Life Expectancy: Advancing the aging biology toolkit
Aging is a universal feature of life. It occurs at the level of both cells and organisms, and is the single greatest risk factor for disease. Researchers have been working to unlock the mysteries of aging for decades, and have identified several key molecular changes that drive age-associated traits, as well as genetic, pharmacological and metabolic changes that control lifespan. Because aging is a complex and lengthy process, most breakthroughs have come from studies on model organisms with short lifespans, including yeast, flies, worms and mice. Remarkably, these studies have shown that age-associated traits and genes regulating lifespan are highly conserved, raising the hope that therapeutic interventions that target aging are a real possibility in the near future (Bitto et al., 2015).
Of all these model systems, the budding yeast, Saccharomyces cerevisiae, is the simplest, and has been used to study aging since the 1950s. At that time, Robert Mortimer and John Johnston used microdissection, a technique that involves separating yeast daughter cells from their mothers after they divide, to demonstrate that yeast undergo a finite number of divisions before they die (Mortimer and Johnston, 1959). This type of aging is called replicative aging, and it is defined by the number of times an individual yeast cell asymmetrically divides to produce a daughter. Since then, researchers have used yeast to uncover a number of age-associated traits and genetic modifiers of lifespan (Wasko and Kaeberlein, 2014).
Despite the many successes of yeast-aging research, the field has always faced a significant challenge: old yeast cells are exceedingly rare in a growing population. Early on, this obstacle limited the experimental approaches researchers used, because they could not obtain enough old cells for analysis. Over the years, several laboratories have made key technical advances that have enabled the field to overcome this obstacle and harness a larger spectrum of techniques beyond microdissection to identify molecular mechanisms associated with aging (Figure 1).
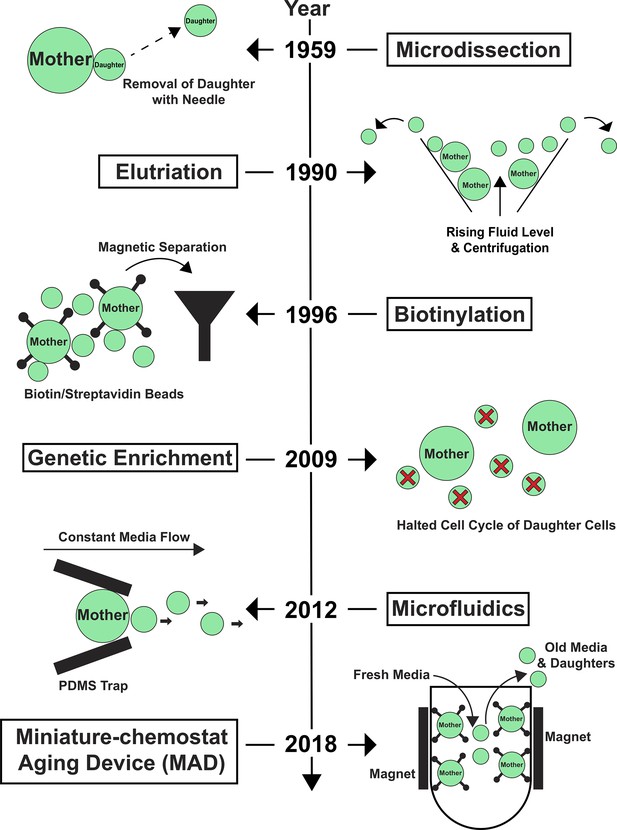
Key technological advances in yeast-aging research.
The development of new tools to study replicative aging in yeast has been crucial to overcome the limitations imposed by the scarcity of old yeast cells in a growing cell population. This timeline depicts broadly adopted technologies that have enabled both single-cell and large-scale measurements using biochemical or genetic approaches to characterize the molecular mechanisms of aging; see main text for more details. Large circles represent mother yeast cells; small circles represent daughter yeast cells; small circles with a red cross represent daughter cells prevented from maturing.
These developments have included: i) microfluidic imaging devices that enable continuous imaging of individual yeast cells over their lifespan (Chen et al., 2017); ii) centrifugation-based approaches that separate populations of old mother cells from young daughters based on size (elutriation; Egilmez et al., 1990); iii) large-scale isolation of aged mother cells by attaching biotin to their cell wall prior to aging (a process known as biotinylation), and then using magnetic microbeads coated with the protein streptavidin to magnetically separate the biotinylated mother cells from their daughters (Smeal et al., 1996); iv) genetic enrichment of aged mother cells by stopping newborn daughter cells from growing (Lindstrom and Gottschling, 2009).
Combined, these techniques have pushed the yeast-aging field to new heights. However, each method has its limitations. For example, while microfluidic devices permit constant media exchange during aging, they are limited to single-cell analysis. On the other hand, genetic enrichment combined with biotin-based purification strategies allows researchers to isolate large numbers of aged cells for a range of analyses. However, this system requires genetically modified yeast strains and does not allow rapid and continuous media flow.
Now, in eLife, Scott McIsaac and colleagues at Calico Life Sciences – including David Hendrickson as first author – report that they have engineered a new aging platform, called the Miniature-chemostat Aging Device (MAD), which pushes the capabilities of the yeast-aging field one step further (Hendrickson et al., 2018). This new device helps to isolate large numbers of yeast cells across a range of ages and genetic backgrounds without the use of genetically modified systems, but with the benefit of continuously renewed media.
Hendrickson et al. achieved this by combining the Miniature-chemostat (Miller et al., 2013) with magnetic-based streptavidin enrichment of mother cells. The MAD approach worked as follows: cells were biotinylated and attached to streptavidin beads prior to aging. The bead-conjugated cells were then loaded into culture tubes fitted with neodymium ring magnets, which trapped the mother cells along the vessel walls, while allowing the daughter cells to be released. The device was connected to a peristaltic pump, which provided fresh media to the confined mother cells while washing away daughters. Mother cells could be released from the magnet at any point during the aging process, and collected for further analysis.
Hendrickson et al. put their new device to the test, performing several genetic and molecular techniques on purified yeast mother cells of various ages and genetic backgrounds. The results confirmed previous observations that aging in yeast is associated with an activation of the core environmental stress response (a set of genes that respond to stress) and the accumulation of ribosomal DNA transcripts (Sinclair and Guarente, 1997; Lesur and Campbell, 2004). They also demonstrated the tremendous potential of this new device to identify unknown age-associated traits by showing that origins of replication (sites were the replication of DNA is initiated) become less accessible with age, and that gene expression from sub-telomeric regions (regions near the end of the chromosomes) increases with age. Moreover, Hendrickson et al. challenged previous observations in the field that global nucleosome occupancy (the density of nucleosomes on DNA) declines with age (Hu et al., 2014).
Overall, yeast-aging research has come a long way since the pioneering studies of Mortimer and Johnston. While there are still significant hurdles to overcome, the development of MAD opens an exciting new era for yeast-aging research.
References
-
Biochemical genetic pathways that modulate aging in multiple speciesCold Spring Harbor Perspectives in Medicine 5:a025114.https://doi.org/10.1101/cshperspect.a025114
-
Microfluidic technologies for yeast replicative lifespan studiesMechanisms of Ageing and Development 161:262–269.https://doi.org/10.1016/j.mad.2016.03.009
-
Preparation and partial characterization of old yeast cellsJournal of Gerontology 45:B9–B17.https://doi.org/10.1093/geronj/45.1.B9
-
The transcriptome of prematurely aging yeast cells is similar to that of telomerase-deficient cellsMolecular Biology of the Cell 15:1297–1312.https://doi.org/10.1091/mbc.e03-10-0742
-
Design and use of multiplexed chemostat arraysJournal of Visualized Experiments e50262.https://doi.org/10.3791/50262
Article and author information
Author details
Publication history
- Version of Record published: November 28, 2018 (version 1)
Copyright
© 2018, Coody et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,870
- Page views
-
- 178
- Downloads
-
- 1
- Citations
Article citation count generated by polling the highest count across the following sources: Crossref, PubMed Central, Scopus.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
- Computational and Systems Biology
Computer models of the human ventricular cardiomyocyte action potential (AP) have reached a level of detail and maturity that has led to an increasing number of applications in the pharmaceutical sector. However, interfacing the models with experimental data can become a significant computational burden. To mitigate the computational burden, the present study introduces a neural network (NN) that emulates the AP for given maximum conductances of selected ion channels, pumps, and exchangers. Its applicability in pharmacological studies was tested on synthetic and experimental data. The NN emulator potentially enables massive speed-ups compared to regular simulations and the forward problem (find drugged AP for pharmacological parameters defined as scaling factors of control maximum conductances) on synthetic data could be solved with average root-mean-square errors (RMSE) of 0.47 mV in normal APs and of 14.5 mV in abnormal APs exhibiting early afterdepolarizations (72.5% of the emulated APs were alining with the abnormality, and the substantial majority of the remaining APs demonstrated pronounced proximity). This demonstrates not only very fast and mostly very accurate AP emulations but also the capability of accounting for discontinuities, a major advantage over existing emulation strategies. Furthermore, the inverse problem (find pharmacological parameters for control and drugged APs through optimization) on synthetic data could be solved with high accuracy shown by a maximum RMSE of 0.22 in the estimated pharmacological parameters. However, notable mismatches were observed between pharmacological parameters estimated from experimental data and distributions obtained from the Comprehensive in vitro Proarrhythmia Assay initiative. This reveals larger inaccuracies which can be attributed particularly to the fact that small tissue preparations were studied while the emulator was trained on single cardiomyocyte data. Overall, our study highlights the potential of NN emulators as powerful tool for an increased efficiency in future quantitative systems pharmacology studies.
-
- Computational and Systems Biology
- Neuroscience
Closed-loop neuronal stimulation has a strong therapeutic potential for neurological disorders such as Parkinson’s disease. However, at the moment, standard stimulation protocols rely on continuous open-loop stimulation and the design of adaptive controllers is an active field of research. Delayed feedback control (DFC), a popular method used to control chaotic systems, has been proposed as a closed-loop technique for desynchronisation of neuronal populations but, so far, was only tested in computational studies. We implement DFC for the first time in neuronal populations and access its efficacy in disrupting unwanted neuronal oscillations. To analyse in detail the performance of this activity control algorithm, we used specialised in vitro platforms with high spatiotemporal monitoring/stimulating capabilities. We show that the conventional DFC in fact worsens the neuronal population oscillatory behaviour, which was never reported before. Conversely, we present an improved control algorithm, adaptive DFC (aDFC), which monitors the ongoing oscillation periodicity and self-tunes accordingly. aDFC effectively disrupts collective neuronal oscillations restoring a more physiological state. Overall, these results support aDFC as a better candidate for therapeutic closed-loop brain stimulation.






