Bone Formation: Sensing the load
The adult skeleton is an extremely dynamic tissue that can adjust its mass and strength to exactly the values needed for efficient movement. For instance, the bones in the dominant arm of a tennis player have a greater mineral density than the bones in their non-dominant arm (Huddleston, 1980). However, bone matter can also be lost if muscles do not get used enough (or if they do not have to support the body against gravity, as happens in space). The ability of bone to sense the mechanical load it has to support, and to adapt to changes in its environment, are central to the body's ability to build the exact amount of bone needed to prevent it from breaking as a result of increased muscle tension or external trauma. But how does bone sense the mechanical load that it carries?
Although the details remain unclear, cells called osteocytes are known to have a prominent role in sensing loads. Osteocytes are descendants of osteoblasts – the cells that build new bone. Whereas osteoblasts reside on the bone surface to deposit new bone, osteocytes are buried deep within mature bone (Figure 1; Long, 2012). Here, they regulate the activity of both osteoblasts and cells called osteoclasts that are responsible for bone resorption (the process by which old bone is broken down). Previous studies have shown that osteocytes alter their transcriptional profile in response to changes in biomechanical strain. For instance, an increase in load is associated with a reduction in the expression of a gene called Sost, which is known to prevent bone formation by osteoblasts (Robling et al., 2008). However, despite much progress, we still do not fully understand how bone is able to sense changes in mechanical load.
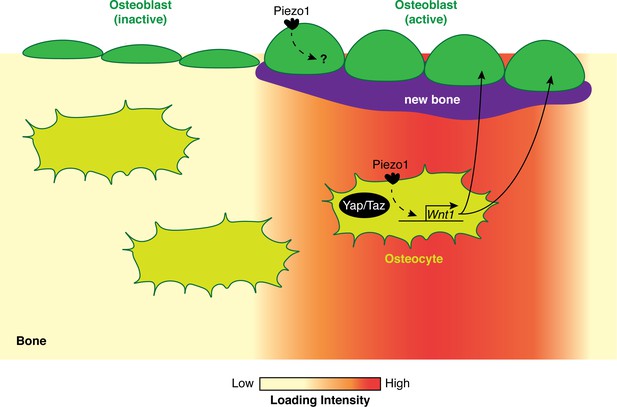
How biomechanical loading stimulates bone formation.
In regions of bone that experience high biomechanical loads (red), the Piezo1 ion channel is activated in both osteoblasts (green) and osteocytes (olive). In osteocytes, the activation of Piezo1 leads to increased Wnt1 expression in a Yap/Taz-dependent manner. Wnt1 then activates the Wnt signaling pathway, which stimulates the formation of new bone (purple) by osteoblasts. In regions of bone that experience average or low biomechanical loads, the osteoblasts are inactive and there is no increase in bone mass.
Now, in eLife, two independent groups report that an ion channel called Piezo1 has a central role in the process of mechanosensation (Li et al., 2019; Sun et al., 2019). Piezo1 is a protein embedded in the cell membrane that responds to mechanical stimulation by changing the influx of calcium and other positive ions. In the first paper, Bailong Xiao (Tsinghua University), Yingxian Li (Chinese Astronaut Research and Training Centre) and co-workers – including Weijia Sun and Shaopeng Chi as joint first authors – report the results of a series of experiments on Piezo1 (Li et al., 2019). First, they observed that the precursors of osteoblasts only respond to mechanical stimulation (which in this case involved 'poking' the cell membrane with a glass pipette) when Piezo1 is present. They also showed that the Piezo1 channel is essential for these cells to differentiate into functional osteoblasts. Next, Sun et al. explored the role played by Piezo1 in vivo by genetically engineering mice in which a signal could be used to delete Piezo1 in osteoblasts and osteocytes. These animals had normal bone resorption, but showed impaired bone strength, formation and structure.
Are these effects due to Piezo1 being a mechanosensitive ion channel? To answer this question, Sun et al. first showed that osteoblasts which lack Piezo1 no longer show the mechanically-induced ion currents seen in osteoblasts that are derived from wild-type mice. More importantly, when the skeleton of animals lacking Piezo1 expression in osteoblasts and osteocytes is exposed to either an increase or a reduction in mechanical load, the associated responses in bone mass are blunted. This shows that the Piezo1 channel is necessary to sense the biomechanical load and to change the rate at which bone is formed.
In the second paper Jinhu Xiong (University of Arkansas for Medical Sciences) and co-workers – including Xuehua Li as first author – also report new findings on Piezo1 (Sun et al., 2019). They placed osteocytes under fluid shear stress (which is a different kind of mechanical stimulation) and used RNA-seq to examine changes in gene expression for a range of calcium channels: the largest changes were observed in Piezo1. Mice in which this channel had been deleted from osteoblasts and osteocytes also showed reduced bone mass and biomechanical strength, confirming the role of Piezo1 in osteoblasts and their descendants. (It is worth noting that the two groups used different approaches to delete Piezo1, but still came to similar conclusions.) Increasing the load on the bones of these mutant animals no longer led to increased bone mass or osteoblast activity, indicating that the channel is at least partially required to 'translate' the signal of increased biomechanical load into increased bone mass.
How would activation of Piezo1 lead to the formation of new bone? Li et al. found that deleting Piezo1 reduces the expression of a signaling protein called Wnt1, which is critical for bone formation (Joeng et al., 2017). In addition, their results suggest that Piezo1 enhances the expression of Wnt1 by increasing the activity of Yap and Taz, two mechanosensitive transcription cofactors that are required for bone formation (Figure 1; Kegelman et al., 2018; Xiong et al., 2018). Lastly, when a drug called Yoda1 was used to activate Piezo1 in adult wild-type mice, the long bones of these animals showed an increase in both Wnt1 levels and bone mass.
Together, the results of Sun et al. and Li et al. strongly suggest that Piezo1 plays a key role in helping the skeleton respond to changes in mechanical loading. However, the results also raise intriguing questions. First, it remains unclear whether Piezo1 mainly mediates mechanosensation in osteoblasts or in osteocytes because we do not have the genetic tools needed to answer these questions. Second, we still do not know if there is a link between the mechanosensing ability of the Piezo1 channel and its role in the differentiation of osteoblasts and osteoprogenitor cells involved in the formation and maintenance of bone (Sugimoto et al., 2017). Third, Piezo1 may not be the only mechanosensitive channel that plays a role in mediating the response of bone to changes in mechanical stimulation. Fourth, we do not fully understand the Yap/Taz-Wnt axis: while Li et al. show that Piezo1 stimulates Wnt1 expression in part through Yap/Taz, it is unclear if this is a direct consequence of Piezo1 activation.
Finally, showing that the effects of biomechanical loads on bone can be mimicked by pharmaceutically activating Piezo1 could be a promising step towards the development of treatments for bone conditions such as osteoporosis. However, we need to proceed carefully as increased Piezo1 expression can stimulate cell death in cartilage and lead to osteoarthritis (Lee et al., 2014), underscoring the need for a complete understanding of the mechanosensation pathway in bone and cartilage.
References
-
Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasisJournal of Clinical Investigation 127:2678–2688.https://doi.org/10.1172/JCI92617
-
Skeletal cell YAP and TAZ combinatorially promote bone developmentFASEB Journal 32:2706–2721.https://doi.org/10.1096/fj.201700872R
-
Building strong bones: Molecular regulation of the osteoblast lineageNature Reviews Molecular Cell Biology 13:27–38.https://doi.org/10.1038/nrm3254
-
Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/SclerostinJournal of Biological Chemistry 283:5866–5875.https://doi.org/10.1074/jbc.M705092200
Article and author information
Author details
Publication history
- Version of Record published: October 7, 2019 (version 1)
Copyright
© 2019, Haelterman and Lim
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,568
- views
-
- 239
- downloads
-
- 7
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
Mechanical load of the skeleton system is essential for the development, growth, and maintenance of bone. However, the molecular mechanism by which mechanical stimuli are converted into osteogenesis and bone formation remains unclear. Here we report that Piezo1, a bona fide mechanotransducer that is critical for various biological processes, plays a critical role in bone formation. Knockout of Piezo1 in osteoblast lineage cells disrupts the osteogenesis of osteoblasts and severely impairs bone structure and strength. Bone loss that is induced by mechanical unloading is blunted in knockout mice. Intriguingly, simulated microgravity treatment reduced the function of osteoblasts by suppressing the expression of Piezo1. Furthermore, osteoporosis patients show reduced expression of Piezo1, which is closely correlated with osteoblast dysfunction. These data collectively suggest that Piezo1 functions as a key mechanotransducer for conferring mechanosensitivity to osteoblasts and determining mechanical-load-dependent bone formation, and represents a novel therapeutic target for treating osteoporosis or mechanical unloading-induced severe bone loss.
-
- Biochemistry and Chemical Biology
- Cell Biology
Mediator of ERBB2-driven Cell Motility 1 (MEMO1) is an evolutionary conserved protein implicated in many biological processes; however, its primary molecular function remains unknown. Importantly, MEMO1 is overexpressed in many types of cancer and was shown to modulate breast cancer metastasis through altered cell motility. To better understand the function of MEMO1 in cancer cells, we analyzed genetic interactions of MEMO1 using gene essentiality data from 1028 cancer cell lines and found multiple iron-related genes exhibiting genetic relationships with MEMO1. We experimentally confirmed several interactions between MEMO1 and iron-related proteins in living cells, most notably, transferrin receptor 2 (TFR2), mitoferrin-2 (SLC25A28), and the global iron response regulator IRP1 (ACO1). These interactions indicate that cells with high MEMO1 expression levels are hypersensitive to the disruptions in iron distribution. Our data also indicate that MEMO1 is involved in ferroptosis and is linked to iron supply to mitochondria. We have found that purified MEMO1 binds iron with high affinity under redox conditions mimicking intracellular environment and solved MEMO1 structures in complex with iron and copper. Our work reveals that the iron coordination mode in MEMO1 is very similar to that of iron-containing extradiol dioxygenases, which also display a similar structural fold. We conclude that MEMO1 is an iron-binding protein that modulates iron homeostasis in cancer cells.






