Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach
Abstract
Antimicrobial peptides (AMPs) are host-encoded antibiotics that combat invading microorganisms. These short, cationic peptides have been implicated in many biological processes, primarily involving innate immunity. In vitro studies have shown AMPs kill bacteria and fungi at physiological concentrations, but little validation has been done in vivo. We utilized CRISPR gene editing to delete most known immune-inducible AMPs of Drosophila, namely: 4 Attacins, 2 Diptericins, Drosocin, Drosomycin, Metchnikowin and Defensin. Using individual and multiple knockouts, including flies lacking these ten AMP genes, we characterize the in vivo function of individual and groups of AMPs against diverse bacterial and fungal pathogens. We found that Drosophila AMPs act primarily against Gram-negative bacteria and fungi, contributing either additively or synergistically. We also describe remarkable specificity wherein certain AMPs contribute the bulk of microbicidal activity against specific pathogens, providing functional demonstrations of highly specific AMP-pathogen interactions in an in vivo setting.
https://doi.org/10.7554/eLife.44341.001eLife digest
All animals – from humans to mice, jellyfish to fruit flies – are armed with an immune system to defend against infections. The immune system’s first line of defence often involves a group of short proteins called antimicrobial peptides. These proteins are found anywhere that germs and microbes come into contact with the body, including the skin, eyes and lungs. In many cases, it is unclear how individual antimicrobial peptides work. For example, which germs are they most effective against? Do they work alone, or in a mixture of other antimicrobial peptides?
To learn more about a protein, scientists can often delete the gene that encodes it and observe what happens. Antimicrobial peptides, however, are small proteins encoded by a large number of very short genes, which makes them difficult to target with most genetic tools. Fortunately, gene editing via the CRISPR/Cas9 system can overcome many of the limitations of more traditional methods; this allowed Hanson et al. to systematically remove the antimicrobial peptide genes from fruit flies to explore how these proteins work.
In the experiments, ten antimicrobial peptide genes known from fruit flies were removed, and the flies were then infected with a variety of bacteria and fungi. Hanson et al. found that the antimicrobial peptides were effective against many bacteria, but unexpectedly they were far more important for controlling one general kind of bacterial infection, but not another kind. Further experiments showed that some of these proteins work alone, targeting only a particular species of microbe. This finding suggested that animals might fight infections by very specific bacteria with a very specific antimicrobial peptide rather than with a mixture.
By understanding how antimicrobial peptides work in more detail, scientists can learn what types of microbes they are most effective against. In the future, this information may eventually lead to the development of new types of antibiotics and better management of diseases that affect important insects, like bumblebees.
https://doi.org/10.7554/eLife.44341.002Introduction
While innate immune mechanisms were neglected during the decades where adaptive immunity captured most of the attention, they have become central to our understanding of immunology. Recent emphasis on innate immunity has, however, mostly focused on the first two phases of the immune response: microbial recognition and associated downstream signaling pathways. In contrast, how innate immune effectors individually or collectively contribute to host resistance has not been investigated to the same extent. The existence of multiple effectors that redundantly contribute to host resistance has hampered their functional characterization by genetic approaches (Lemaitre and Hoffmann, 2007). The single mutation methodology that still prevails today has obvious limits in the study of immune effectors, which often belong to large gene families. As such, our current understanding of the logic underlying the roles of immune effectors is only poorly defined. As a consequence, the key parameters that influence host survival associated with a successful immune response are not well characterized. In this paper, we harnessed the power of the CRISPR gene editing approach to study the function of Drosophila antimicrobial peptides in host defence both individually and collectively.
Antimicrobial peptides (AMPs) are small, cationic, usually amphipathic peptides that contribute to innate immune defence in plants and animals (Imler and Bulet, 2005; Guaní-Guerra et al., 2010; Rolff and Schmid-Hempel, 2016). They display potent antimicrobial activity in vitro by disrupting negatively charged microbial membranes, but AMPs can also target specific microbial processes (Park et al., 1998; Kragol et al., 2001; Rahnamaeian et al., 2015). Their expression is induced to very high levels upon challenge to provide microbicidal concentrations in the μM range. Numerous studies have revealed unique roles that AMPs may play in host physiology including anti-tumor activity (Suttmann et al., 2008; Kuroda et al., 2015; Araki et al., 2018; Parvy et al., 2019), inflammation in aging (Cao et al., 2013; Kounatidis et al., 2017; E et al., 2018), involvement in memory (Bozler et al., 2017; Barajas-Azpeleta et al., 2018), mammalian immune signaling (van Wetering et al., 2002; Tjabringa et al., 2003), wound-healing (Tokumaru et al., 2005; Chung et al., 2017), regulation of the host microbiota (Login et al., 2011; Mergaert et al., 2017), tolerance to oxidative stress (Zhao et al., 2011; Zheng et al., 2007), and of course microbicidal activity (Imler and Bulet, 2005; Wimley, 2010). The fact that AMP genes are immune inducible and expressed at high levels has led to the common assumption they play a vital role in the innate immune response. However, little is known in most cases about how AMPs individually or collectively contribute to animal host defence. In vivo functional analysis of AMPs has been hampered by the sheer number and small size of these genes, making them difficult to mutate with traditional genetic tools (but e.g. see Hoeckendorf et al., 2012).
Since the first animal AMPs were discovered in silk moths (Steiner et al., 1981), insects and particularly Drosophila melanogaster have emerged as a powerful model for characterizing their function. There are currently seven well-characterized families of inducible AMPs in D. melanogaster, but we note that many genes encoding small peptides are strongly upregulated upon infection and are awaiting description (De Gregorio et al., 2002). The activities of the seven known AMP families of Drosophila have been determined either in vitro by using peptides directly purified from flies or produced in heterologous systems, or deduced by comparison with homologous peptides isolated in other insect species: Drosomycin and Metchnikowin show antifungal activity (Fehlbaum et al., 1994; Levashina et al., 1995); Cecropins (four inducible genes) and Defensin have both antibacterial and some antifungal activities (Hultmark et al., 1980; Ekengren and Hultmark, 1999; Cociancich et al., 1993; Tzou et al., 2002); and Drosocin, Attacins (four genes) and Diptericins (two genes) primarily exhibit antibacterial activity (Kragol et al., 2001; Asling et al., 1995; Cudic et al., 1999; Hedengren et al., 2000; Bulet et al., 1996). In Drosophila, these AMPs are produced either locally at various surface epithelia in contact with environmental microbes (Tzou et al., 2000; Basset et al., 2000; Gendrin et al., 2009), or secreted systemically into the hemolymph, the insect blood. During systemic infection, these 14 antimicrobial peptides are strongly induced in the fat body, an organ analogous to the mammalian liver.
The systemic production of AMPs is regulated at the transcriptional level by two NF-κB pathways, the Toll and Imd pathways, which are activated by different classes of microbes. The Toll pathway is predominantly responsive to Gram-positive bacteria and fungi, and accordingly plays a major role in defence against these microbes. In contrast, the Imd pathway is activated by Gram-negative bacteria and a subset of Gram-positive bacteria with DAP-type peptidoglycan, and mutations affecting this pathway cause profound susceptibility to Gram-negative bacteria (De Gregorio et al., 2002; Lemaitre et al., 1997). However, the expression pattern of AMP genes is complex as each gene is expressed with different kinetics and can often receive transcriptional input from both pathways (De Gregorio et al., 2002; Leulier et al., 2000). This ranges from Diptericin, which is tightly regulated by the Imd pathway, to Drosomycin, whose expression is mostly regulated by the Toll pathway (Lemaitre et al., 1997), except at surface epithelia where Drosomycin is under the control of Imd signaling (Ferrandon et al., 1998; Tzou et al., 2000). While a critical role of AMPs in Drosophila host defence is supported by transgenic flies overexpressing a single AMP (Tzou et al., 2002), the specific contributions of each of these AMPs has not been tested. Indeed loss-of-function mutants for most AMP genes were not previously available due to their small size, making them difficult to mutate before the advent of CRISPR/Cas9 technology. Despite this, the great susceptibility to infection of mutants with defective Toll and Imd pathways is commonly attributed to the loss of the AMPs they regulate, though these pathways control hundreds of genes awaiting characterization (De Gregorio et al., 2002). Strikingly, Clemmons et al. (2015) recently reported that flies lacking a set of uncharacterized Toll-responsive peptides (named Bomanins) succumb to infection by Gram-positive bacteria and fungi at rates similar to Toll-deficient mutants (Clemmons et al., 2015). This provocatively suggests that Bomanins, and not AMPs, might be the predominant effectors downstream of the Toll pathway; yet synthesized Bomanins do not display antimicrobial activity in vitro (Lindsay et al., 2018). Thus, while today the fly represents one of the best-characterized animal immune systems, the contribution of AMPs as immune effectors is poorly defined as we still do not understand why Toll and Imd pathway mutants succumb to infection.
In this paper, we took advantage of recent gene editing technologies to delete most of the known immune inducible AMP genes of Drosophila. Using single and multiple knockouts, as well as a variety of bacterial and fungal pathogens, we have characterized the in vivo function of individual and groups of antimicrobial peptides. We reveal that AMPs can play highly specific roles in defence, being vital for surviving certain infections yet dispensable against others. We highlight key interactions amongst immune effectors and pathogens and reveal to what extent these defence peptides act in concert or alone.
Results
Generation and characterization of AMP mutants
We generated null mutants for 10 of the 14 known Drosophila antimicrobial peptide genes that are induced upon systemic infection. These include five single gene mutations affecting Defensin (DefSK3), Attacin C (AttCMi), Metchnikowin (MtkR1), Attacin D (AttDSK1) and Drosomycin (DrsR1), respectively, and two small deletions removing both Diptericins DptA and DptB (DptSK1), or the gene cluster containing Drosocin, and Attacins AttA and AttB (Dro-AttABSK2). The function of Cecropins were not assessed in this manuscript. All mutations/deletions were made using the CRISPR editing approach with the exception of Attacin C, which was disrupted by insertion of a Minos transposable element (Bellen et al., 2011), and the Drosomycin and Metchnikowin deletions generated by homologous recombination (Figure 1A and Figure 1—figure supplement 1). To disentangle the role of Drosocin and AttA/AttB in the Dro-AttABSK2 deletion, we also generated an individual Drosocin mutant (DroSK4); for complete information, see Figure 1—figure supplement 1. We then isogenized these mutations for at least seven generations into the w1118 DrosDel isogenic genetic background (Ryder et al., 2004) (iso w1118). Then, we recombined these seven independent mutations into a background lacking these 10 inducible AMPs referred to as ‘ΔAMPs.’ ΔAMPs flies were viable and showed no morphological defects. To confirm the absence of AMPs in our ΔAMPs background, we performed a MALDI-TOF analysis of hemolymph from both unchallenged and immune-challenged flies infected by a mixture of Escherichia coli and Micrococcus luteus. This analysis revealed the presence of peaks induced upon challenge corresponding to AMPs in wild-type but not ΔAMPs flies. Importantly, it also confirmed that induction of most other immune-induced molecules (IMs) (Uttenweiler-Joseph et al., 1998), was unaffected in ΔAMPs flies (Figure 1B). Of note, we failed to observe two IMs, IM7 and IM21, in our ΔAMPs flies, suggesting that these unknown peptides are secondary products of AMP genes. We further confirmed that Toll and Imd NF-κB signaling pathways were intact in ΔAMPs flies by measuring the expression of target genes of these pathways (Figure 1C–D). This demonstrates that Drosophila AMPs are not signaling molecules required for Toll or Imd pathway activity. We also assessed the role of AMPs in the melanization response, wound clotting, and hemocyte populations. After clean injury, ΔAMPs flies survive as wild-type (Figure 1—figure supplement 2A). We found no defect in melanization (χ2, p=0.34, Figure 1—figure supplement 2B) as both adults and larvae strongly melanize the cuticle following clean injury (Figure 1—figure supplement 2C). Furthermore, we visualized the rapid formation of clot fibers ex vivo using the hanging drop assay and PNA staining (Scherfer et al., 2004) in hemolymph of both wild-type and ΔAMPs larvae (Figure 1—figure supplement 2D). Hemocyte counting (i.e. crystal cells, FACS) did not reveal any deficiency in hemocyte populations of ΔAMPs larvae (Figure 1—figure supplement 2E,F, and not shown). Altogether, our study suggests that Drosophila AMPs are primarily immune effectors, and not regulators of innate immunity.
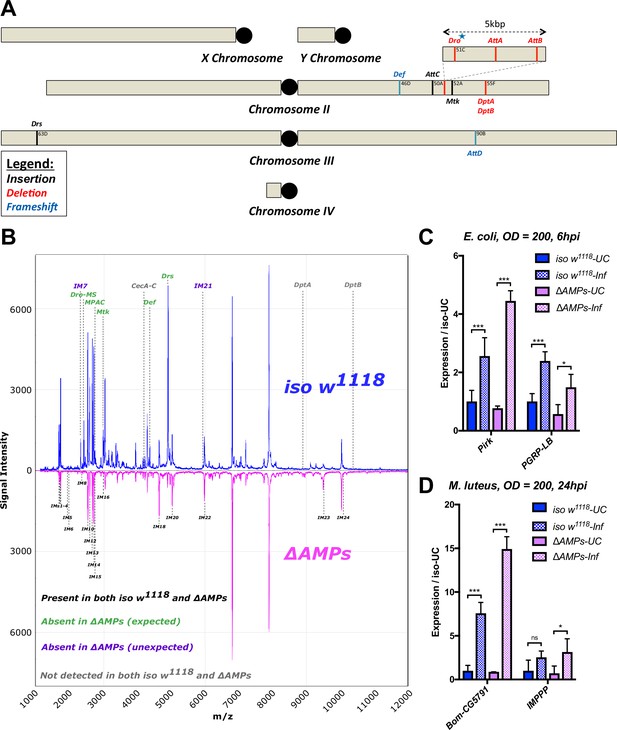
Description of AMP mutants.
(A) Chromosomal locations of AMP genes that were deleted. Each mutation is color-coded with the mutagenic agent: black, a Minos insertion or homologous recombination, red, CRISPR-CAS9-mediated deletion, and blue CRISPR CAS9 mediated indel causing a nonsense peptide. (B) A representative MALDI-TOF analysis of hemolymph samples from immune-challenged (1:1 E. coli and M. luteus at OD600 = 200) iso w1118 and ΔAMPs flies as described in Uttenweiler-Joseph et al. (1998). No AMP-derived products were detected in the hemolymph samples of ΔAMPs flies. No signals for IM7, nor IM21 were observed in the hemolymph samples of ΔAMPs mutants suggesting that these uncharacterized immune-induced molecules are the products of AMP genes. The Imd pathway (C) and Toll pathway (D) are functional and respond to immune challenge in ΔAMPs flies. We used alternate readouts to monitor the Toll and Imd pathways: pirk and PGRP-LB for Imd pathway and CG5791 (Bomanin) and IMPPP for Toll signaling (De Gregorio et al., 2002; Hanson et al., 2016). UC = unchallenged, Inf = infected. hpi = hours post-infection. Expression normalized with iso w1118-UC set to a value of 1.
AMPs are essential for combating Gram-negative bacterial infection
We used these ΔAMPs flies to explore the role that AMPs play in defence against pathogens during systemic infection. We first focused our attention on Gram-negative bacterial infections, which are combatted by Imd pathway-mediated defence in Drosophila (Lemaitre and Hoffmann, 2007). We challenged wild-type and ΔAMPs flies with six different Gram-negative bacterial species, using inoculation doses (given as OD600) selected such that at least some wild-type flies were killed. In our survival experiments, we also include Oregon R (OR-R) as an alternate wild-type for comparison, and Relish mutants (RelE20) that lack a functional Imd response and are known to be very susceptible to this class of bacteria (Hedengren et al., 1999) (Figure 2). Globally, ΔAMPs flies were extremely susceptible to all Gram-negative pathogens tested (Figure 2, light blue plots). The susceptibility of AMP-deficient flies to Gram-negative bacteria largely mirrored that of RelE20 flies. For all Gram-negative infections tested, ΔAMPs flies show a higher bacterial count at 18 hr post-infection (hpi) indicating that AMPs actively inhibit bacterial growth, as expected of ‘antimicrobial peptides’ (Figure 2—figure supplement 1A). Use of GFP-expressing bacteria show that bacterial growth in ΔAMPs flies radiates from the wound site until spreading systemically (Figure 2—figure supplement 1B,C). Collectively, the use of AMP-deficient flies reveals that AMPs are major players in resistance to Gram-negative bacteria, and likely constitute an essential component of the Imd pathway’s contribution for survival against these germs.
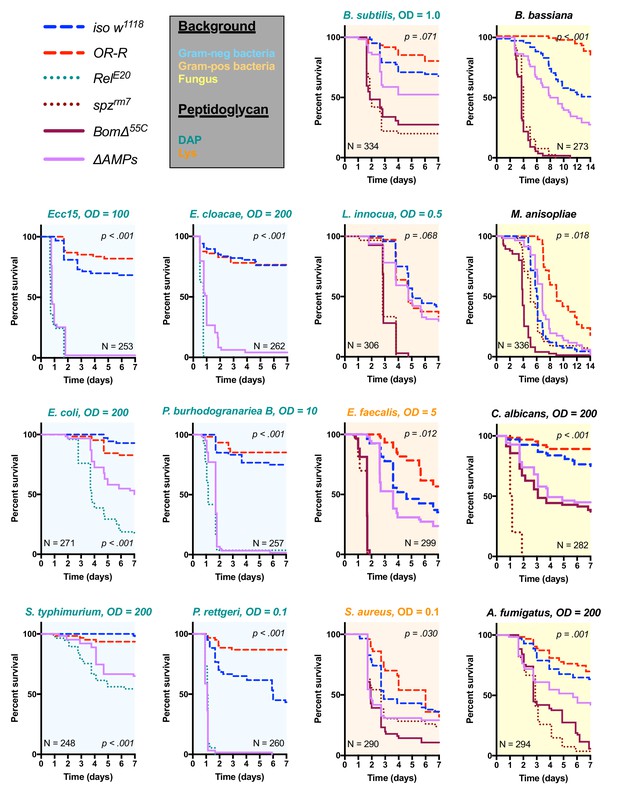
Survival of ΔAMPs flies to diverse microbial challenges.
Control lines for survival experiments included two wild-types (w;Drosdel (iso w1118) and Oregon R (OR-R) as an alternate wild-type), mutants for the Imd response (RelE20), mutants for Toll signaling (spzrm7), and mutants for Bomanins (BomΔ55C). ΔAMPs flies are extremely susceptible to infection with Gram-negative bacteria (blue backgrounds). Unexpectedly, ΔAMPs flies were not markedly susceptible to infection with Gram-positive bacteria (orange backgrounds), while BomΔ55C flies were extremely susceptible, often mirroring spzrm7 mutants. This pattern of BomΔ55C susceptibility held true for fungal infections (yellow backgrounds). ΔAMPs flies are somewhat susceptible to fungal infections, but the severity shifts with different fungi. Pellet densities are reported for all systemic infections in OD at 600 nm. p-Values are given for ΔAMPs flies compared to iso w1118 using a Cox-proportional hazards model. N = total number of flies in experiments. A full description of p-values relative to iso w1118 can be found in Figure 2—source data 1.
-
Figure 2—source data 1
p-Values from Figure 2A relative to iso w1118.
- https://doi.org/10.7554/eLife.44341.008
Bomanins and to a lesser extent AMPs contribute to resistance against Gram-positive bacteria and fungi
Previous studies have shown that resistance to Gram-positive bacteria and fungi in Drosophila is mostly mediated by the Toll pathway, although the Imd pathway also contributes to some extent (Lemaitre et al., 1997; Leulier et al., 2000; Rutschmann et al., 2002; Tanji et al., 2007). Moreover, a deletion removing ten uncharacterized Bomanins (BomΔ55C) induces a strong susceptibility to both Gram-positive bacteria and fungi (Clemmons et al., 2015), suggesting that Bomanins are major players downstream of Toll in the defence against these germs. This prompted us to explore the role of antimicrobial peptides in defence against Gram-positive bacteria and fungi. In these experiments, we additionally included spätzle mutant flies (spzrm7) lacking Toll signaling as susceptible controls. We first challenged wild-type and ΔAMPs flies with two lysine-type (E. faecalis, S. aureus) and two DAP-type (B. subtilis, L. innocua) peptidoglycan-containing Gram-positive bacterial species. We observed that ΔAMPs flies display only weak or no increased susceptibility to infection with these Gram-positive bacterial species, as ΔAMPs survival rates were closer to the wild-type than to spzrm7 mutants lacking a functional Toll pathway (Figure 2, orange plots), with the exception of S. aureus. Meanwhile, BomΔ55C mutants consistently phenocopied spzrm7 flies, confirming the important contribution of these peptides in defence against Gram-positive bacteria (Clemmons et al., 2015).
Next, we monitored the survival of ΔAMPs to the yeast Candida albicans, the opportunistic fungus Aspergillus fumigatus and two entomopathogenic fungi, Beauveria bassiana, and Metarhizium anisopliae. For the latter two, we used a natural mode of infection by spreading spores on the cuticle (Lemaitre et al., 1997). ΔAMPs flies were more susceptible to fungal infections with B. bassiana, A. fumigatus, and C. albicans, but not M. anisopliae (Figure 2, yellow plots). In all instances, BomΔ55C mutants were as or more susceptible to fungal infection than ΔAMPs flies, approaching Toll-deficient mutant levels. Collectively, our data demonstrate that AMPs are major immune effectors in defence against Gram-negative bacteria and have a less essential role in defence against bacteria and fungi.
A combinatory approach to explore AMP interactions
The impact of the ΔAMPs deletion on survival could be due to the action of certain AMPs having a specific effect, or more likely due to the combinatory action of co-expressed AMPs. Indeed, cooperation of AMPs to potentiate their microbicidal activity has been suggested by numerous in vitro approaches (Rahnamaeian et al., 2015; Yu et al., 2016; Mohan et al., 2014), but rarely in an in vivo context (Zanchi et al., 2017). Having shown that AMPs as a whole significantly contribute to fly defence, we next explored the contribution of individual peptides to this effect. To tackle this question in a systematic manner, we performed survival analyses using fly lines lacking one or several AMPs, focusing on pathogens with a range of virulence that we previously showed to be sensitive to the action of AMPs. This includes the yeast C. albicans and the Gram-negative bacterial species P. burhodogranariea, P. rettgeri, Ecc15, and E. cloacae. Given seven independent AMP mutations, over 100 combinations of mutants are possible, making a systematic analysis of AMP interactions a logistical nightmare. Therefore, we designed an approach that would allow us to characterize their contributions to defence by deleting groups of AMPs. To this end, we generated three groups of combined mutants: A) flies lacking Defensin (Group A); Defensin is regulated by Imd signalling but is primarily active against Gram-positive bacteria in vitro (Imler and Bulet, 2005). B) Flies lacking three antibacterial and structurally related AMP families: the Proline-rich Drosocin and the Proline- and Glycine-rich Diptericins and Attacins (Group B, regulated by the Imd pathway). C) Flies lacking the two antifungal peptide genes Metchnikowin and Drosomycin (Group C, mostly regulated by the Toll pathway). We then combined these three groups to generate flies lacking AMPs from groups A and B (AB), A and C (AC), or B and C (BC). Finally, flies lacking all three groups are our ΔAMPs flies, which are highly susceptible to a number of infections. By screening these seven genotypes as well as individual mutants, we were able to assess potential interactions between AMPs of different groups, as well as decipher the function of individual AMPs.
Drosomycin and metchnikowin additively contribute to defence against the yeast C. albicans
We first applied this AMP-groups approach to infections with the relatively avirulent yeast C. albicans. Previous studies have shown that Toll, but not Imd, contributes to defence against this fungus (Gottar et al., 2006; Glittenberg et al., 2011). Thus, we suspected that the two antifungal peptides, Drosomycin and Metchnikowin, could play a significant role in the susceptibility of ΔAMPs flies to this yeast. Consistent with this, Group C flies lacking Metchnikowin and Drosomycin were more susceptible to infection (p<0.001 relative to iso w1118) with a survival rate similar to ΔAMPs flies (Figure 3A). Curiously, AC-deficient flies that also lack Defensin survived better than Group C-deficient flies (Log-Rank p=0.014). We have no explanation for this interaction, but this could be due to i) a better canalization of the immune response by preventing the induction of ineffective AMPs, ii) complex biochemical interactions amongst the AMPs involved affecting either the host or pathogen or iii) differences in genetic background generated by additional recombination. We then investigated the individual contributions of Metchnikowin and Drosomycin to survival to C. albicans. We found that both MtkR1 and DrsR1 individual mutants were somewhat susceptible to infection, but notably only Mtk; Drs compound mutants reached ΔAMPs levels of susceptibility (Figure 3B). This co-occurring loss of resistance appears to be primarily additive (Mutant, Cox Hazard Ratio (HR), p-value: MtkR1, HR =+1.17, p=0.008; DrsR1, HR =+1.85, p<0.001; Mtk*Drs, HR = −0.80, p=0.116). We observed that Group C deficient flies eventually succumb to uncontrolled C. albicans growth by monitoring yeast titre, indicating that these AMPs indeed act by suppressing yeast growth (Figure 3C).
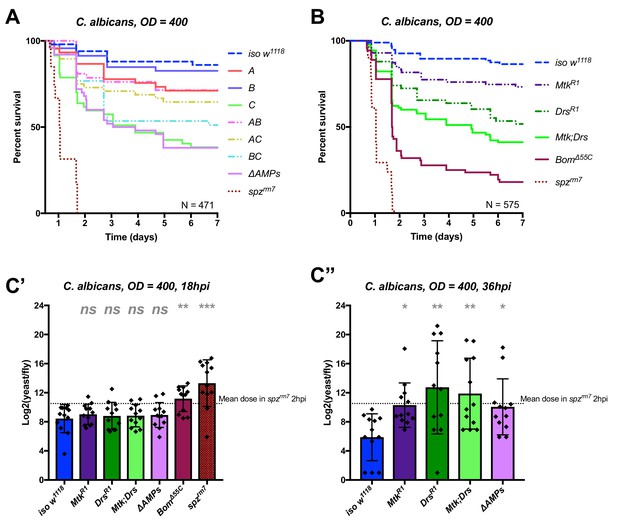
Identification of AMPs involved in the susceptibility of ΔAMPs flies to C. albicans.
(A) Survival of mutants for groups of AMPs reveals that loss of only Toll-responsive Group C peptides (Metchnikowin and Drosomycin) is required to recapitulate the susceptibility of ΔAMPs flies. Co-occurring loss of groups A and C has a net protective effect (A*C: HR = −1.71, p=0.002). (B) Further dissection of Group C mutations reveals that both Metchnikowin and Drosomycin contribute to resist C. albicans survival (p=0.008 and p<0.001, respectively). The interaction of Metchnikowin and Drosomycin was not different from the sum of their individual effects (Mtk*Drs: HR = −0.80, p=0.116). (C) Fungal loads of individual flies at 18 hpi. At this time point, BomΔ55C mutants and spzrm7 flies have already failed to constrain C. albicans growth (C’). Fungal titres at 36hpi (C’’), a time point closer to mortality for many AMP mutants, show that some AMP mutants fail to control fungal load, while wild-type flies consistently controlled fungal titre. One-way ANOVA: not significant = ns, p<0.05 = *, p<0.01 = **, and p<0.001 = *** relative to iso w1118.
In conclusion, our study provides an in vivo validation of the potent antifungal activities of Metchnikowin and Drosomycin (Fehlbaum et al., 1994; Levashina et al., 1995), and highlights a clear example of additive cooperation of AMPs.
AMPs synergistically contribute to defence against P. burhodogranariea
We next analyzed the contribution of AMPs in resistance to infection with the moderately virulent Gram-negative bacterium P. burhodogranariea. We found that Group B mutants lacking Drosocin, the two Diptericins, and the four Attacins, were as susceptible to infection as ΔAMPs flies (Figure 4A), while flies lacking the antifungal peptides Drosomycin and Metchnikowin (Toll-regulated, Group C) resisted the infection as wild-type. Flies lacking Defensin (Group A) showed an intermediate susceptibility, but behave as wild-type in the additional absence of Toll Group C peptides (Group AC). Thus, we again observed a better survival rate with the co-occurring loss of Group A and C peptides (see possible explanation above). In this case, Group A flies were susceptible while AC flies were not.
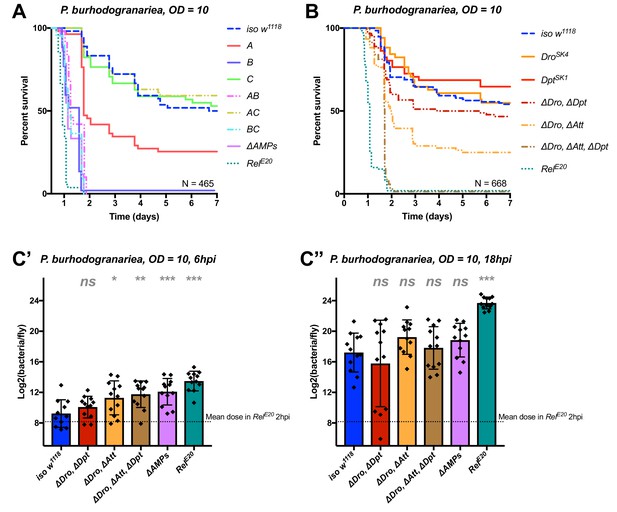
Identification of AMPs involved in the susceptibility of ΔAMPs flies to P. burhodogranariea.
(A) Survival of mutants for groups of AMPs reveals that loss of Imd-responsive Group B peptides (Drosocin, Attacins, and Diptericins) recapitulates the susceptibility of ΔAMPs flies. Loss of the Group A peptide Defensin also resulted in strong susceptibility (p<0.001) (and see Figure 4—figure supplement 1). (B) Further dissection of AMPs deleted in Group B reveals that only the loss of all Drosocin, Attacin, and Diptericin gene families leads to susceptibility similar to ΔAMPs flies. Simultaneous loss of Attacins and Diptericins results in a synergistic loss of resistance (ΔAtt*ΔDpt: HR =+1.45, p<0.001). (C) Bacterial loads of individual flies at 6 hpi (C’). At this time point, most AMP mutants had significantly higher bacterial loads compared to wild-type flies. At 18 hpi (C’’), differences in bacterial load are reduced, likely owing to the high chronic load P. burhodogranariea establishes even in surviving flies (Duneau et al., 2017). Meanwhile RelE20 flies succumb ~18 hr earlier than ΔAMPs flies in survival experiments, and already have significantly higher loads. One-way ANOVA: not significant = ns, p<0.05 = *, p<0.01 = **, and p<0.001 = *** relative to iso w1118.
Following the observation that Group B flies were as susceptible as ΔAMPs flies, we sought to better decipher the contribution of each Group B AMP to resistance to P. burhodogranariea. We observed that mutants for Drosocin alone (DroSK4), or the DiptericinA/B deficiency were not susceptible to this bacterium (Figure 4B). We additionally saw no marked susceptibility of Drosocin-Attacin A/B-deficient flies, nor Attacin C or Attacin D mutants (not shown). Interestingly, we found that compound mutants lacking Drosocin and Attacins A, B, C, and D (Figure 4B: ‘ΔDro, ΔAtt’), or Drosocin and Diptericins DptA and DptB (‘ΔDro, ΔDpt’) displayed an intermediate susceptibility. Only the Group B mutants lacking Drosocin, all Attacins, and both Diptericins (ΔDro, ΔAtt, ΔDpt) phenocopied ΔAMPs flies (Figure 4B), with synergistic statistical interactions observed upon co-occurring loss of Attacins and Diptericins (ΔAtt*ΔDpt: HR =+1.45, p<0.001); we emphasize here that this synergistic interaction solely reflects that the effect on survival of combining these mutations is greater than the sum effect of the individual mutations (discussed later). By 6hpi, bacterial titres of individual flies already showed significant differences in the most susceptible genotypes (Figure 4C), although these differences were reduced by 18 hpi likely owing to the high chronic load P. burhodogranariea establishes in surviving flies (Duneau et al., 2017; also see Figure 2—figure supplement 1A).
Collectively, the use of various compound mutants reveals that several Imd-responsive AMPs, notably Drosocin, Attacins, and Diptericins, jointly contribute to defence against P. burhodogranariea infection. A strong susceptibility of Group B flies was also observed upon infection with Ecc15, another Gram-negative bacterium commonly used to infect flies (Neyen et al., 2014) (Figure 4—figure supplement 1B ).
Diptericins alone contribute to defence against P. rettgeri
We continued our exploration of AMP interactions using our AMP groups approach with the fairly virulent P. rettgeri (strain Dmel), a strain isolated from wild-caught Drosophila hemolymph (Juneja and Lazzaro, 2009). We were especially interested by this bacterium as previous studies (Unckless et al., 2015; Unckless and Lazzaro, 2016) have shown a correlation between susceptibility to P. rettgeri and a polymorphism in the Diptericin A gene pointing to a specific AMP-pathogen interaction. Use of compound mutants revealed only loss of Group B AMPs was needed to reach the susceptibility of ΔAMPs and RelE20 flies (Figure 5A). Use of individual mutant lines, however, revealed a pattern overtly different from P. burhodogranariea, as the sole Diptericin A/B deficiency caused susceptibility similar to Group B, ΔAMPs, and RelE20 flies (Figure 5B,C). We further confirmed this susceptibility using a DptA RNAi construct (Figure 5—figure supplement 1A, B). Moreover, flies carrying the DptSK1 mutation over a deficiency (Df(2R)Exel6067) were also highly susceptible to P. rettgeri (Figure 5D). Interestingly, flies that were heterozygotes for DptSK1 or the Df(2R)Exel6067 that still have one copy of the two Diptericins were markedly susceptible to infection with P. rettgeri (Figure 5D). This indicates that a full transcriptional output of Diptericin is required over the course of the infection to resist P. rettgeri (Figure 5E). Altogether, our results suggest that only the Diptericin gene family, amongst the many AMPs regulated by the Imd pathway, provides the full AMP-based contribution to defence against this bacterium. To test this hypothesis, we generated a fly line lacking all the AMPs except DptA and DptB (ΔAMPs+Dpt). Strikingly, ΔAMPs+Dpt flies have the same survival rate as wild-type flies, further emphasizing the specificity of this interaction (Figure 5B). Bacterial counts confirm that the susceptibility of these Diptericin mutants arises from an inability of the host to suppress bacterial growth (Figure 5C).
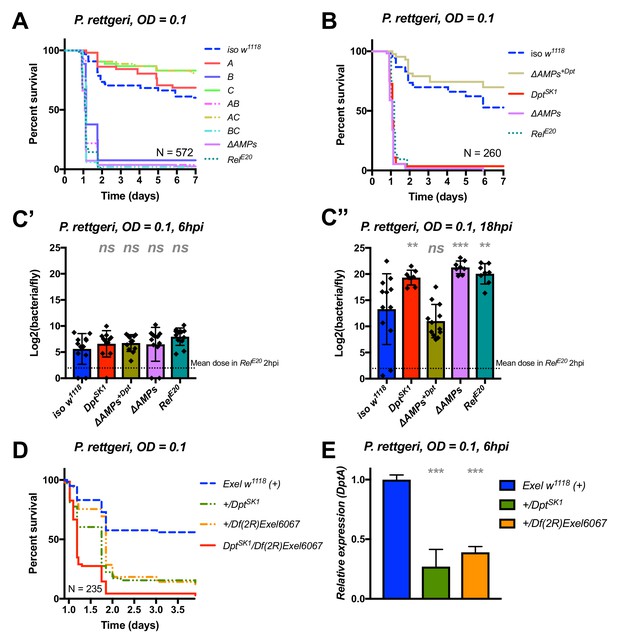
Identification of AMPs involved in the susceptibility of ΔAMPs flies to P. rettgeri.
(A) Survival of mutants for groups of AMPs reveals that only loss of Imd-responsive Group B peptides (Drosocin, Attacins, and Diptericins) recapitulates the susceptibility of ΔAMPs flies. (B) Further dissection of the mutations affected in Group B reveals that only the loss of Diptericins (DptSK1) leads to susceptibility similar to ΔAMPs flies. Remarkably, flies lacking all other AMPs (ΔAMPs+Dpt) resist as wild-type. (C) Bacterial loads of individual flies are similar at 6hpi (C’), but by 18hpi (C’’), Dpt mutants and RelE20 flies have all failed to control P. rettgeri growth. (D) Heterozygote flies for DptSK1 and a deficiency including the Diptericins and flanking genes (Df(2R)Exel6067) recapitulates the susceptibility of Diptericin mutants. Intriguingly, heterozygotes with one functional copy of the Diptericins (+/DptSK1 or +/Df(2R)Exel6067) are nonetheless highly susceptible to infection. (E) Diptericin A transcriptional output is strongly reduced in heterozygotes 6 hpi compared to wild-type flies. One-way ANOVA: not significant = ns, p<0.05 = *, p<0.01 = **, and p<0.001 = *** relative to iso w1118.
Collectively, our study shows that Diptericins are critical to resist P. rettgeri, while they play an important but less essential role in defence against P. burhodogranariea infection. We were curious whether Diptericin’s major contribution to defence observed with P. rettgeri could be generalized to other members of the genus Providencia. An exclusive role for Diptericins was also found for the more virulent P. stuartii (Figure 5—figure supplement 1C), but not for other Providencia species tested (P. burhodogranariea, P. alcalifaciens, P. sneebia, P. vermicola) (data not shown).
Drosocin is critical to resist infection with E. cloacae
In the course of our exploration of AMP-pathogen interactions, we identified another highly specific interaction between E. cloacae and Drosocin. Use of compound mutants revealed that alone, Group B flies were already susceptible to E. cloacae. Meanwhile, Group AB flies additionally lacking Defensin reached ΔAMPs levels of susceptibility, while Group A and Group C flies resisted as wild-typeMeanwhile, Group AB flies reached ΔAMPs levels of susceptibility, while Group A and Group C flies resisted as wild-type (Figure 6A). The high susceptibility of Group AB flies results from a synergistic statistical interaction amongst Group A (Defensin) and Group B peptides in defence against E. cloacae (A*B, HR =+2.55, p=0.003).
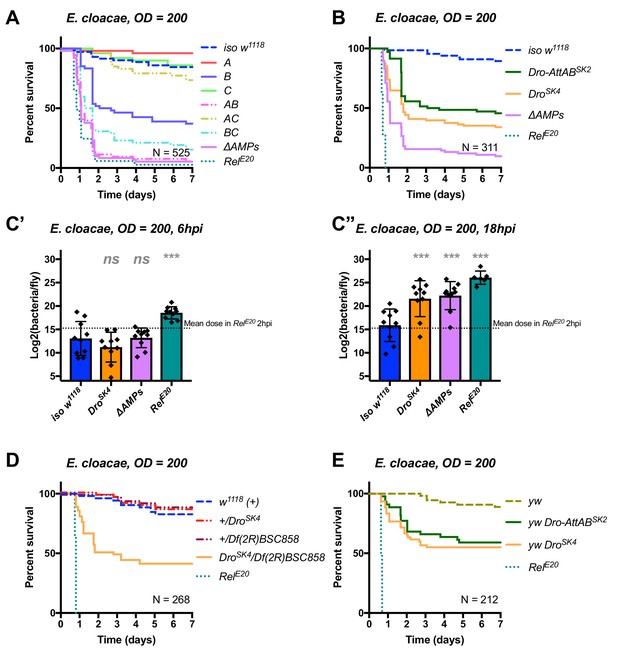
Identification of AMPs involved in the susceptibility of ΔAMPs flies to E.cloacae.
(A) Survival of mutants for groups of AMPs reveals that loss of Imd-responsive Group B peptides (Drosocin, Attacins, and Diptericins) results in a strong susceptibility to infection (p<0.001), while loss of Group A or C peptides alone resists as wild-type (p>0.1 each). Group AB flies were as susceptible as ΔAMPs flies, and we observed a synergistic interaction between Group A and B mutations (A*B: HR =+2.55, p=0.003). (B) Further dissection of the mutations in Group B revealed that loss of Drosocin alone (DroSK4), or a deficiency lacking both Drosocin and Attacins AttA and AttB (Dro-AttABSK2) recapitulates the susceptibility of Group B flies. (C) By 18hpi, bacterial loads in individual Drosocin mutants or RelE20 flies are significantly higher than wild-type. (D) Heterozygote flies for DroSK4 and Df(2R)BSC858 (a deficiency removing Drosocin, Attacins AttA and AttB, and other genes) are strongly susceptible to E. cloacae infection. (E) Drosocin mutants in an alternate genetic background (yw) are susceptible to E. cloacae. One-way ANOVA: not significant = ns, and p<0.001 = *** relative to iso w1118.
We chose to further explore the AMPs deleted in Group B flies, as alone this genotype already displayed a strong susceptibility. Use of individual mutant lines revealed that mutants for Drosocin alone (DroSK4) or the Drosocin-Attacin A/B deficiency (Dro-AttABSK2), but not AttC, AttD, nor DptSK1 (not shown), recapitulate the susceptibility observed in Group B flies (Figure 6B). At 18 hpi, both DroSK4 and ΔAMPs flies had significantly higher bacterial loads compared to wild-type flies, while RelE20 mutants were already moribund with much higher bacterial loads (Figure 6C). Indeed, the deletion of Drosocin alone alters the fly’s ability to control the otherwise avirulent E. cloacae upon inoculations using OD = 200 (~39,000 bacteria, Figure 6A–C) or even OD = 10 (~7000 bacteria, Figure 6—figure supplement 1A).
We confirmed the high susceptibility of Drosocin mutant flies to E. cloacae in various contexts: transheterozygote flies carrying DroSK4 over a Drosocin deficiency (Df(2R)BSC858) that also lacks flanking genes including AttA and AttB ((Figure 6D), the Dro SK4 mutations in an alternate genetic background (yw, Figure 6E), and, Drosocin RNAi (Figure 6—figure supplement 1B,C). Thus, we recovered two highly specific AMP-pathogen interactions: Diptericins are essential to combat P. rettgeri infection, while Drosocin is paramount to surviving E. cloacae infection.
Discussion
A combinatory approach to study AMPs
Despite the recent emphasis on innate immunity, little is known on how immune effectors contribute individually or collectively to host defence, exemplified by the lack of in depth in vivo functional characterization of Drosophila AMPs. Taking advantage of new gene editing approaches, we developed a systematic mutation approach to study the function of Drosophila AMPs. With seven distinct mutations, we were able to generate a fly line lacking 10 AMPs that are known to be strongly induced during the systemic immune response. A striking first finding is that ΔAMPs flies were perfectly healthy and have an otherwise wild-type immune response. This indicates that in contrast to mammals (van Wetering et al., 2002), these Drosophila AMPs are not likely to function as signaling molecules. Using a systemic mode of infection that induces AMP expression in the fat body and hemocytes, we found that most flies lacking a single AMP family exhibited a higher susceptibility to certain pathogens consistent with their in vitro activity. We found activity of Diptericins against P. rettgeri, Drosocin against E. cloacae, Drosomycin and Metchnikowin against C. albicans, and Defensin against P. burhodogranariea. In most cases, the susceptibility of single mutants was slight, and the contribution of individual AMPs could be revealed only when combined to other AMP mutations as illustrated by the susceptibility of Drosocin, Attacin, and Diptericin combined mutants to P. burhodogranariea. Thus, the use of compound rather than single mutations provides a better strategy to decipher the contribution of AMPs to host defence. Our findings are consistent with a previous study using flies that constitutively expressed individual peptides (Tzou et al., 2002), which showed an activity of Drosomycin against A. fumigatus and Attacin against Ecc15. Beyond the systemic immune response, AMPs are also expressed in many tissues such as the gut and trachea (Ferrandon et al., 1998; Tzou et al., 2000). Future studies should investigate the role of AMPs in these local epithelial immune responses.
AMPs and Bomanins are essential contributors to Toll and Imd pathway mediated host defence
The Toll and Imd pathways provide a paradigm of innate immunity, illustrating how two distinct pathways link pathogen recognition to distinct but overlapping sets of downstream immune effectors (Lemaitre and Hoffmann, 2007; Buchon et al., 2014). However, a method of deciphering the contributions of the different downstream effectors to the specificity of these pathways remained out of reach, as mutations in these immune effectors were lacking. Our study shows that AMPs contribute greatly to resistance to Gram-negative bacteria. Consistent with this, ΔAMPs flies are almost as susceptible as Imd-deficient mutants to most Gram-negative bacteria. In contrast, flies lacking AMPs were only slightly more susceptible to Gram-positive bacteria and fungal infections compared to wild-type flies, and this susceptibility rarely approached the susceptibility of Bomanin mutants. It is possible that additional loss of Cecropins would further increase the sensitivity of ΔAMPs flies to bacteria or fungi. This may be due to the cell walls of Gram-negative bacteria being thinner and more fluid than the rigid cell walls of Gram-positive bacteria (Fayaz et al., 2010), consequently making Gram-negative bacteria more prone to the action of pore-forming cationic peptides. It would be interesting to know if the specificity of AMPs to primarily combatting Gram-negative bacteria is also true in other species.
Based on our study and Clemmons et al. (2015), we can now explain the susceptibility of Toll and Imd mutants at the level of the effectors, as we show that mutations affecting Imd-pathway responsive antibacterial peptide genes are highly susceptible to Gram-negative bacteria while the Toll-responsive targets Drosomycin, Metchnikowin, and especially the Bomanins, confer resistance to fungi and Gram-positive bacteria. Thus, the susceptibility of these two pathways to different sets of microbes not only reflects specificity at the level of recognition, but can now also be translated to the activities of downstream effectors. It remains to be seen how Bomanins contribute to the microbicidal activity of immune-induced hemolymph, as attempts to synthesize Bomanins have not revealed direct antimicrobial activity (Lindsay et al., 2018). It should also be noted that many putative effectors downstream of Toll and Imd remain uncharacterized, and so could also contribute to host defence beyond these AMPs and Bomanins.
AMPs act additively and synergistically to suppress bacterial growth in vivo
In the last few years, numerous in vitro studies have focused on the potential for synergistic interactions of AMPs in microbial killing (Rahnamaeian et al., 2015; Yu et al., 2016; Zanchi et al., 2017; Yan and Hancock, 2001; Nuding et al., 2014; Zerweck et al., 2017; Chen et al., 2005; Stewart et al., 2014; Zdybicka-Barabas et al., 2012). Our collection of AMP mutant fly lines placed us in an ideal position to investigate AMP interactions in an in vivo setting. While Toll-responsive AMPs (Group C: Metchnikowin, Drosomycin) additively contributed to defence against the yeast C. albicans, we found that certain combinations of AMPs have synergistic contributions to defence against P. burhodogranariea. Synergistic loss of resistance may arise in two general fashions: first, co-operation of AMPs using similar mechanisms of action may breach a threshold microbicidal activity whereupon pathogens are no longer able to resist. This may be the case for the synergistic effect of Diptericins and Attacins against P. burhodogranariea, as only co-occurring loss of both these related glycine-rich peptide families (Hedengren et al., 2000) led to complete loss of resistance. Alternatively, synergy may arise due to complementary mechanisms of action, whereupon one AMP potentiates the other AMP’s ability to act. For instance, the action of the bumblebee AMP Abaecin, which binds to the molecular chaperone DnaK to inhibit bacterial DNA replication, is potentiated by the presence of the pore-forming peptide Hymenoptaecin (Rahnamaeian et al., 2016). Drosophila Drosocin is highly similar to Abaecin and the related peptide Apidecin, including O-glycosylation of a critical threonine residue (Imler and Bulet, 2005; Hanson et al., 2016), and thus likely acts in a similar fashion. Furthermore, Drosophila Attacin C is maturated into both a glycine-rich peptide and a Drosocin-like peptide called MPAC (Rabel et al., 2004). As such, co-occuring loss of Drosocin, MPAC, and other possible MPAC-like peptides encoded by the Attacin/Diptericin superfamily may be responsible for the synergistic loss of resistance in Drosocin, Attacin, Diptericin combined mutants.
AMPs can act with great specificity against certain pathogens
It is commonly thought that the innate immune response lacks the specificity of the adaptive immune system, which mounts directed defences against specific pathogens. Accordingly for innate immunity, the diversity of immune-inducible AMPs can be justified by the need for generalist and/or co-operative mechanisms of microbial killing. However, an alternate explanation may be that innate immunity expresses diverse AMPs in an attempt to hit the pathogen with a ‘silver bullet:’ an AMP specifically attuned to defend against that pathogen. Here, we provide a demonstration in an in vivo setting that such a strategy may actually be employed by the innate immune system. Remarkably, we recovered not just one, but two examples of exquisite specificity in our laborious but relatively limited assays.
Diptericin has previously been highlighted for its important role in defence against P. rettgeri (Unckless et al., 2016), but it was previously unknown whether other AMPs may confer defence in this infection model. Astoundingly, flies mutant for the other inducible AMPs resisted P. rettgeri infection as wild-type, while only Diptericin mutants succumbed to infection. This means that Diptericins do not co-operate with these other AMPs in defence against P. rettgeri and are solely responsible for defence in this specific host-pathogen interaction. Moreover, +/DptSK1 heterozygote flies were nonetheless extremely susceptible to infection, demonstrating that a full transcriptional output over the course of infection is required to effectively prevent pathogen growth. A previous study has shown that ~7 hpi appears to be the critical time point at which P. rettgeri either grows unimpeded or the infection is controlled (Duneau et al., 2017). This time point correlates with the time at which the Diptericin transcriptional output is in full-force (Lemaitre et al., 1997). Thus, a lag in the transcriptional response in DptSK1/+ flies likely prevents the host from reaching a competent Diptericin concentration, indicating that Diptericin expression level is a key factor in successful host defence.
We also show that Drosocin is specifically required for defence against E. cloacae. This striking finding validates previous biochemical analyses showing Drosocin in vitro activity against several Enterobacteriaceae, including E. cloacae (Bulet et al., 1996). As ΔAMPs flies are more susceptible than Drosocin single mutants, other AMPs also contribute to Drosocin-mediated control of E. cloacae. As highlighted above, Drosocin is similar to other Proline-rich AMPs (e.g. Abaecin, Pyrrhocoricin) that have been shown to target bacterial DnaK (Kragol et al., 2001; Rahnamaeian et al., 2015). Alone, these peptides still penetrate bacteria cell walls through their uptake by bacterial permeases (Rahnamaeian et al., 2016; Narayanan et al., 2014). Thus, while Drosocin would benefit from the presence of pore-forming toxins to enter bacterial cells (Rahnamaeian et al., 2016), the veritable ‘stake to the heart’ is likely the plunging of Drosocin itself into vital bacterial machinery.
On the role of AMPs in host defence
It has often been questioned why flies should need so many AMPs (Lemaitre and Hoffmann, 2007; Rolff and Schmid-Hempel, 2016; Unckless and Lazzaro, 2016). A common idea, supported by in vitro experiments (Rahnamaeian et al., 2015; Yan and Hancock, 2001; Zdybicka-Barabas et al., 2012) is that AMPs work as cocktails, wherein multiple effectors are needed to kill invading pathogens. However, we find support for an alternative hypothesis that suggests AMP diversity may be due to highly specific interactions between AMPs and subsets of pathogens that they target. Burgeoning support for this idea also comes from recent evolutionary studies that show Drosophila and vertebrate AMPs experience positive selection (Unckless et al., 2015; Unckless and Lazzaro, 2016; Hanson et al., 2016; Chapman et al., 2018; Hellgren and Sheldon, 2011; Tennessen and Blouin, 2008; Sackton, 2019), a hallmark of host-pathogen evolutionary conflict. Our functional demonstrations of AMP-pathogen specificity, using naturally relevant pathogens (Juneja and Lazzaro, 2009; Cox and Gilmore, 2007), suggest that such specificity is fairly common, and that certain AMPs can act as the arbiters of life or death upon infection by certain pathogens. This stands in contrast to the classical view that the AMP response contains such redundancy that single peptides should have little effect on organism-level immunity (Rolff and Schmid-Hempel, 2016; Unckless et al., 2015; Tzou et al., 2000; Unckless and Lazzaro, 2016). Nevertheless, it seems these immune effectors play non-redundant roles in defence.
By providing a long-awaited in vivo functional validation for the role of AMPs in host defence, we also pave the way for a better understanding of the functions of immune effectors. Our approach of using multiple compound mutants, now possible with the development of new genome editing approaches, was especially effective to decipher the logic of immune effectors. Understanding the role of AMPs in innate immunity holds great promise for the development of novel antibiotics (Chung et al., 2017; Mylonakis et al., 2016; Mahlapuu et al., 2016), insight into autoimmune diseases (Schluesener et al., 1993; Gilliet and Lande, 2008; Sun et al., 2015; Kumar et al., 2016), and given their potential for remarkably specific interactions, perhaps in predicting key parameters that predispose individuals or populations to certain kinds of infections (Unckless et al., 2015; Unckless and Lazzaro, 2016; Chapman et al., 2018). Finally, our set of isogenized AMP mutant lines provides long-awaited tools to decipher the role of AMPs not only in systemic immunity, but also in local immune responses, and the various roles that AMPs may play in aging, neurodegeneration, anti-tumor activity, regulation of the microbiota and more, where disparate evidence has pointed to their involvement.
Materials and methods
Drosophila genetics and mutant generation
Request a detailed protocolThe DrosDel (Ryder et al., 2004) isogenic w1118 (iso w1118) wild type was used as a genetic background for mutant isogenization. Alternate wild-types used throughout include Oregon R (OR-R), w1118 from the Vienna Drosophila Resource Centre, and the Canton-S isogenic line Exelexis w1118, which was kindly provided by Brian McCabe. BomΔ55C mutants were generously provided by Steven Wasserman, and BomΔ55C was isogenized into the iso w1118 background. RelE20 and spzrm7 iso w1118 flies were provided by Luis Teixeira (Hedengren et al., 1999; Ferreira et al., 2014). Prophenoloxidase mutants (ΔPPO) are described in Dudzic et al. (2015). P-element mediated homologous recombination according to Baena-Lopez et al. (2013) was used to generate mutants for Mtk (MtkR1) and Drs (DrsR1). Plasmids were provided by Mickael Poidevin. Attacin C mutants (AttCMi, #25598), the Diptericin deficiency (Df(2R)Exel6067, #7549), the Drosocin deficiency (Df(2R)BSC858, #27928), UAS-Diptericin RNAi (DptRNAi, #53923), UAS-Drosocin RNAi (DroRNAi, #67223), and Actin5C-Gal4 (ActGal4, #4414) were ordered from the Bloomington stock centre (stock #s included). CRISPR mutations were performed by Shu Kondo according to Kondo and Ueda (Kondo and Ueda, 2013), and full descriptions are given in Figure 1—figure supplement 1. In brief, flies deficient for Drosocin, Attacin A,and Attacin B (Dro-AttABSK2), and Diptericin A and Diptericin B (DptSK1) were produced by gene region deletion specific to those AMPs without affecting other genes. Single mutants for Defensin (DefSK3), Drosocin (DroSK4), and Attacin D (AttDSK1) are small indels resulting in the production of short (80–107 residues) nonsense peptides. Mutations were isogenized for a minimum of seven generations into the iso w1118 background prior to subsequent recombination. It should be noted that Group A flies were initially thought to be a double mutant for both Defensin and the Cecropin cluster, resulting from a combination of DefSK3 and a CRISPR-induced Cecropin deletion (called CecSK6). It was subsequently shown that CecSK6 is a complex aberration at the Cecropin locus that retains a wild-type copy of the Cecropin cluster. This re-arranged Cecropin locus does not contribute significantly to the susceptibility of Group A flies, as Group A was not different from DefSK3 alone (Log-Rank p=0.818; Figure 4—figure supplement 1A). Thus, group A flies were considered as single DefSK3 mutants.
Microbial culture conditions
Request a detailed protocolBacteria were grown overnight on a shaking plate at 200 rpm in their respective growth media and temperature conditions, and then pelleted by centrifugation at 4°C. These bacterial pellets were diluted to the desired optical density at 600 nm (OD) as indicated. The following bacteria were grown at 37°C in LB media: Escherichia coli strain 1106, Salmonella typhimurium, Enterobacter cloacae β12, Providencia rettgeri strain Dmel, Providencia burhodogranariea strain B, Providencia stuartii strain DSM 4539, Providencia sneebia strain Dmel, Providencia alcalifaciens strain Dmel, Providencia vermicola strain DSM 17385, Bacillus subtilis, and Staphylococcus aureus. Erwinia carotovora carotovora (Ecc15) and Micrococcus luteus were grown overnight in LB at 29°C. Enterococcus faecalis and Listeria innocua were cultured in BHI medium at 37°C. Candida albicans was cultured in YPG medium at 37°C. Aspergillus fumigatus was grown at room temperature on Malt Agar, and spores were collected in sterile PBS rinses, pelleted by centrifugation, and then resuspended to the desired OD in PBS. The entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae were grown on Malt Agar at room temperature until sporulation.
Systemic infections and survival
Request a detailed protocolSystemic infections were performed by pricking 3- to 5-day-old adult males in the thorax with a 100-μm-thick insect pin dipped into a concentrated pellet of bacteria or fungal spores. Infected flies were subsequently maintained at 25°C for experiments. For infections with B. bassiana and M. anisopliae, flies were anesthetized and then shaken on a sporulating plate of fungi for 30 s. At least two replicate survival experiments were performed for each infection, with 20–35 flies per vial on standard fly medium without yeast. Survivals were scored twice daily, with additional scoring at sensitive time points. Comparisons of iso w1118 wild-type to ΔAMPs mutants were made using a Cox-proportional hazard (CoxPH) model, where independent experiments were included as covariates, and covariates were removed if not significant (p>0.05). Direct comparisons were performed using Log-Rank tests in Prism seven software. The effect size and direction is included as the CoxPH hazard ratio (HR) where relevant, with a positive effect indicating increased susceptibility. CoxPH models were used to test for synergistic contributions of AMPs to survival in R 3.4.4. Total sample size (N) is given for each experiment as indicated.
Quantification of microbial load
Request a detailed protocolThe native Drosophila microbiota does not readily grow overnight on LB, allowing for a simple assay to estimate bacterial load. Flies were infected with bacteria at the indicated OD as described, and allowed to recover. At the indicated time post-infection, flies were anesthetized using CO2 and surface sterilized by washing them in 70% ethanol. Ethanol was removed, and then flies were homogenized using a Precellys bead beater at 6500 rpm for 30 s in LB broth, with 300 μl for individual samples, or 500 μl for pools of 5–7 flies. These homogenates were serially diluted and 150 μl was plated on LB agar. Bacterial plates were incubated overnight, and colony-forming units (CFUs) were counted manually. Statistical analyses were performed using One-way ANOVA with Sidak’s correction. p-Values are reported as <0.05 = *,<0.01 = **, and <0.001 = ***. For C. albicans, BiGGY agar was used instead to select for Candida colonies from fly homogenates.
Gene expression by qPCR
Request a detailed protocolFlies were infected by pricking flies with a needle dipped in a pellet of either E. coli or M. luteus (OD600 = 200), and frozen at −20°C 6 hr and 24 hr post-infection, respectively. Total RNA was then extracted from pooled samples of five flies each using TRIzol reagent, and re-suspended in MilliQ dH2O. Reverse transcription was performed using 0.5 mg total RNA in 10 μl reactions using PrimeScript RT (TAKARA) with random hexamer and oligo dT primers. Quantitative PCR was performed on a LightCycler 480 (Roche) in 96-well plates using Applied Biosystems SYBR Select Master Mix. Values represent the mean from three replicate experiments. Error bars represent one standard deviation from the mean. Primers used in this study can be found in Supplementary file 1. Statistical analyses were performed using one-way ANOVA with Tukey post-hoc comparisons. p-Values are reported as not significant = ns,<0.05 = *,<0.01 = **, and <0.001 = ***. qPCR primers and sources (Kounatidis et al., 2017; Hanson et al., 2016; Iatsenko et al., 2016) are included in Supplementary file 1.
MALDI-TOF peptide analysis
Request a detailed protocolTwo methods were used to collect hemolymph from adult flies: in the first method, pools of five adult females were pricked twice in the thorax and once in the abdomen. Wounded flies were then spun down with 15 μl of 0.1% trifluoroacetic acid (TFA) at 21000 RCF at 4°C in a mini-column fitted with a 10 μm pore to prevent contamination by circulating hemocytes. These samples were frozen at −20°C until analysis, and three biological replicates were performed with four technical replicates. In the second method, approximately 20 nl of fresh hemolymph was extracted from individual adult males using a Nanoject, and immediately added to 1 μl of 1% TFA, and the matrix was added after drying. Peptide expression was visualized as described in Uttenweiler-Joseph et al. (1998). Both methods produced similar results, and representative expression profiles are given.
Melanization and hemocyte characterization, image acquisition
Request a detailed protocolMelanization assays (Dudzic et al., 2018) and peanut agglutinin (PNA) clot staining (Scherfer et al., 2004) was performed as previously described. In brief, flies or L3 larvae were pricked, and the level of melanization was assessed at the wound site. We used FACS sorting to count circulating hemocytes. For sessile crystal cell visualization, L3 larvae were cooked in dH2O at 70°C for 20 min, and crystal cells were visualized on a Leica DFC300FX camera using Leica Application Suite and counted manually.
Data availability
Data generated or analysed during this study are included in the manuscript and supporting files. Source data has been provided for Figure 2.
References
-
Identification of early genes in the Drosophila immune response by PCR-based differential display: the attacin A gene and the evolution of attacin-like proteinsInsect Biochemistry and Molecular Biology 25:511–518.https://doi.org/10.1016/0965-1748(94)00091-C
-
Antimicrobial peptides modulate long-term memoryPLOS Genetics 14:e1007440.https://doi.org/10.1371/journal.pgen.1007440
-
The phytopathogenic bacteria erwinia carotovora infects Drosophila and activates an immune responseProceedings of the National Academy of Sciences 97:3376–3381.https://doi.org/10.1073/pnas.97.7.3376
-
Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiologyNature Reviews Immunology 14:796–810.https://doi.org/10.1038/nri3763
-
Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in micrococcus luteusThe Journal of Biological Chemistry 268:19239–19245.
-
Chemical synthesis, antibacterial activity and conformation of diptericin, an 82-mer peptide originally isolated from insectsEuropean Journal of Biochemistry 266:549–558.https://doi.org/10.1046/j.1432-1327.1999.00894.x
-
Drosophila cecropin as an antifungal agentInsect Biochemistry and Molecular Biology 29:965–972.https://doi.org/10.1016/S0965-1748(99)00071-5
-
Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteriaNanomedicine: Nanotechnology, Biology and Medicine 6:103–109.https://doi.org/10.1016/j.nano.2009.04.006
-
Insect immunity. septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptidesThe Journal of Biological Chemistry 269:33159–33163.
-
Antimicrobial peptides and self-DNA in autoimmune skin inflammationCurrent Opinion in Immunology 20:401–407.https://doi.org/10.1016/j.coi.2008.06.008
-
Expression and evolution of the Drosophila attacin/diptericin gene familyBiochemical and Biophysical Research Communications 279:574–581.https://doi.org/10.1006/bbrc.2000.3988
-
Locus-specific protocol for nine different innate immune genes (antimicrobial peptides: β-defensins) across passerine bird species reveals within-species coding variation and a case of trans-species polymorphismsMolecular Ecology Resources 11:686–692.https://doi.org/10.1111/j.1755-0998.2011.02995.x
-
Antimicrobial peptides in Drosophila: structures, activities and gene regulationChemical Immunology and Allergy 86:1–21.https://doi.org/10.1159/000086648
-
Providencia sneebia sp. nov. and providencia burhodogranariea sp. nov., isolated from wild Drosophila melanogasterInternational Journal of Systematic and Evolutionary Microbiology 59:1108–1111.https://doi.org/10.1099/ijs.0.000117-0
-
Amyloid-β peptide protects against microbial infection in mouse and worm models of alzheimer's diseaseScience Translational Medicine 8:340ra72.https://doi.org/10.1126/scitranslmed.aaf1059
-
The host defense of Drosophila melanogasterAnnual Review of Immunology 25:697–743.https://doi.org/10.1146/annurev.immunol.25.022106.141615
-
Metchnikowin, a novel immune-inducible proline-rich peptide from Drosophila with antibacterial and antifungal propertiesEuropean Journal of Biochemistry 233:694–700.https://doi.org/10.1111/j.1432-1033.1995.694_2.x
-
Short-Form bomanins mediate humoral immunity in DrosophilaJournal of Innate Immunity 10:306–314.https://doi.org/10.1159/000489831
-
Antimicrobial peptides: an emerging category of therapeutic agentsFrontiers in Cellular and Infection Microbiology 6:.https://doi.org/10.3389/fcimb.2016.00194
-
Metabolic integration of bacterial endosymbionts through antimicrobial peptidesTrends in Microbiology 25:703–712.https://doi.org/10.1016/j.tim.2017.04.007
-
Enhanced antimicrobial activity of peptide-cocktails against common bacterial contaminants of ex vivo stored plateletsClinical Microbiology and Infection 20:O39–O46.https://doi.org/10.1111/1469-0691.12326
-
Diversity, evolution and medical applications of insect antimicrobial peptidesPhilosophical Transactions of the Royal Society B: Biological Sciences 371:20150290.https://doi.org/10.1098/rstb.2015.0290
-
Staphylococcus aureus exploits epidermal barrier defects in atopic dermatitis to trigger cytokine expressionJournal of Investigative Dermatology 136:2192–2200.https://doi.org/10.1016/j.jid.2016.05.127
-
Mechanism of Escherichia coli resistance to pyrrhocoricinAntimicrobial Agents and Chemotherapy 58:2754–2762.https://doi.org/10.1128/AAC.02565-13
-
Synergistic effects of antimicrobial peptides and antibiotics against clostridium difficileAntimicrobial Agents and Chemotherapy 58:5719–5725.https://doi.org/10.1128/AAC.02542-14
-
Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functionsBiochemical and Biophysical Research Communications 244:253–257.https://doi.org/10.1006/bbrc.1998.8159
-
Primary structure and in vitro antibacterial properties of the Drosophila melanogaster attacin C Pro-domainThe Journal of Biological Chemistry 279:14853–14859.https://doi.org/10.1074/jbc.M313608200
-
Insect antimicrobial peptides show potentiating functional interactions against Gram-negative bacteriaProceedings of the Royal Society B: Biological Sciences 282:20150293.https://doi.org/10.1098/rspb.2015.0293
-
Perspectives on the evolutionary ecology of arthropod antimicrobial peptidesPhilosophical Transactions of the Royal Society B: Biological Sciences 371:20150297.https://doi.org/10.1098/rstb.2015.0297
-
Cutting edge: the toll pathway is required for resistance to gram-positive bacterial infections in DrosophilaThe Journal of Immunology 168:1542–1546.https://doi.org/10.4049/jimmunol.168.4.1542
-
Comparative genomics and transcriptomics of host–pathogen interactions in insects: evolutionary insights and future directionsCurrent Opinion in Insect Science 31:106–113.https://doi.org/10.1016/j.cois.2018.12.007
-
Leukocytic antimicrobial peptides kill autoimmune T cellsJournal of Neuroimmunology 47:199–202.https://doi.org/10.1016/0165-5728(93)90030-3
-
The perforin pore facilitates the delivery of cationic cargosJournal of Biological Chemistry 289:9172–9181.https://doi.org/10.1074/jbc.M113.544890
-
Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogasterMolecular and Cellular Biology 27:4578–4588.https://doi.org/10.1128/MCB.01814-06
-
Balancing selection at a frog antimicrobial peptide locus: fluctuating immune effector alleles?Molecular Biology and Evolution 25:2669–2680.https://doi.org/10.1093/molbev/msn208
-
The potential for adaptive maintenance of diversity in insect antimicrobial peptidesPhilosophical Transactions of the Royal Society B: Biological Sciences 371:20150291.https://doi.org/10.1098/rstb.2015.0291
-
Synergistic interactions between mammalian antimicrobial defense peptidesAntimicrobial Agents and Chemotherapy 45:1558–1560.https://doi.org/10.1128/AAC.45.5.1558-1560.2001
-
The more the better? combination effects of antimicrobial peptidesAntimicrob Agents Chemother.https://doi.org/10.1128/AAC.02434-15
-
Synergistic action of galleria mellonella anionic peptide 2 and lysozyme against Gram-negative bacteriaBiochimica Et Biophysica Acta (BBA) - Biomembranes 1818:2623–2635.https://doi.org/10.1016/j.bbamem.2012.06.008
-
Antimicrobial peptides increase tolerance to oxidant stress in Drosophila melanogasterJournal of Biological Chemistry 286:6211–6218.https://doi.org/10.1074/jbc.M110.181206
-
Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophilsBritish Journal of Dermatology 157:1124–1131.https://doi.org/10.1111/j.1365-2133.2007.08196.x
Article and author information
Author details
Funding
The authors declare that there was no funding for this work
Acknowledgements
We thank Marc Moniatte and the EPFL proteomics core facility for assistance with MALDI-TOF analysis, Claudia Melcarne for assistance with hemocyte characterization, and Igor Iatsenko for help in preparation of critical reagents. Brian Lazzaro generously provided Providencia species used in this study. We thank Hannah Westlake for useful comments on the manuscript. MAH would like to extend special thanks to Jan Dudzic for many illuminating discussions had over coffee.
Version history
- Received: December 12, 2018
- Accepted: February 13, 2019
- Accepted Manuscript published: February 26, 2019 (version 1)
- Version of Record published: March 4, 2019 (version 2)
- Version of Record updated: May 28, 2019 (version 3)
Copyright
© 2019, Hanson et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 10,920
- Page views
-
- 1,588
- Downloads
-
- 124
- Citations
Article citation count generated by polling the highest count across the following sources: Scopus, PubMed Central, Crossref.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Developmental Biology
- Immunology and Inflammation
During embryogenesis, the fetal liver becomes the main hematopoietic organ, where stem and progenitor cells as well as immature and mature immune cells form an intricate cellular network. Hematopoietic stem cells (HSCs) reside in a specialized niche, which is essential for their proliferation and differentiation. However, the cellular and molecular determinants contributing to this fetal HSC niche remain largely unknown. Macrophages are the first differentiated hematopoietic cells found in the developing liver, where they are important for fetal erythropoiesis by promoting erythrocyte maturation and phagocytosing expelled nuclei. Yet, whether macrophages play a role in fetal hematopoiesis beyond serving as a niche for maturing erythroblasts remains elusive. Here, we investigate the heterogeneity of macrophage populations in the murine fetal liver to define their specific roles during hematopoiesis. Using a single-cell omics approach combined with spatial proteomics and genetic fate-mapping models, we found that fetal liver macrophages cluster into distinct yolk sac-derived subpopulations and that long-term HSCs are interacting preferentially with one of the macrophage subpopulations. Fetal livers lacking macrophages show a delay in erythropoiesis and have an increased number of granulocytes, which can be attributed to transcriptional reprogramming and altered differentiation potential of long-term HSCs. Together, our data provide a detailed map of fetal liver macrophage subpopulations and implicate macrophages as part of the fetal HSC niche.






