and ongoing vaccination programs, V. cholerae still causes major pandemics of cholera in high-risk areas such as Yemen, where over half a million people have recently been infected, and thousands have been killed (World Health Organization, 2017a; World Health Organization, 2017b). The bacterium spreads rapidly through contaminated water sources and intestinal infections – a cycle that is promoted by the formation of biofilms.
Because the lifecycle of V. cholerae depends on switching between planktonic and biofilm states, the components of the matrix must remain dynamic. Two of the most important components are a protein called RbmA and a polysaccharide called VPS (Figure 1A; Teschler et al., 2015). Each component performs a specific role, which is partly determined by its position during the formation of the biofilm. RbmA helps cells to stick together and to form clusters, while VPS is needed to develop a three-dimensional cellular structure. In the matrix, RbmA can exist as a monomer or two RbmA proteins can combine to form a dimer (Figure 1B,C; Giglio et al., 2013). However, until now it was not known how these different states influence the architecture of the biofilm, or how RbmA forms dimers in the first place.
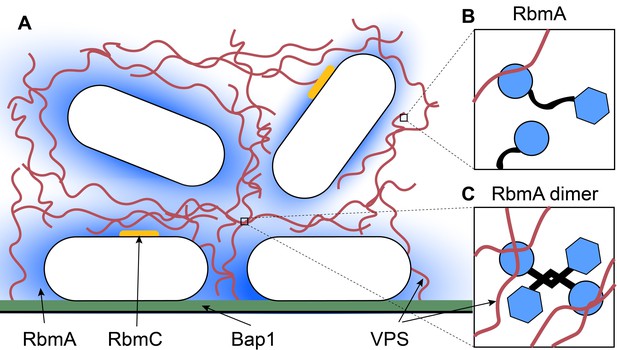
Inside a biofilm.
(A) Vibrio cholerae (white) secretes four main matrix proteins: RbmA (blue), RbmC (orange), Bap1 (green) and VPS (red strands). (B) An RbmA monomer (blue and black) binds to VPS (red). (C) An RbmA dimer can bind to several strands of VPS to create a stable mesh across the matrix.
Fong et al. demonstrated that far from being inert, the matrix components are dynamic structures that interact with each other to maintain the biofilm architecture. Using a technique called nuclear magnetic resonance spectroscopy, Fong et al. found that the dimers can adopt one of two conformations – an ordered loop or a disordered loop. However, only the ordered-loop conformation can enable RbmA to form the antiparallel dimers, which are less prone to degradation than the disordered-loop monomers.
Fong et al. further discovered that both the monomers and the dimers have the ability to bind to VPS. However, RbmA dimers cross-link VPS to form a more solid complex that is anchored into the matrix. When Fong et al. genetically modified RbmA to only form dimers, the proteins concentrated in the center of the matrix instead of building a stable mesh across it. This affected the mechanical properties of the matrix, and the biofilms appeared smooth with a lower cell density. However, removing RbmA led to visually similar biofilms, despite the absence of the cross-linked protein complexes.
These findings raise a plethora of questions: for example, what causes the changes in the viscous properties of the matrix – the RbmA dimer or VPS? And does the position of RbmA in the matrix cause the architectural defects in mutant biofilms? It remains unclear what triggers the formation of the RbmA dimer. One possibility may be that the increasing internal forces generated within a growing biofilm could toggle the conformational change, in a manner that is similar to other bacterial adhesion proteins (Persat et al., 2015). If this was indeed the case, the biofilm matrix could be considered as a ‘smart hydrogel’ that can modulate adhesion depending on its mechanical environment.
The study by Fong et al. builds a foundation for future studies exploring the role of further matrix components of V. cholerae and other organisms. The principles of biofilm formation might not be specific to the microbial world – other living systems also depend on the dynamic properties of their extracellular matrices. In humans, for example, the architecture and dynamics of the extracellular matrix secreted by embryonic cells tightly control how the tissue and organs develop (Rozario and DeSimone, 2010). It has also been shown that force-induced dynamic interactions between extracellular matrix components play a central role in wound healing (Kubow et al., 2015). There is clearly much that we can learn by digging deeper into the matrix.
References
-
Microbial biofilmsAnnual Review of Microbiology 49:711–745.https://doi.org/10.1146/annurev.mi.49.100195.003431
-
Structural basis for biofilm formation via the Vibrio cholerae matrix protein RbmAJournal of Bacteriology 195:3277–3286.https://doi.org/10.1128/JB.00374-13
-
The extracellular matrix in development and morphogenesis: a dynamic viewDevelopmental Biology 341:126–140.https://doi.org/10.1016/j.ydbio.2009.10.026
-
Living in the matrix: assembly and control of Vibrio cholerae biofilmsNature Reviews Microbiology 13:255–268.https://doi.org/10.1038/nrmicro3433
Article and author information
Author details
Publication history
- Version of Record published: September 19, 2017 (version 1)
Copyright
© 2017, Pierrat et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,615
- views
-
- 202
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
- Structural Biology and Molecular Biophysics
Mutations in the human PURA gene cause the neurodevelopmental PURA syndrome. In contrast to several other monogenetic disorders, almost all reported mutations in this nucleic acid-binding protein result in the full disease penetrance. In this study, we observed that patient mutations across PURA impair its previously reported co-localization with processing bodies. These mutations either destroyed the folding integrity, RNA binding, or dimerization of PURA. We also solved the crystal structures of the N- and C-terminal PUR domains of human PURA and combined them with molecular dynamics simulations and nuclear magnetic resonance measurements. The observed unusually high dynamics and structural promiscuity of PURA indicated that this protein is particularly susceptible to mutations impairing its structural integrity. It offers an explanation why even conservative mutations across PURA result in the full penetrance of symptoms in patients with PURA syndrome.
-
- Microbiology and Infectious Disease
- Structural Biology and Molecular Biophysics
African trypanosomes replicate within infected mammals where they are exposed to the complement system. This system centres around complement C3, which is present in a soluble form in serum but becomes covalently deposited onto the surfaces of pathogens after proteolytic cleavage to C3b. Membrane-associated C3b triggers different complement-mediated effectors which promote pathogen clearance. To counter complement-mediated clearance, African trypanosomes have a cell surface receptor, ISG65, which binds to C3b and which decreases the rate of trypanosome clearance in an infection model. However, the mechanism by which ISG65 reduces C3b function has not been determined. We reveal through cryogenic electron microscopy that ISG65 has two distinct binding sites for C3b, only one of which is available in C3 and C3d. We show that ISG65 does not block the formation of C3b or the function of the C3 convertase which catalyses the surface deposition of C3b. However, we show that ISG65 forms a specific conjugate with C3b, perhaps acting as a decoy. ISG65 also occludes the binding sites for complement receptors 2 and 3, which may disrupt recruitment of immune cells, including B cells, phagocytes, and granulocytes. This suggests that ISG65 protects trypanosomes by combining multiple approaches to dampen the complement cascade.






