The Natural History of Model Organisms: Insights into the evolution of social systems and species from baboon studies
- Article
- Figures and data
- Abstract
- Introduction
- Systematic classification and distribution
- Morphology
- Ecology
- Phylogeography
- Variation in social organization and behavior
- Variation in social cognition
- Sociality, health, aging and fitness
- Functional genomics
- Baboons in the Anthropocene
- Conclusion
- Data availability
- References
- Decision letter
- Author response
- Article and author information
- Metrics
Abstract
Baboons, members of the genus Papio, comprise six closely related species distributed throughout sub-Saharan Africa and southwest Arabia. The species exhibit more ecological flexibility and a wider range of social systems than many other primates. This article summarizes our current knowledge of the natural history of baboons and highlights directions for future research. We suggest that baboons can serve as a valuable model for complex evolutionary processes, such as speciation and hybridization. The evolution of baboons has been heavily shaped by climatic changes and population expansion and fragmentation in the African savanna environment, similar to the processes that acted during human evolution. With accumulating long-term data, and new data from previously understudied species, baboons are ideally suited for investigating the links between sociality, health, longevity and reproductive success. To achieve these aims, we propose a closer integration of studies at the proximate level, including functional genomics, with behavioral and ecological studies.
https://doi.org/10.7554/eLife.50989.001Introduction
Humans have been captivated by baboons for thousands of years: from ancient Egypt, where the god of wisdom, Thoth, was depicted with a baboon head, to the mid-19th century when Charles Darwin remarked, "He who understands baboon would do more towards metaphysics than Locke" (Darwin, 1838). At the beginning of the 20th century, the South African naturalist Eugene Marais provided one of the first detailed accounts of free-ranging baboons (Marais, 1939), and by the 1950s, baboons had become the subject of more systematic scientific enquiry, both in the field and in captivity. This was the decade that the American physical anthropologist Sherwood Washburn and his student Irven DeVore set out to investigate baboons in Kenya (Vore and Washburn, 1961). Washburn reasoned that these ground-living primates were a good model for early human adaptations because they evolved in African savannas alongside ancestral hominins. Meanwhile, increasing interest in the use of non-human primates as biomedical models for humans led to the funding in 1958 of a three-year proposal titled "Initiation and Support of Colony of Baboons" by the US National Institutes of Health, with the first group of baboons shipped to the United States from Kenya in 1960 (VandeBerg, 2009). Since then, research in captivity on baboons as a biomedical model has been complemented by extensive fieldwork on baboon populations across Africa. Knowledge of the links between health and fitness in baboons under natural circumstances, including natural levels of genotypic and phenotypic variation, appears critical to put results from captive studies into context. An understanding of the evolution and life history of these animals in the wild also allows the scientific community to assess the validity of results derived from captive populations.
While the earlier field studies set out to uncover a baboon archetype, subsequent research has revealed that there is no such thing as "the baboon". Indeed, many would argue that the value of this genus lies precisely in the substantial variation in the social systems, life histories and ecologies within and between the baboon species (see Box 1 for a glossary of specialist terms used in this article). Collectively, these characteristics make baboons an excellent model organism for investigating a range of fundamental biological processes, such as physiological and behavioral adaptation, hybridization and speciation with gene flow (Alfred and Baldwin, 2015). In this way, the baboon model constitutes an important complement to other mammalian model organisms, such as wild house mice(Mus musculus; Phifer-Rixey and Nachman, 2015) and deer mice (genus Peromyscus; Bedford and Hoekstra, 2015).
Box 1.
Glossary.
Admixture: Genetic admixture refers to the exchange of genetic information among two populations or taxa that had been reproductively isolated and which genetically diverged (see introgressive hybridization).
Consortship: Consortships occur when females are sexually receptive and involve a male and female pair who associate in close proximity, often mating repeatedly. Typically, male-female consort pairs travel, feed and rest together. Consortships can last for hours or days.
Genetic architecture: Refers to the underlying genetic basis of a phenotypic trait (morphological, physiological, behavioral) and the variation in the respective trait.
Ghost lineage: A term from paleontology and phylogenetics. It refers to a phylogenetic lineage that has no fossil record or living representatives, but is inferred to have existed, for example, by whole-genome analyses of related taxa.
Hybridization: The interbreeding between two differentiated populations, usually closely related species, resulting in the combination of genetic material from previously isolated gene pools.
Introgression or introgressive hybridization: Observed between species or between genetically well-separated populations. It refers to the movement of genes, or gene flow, from one species into the gene pool of another by the repeated backcrossing of interspecific hybrids with one of their parent species.
Life history: The life history of an organism is a characterization of its patterns of development, reproduction, aging and mortality. Key measures of primate life history include length of gestation, age at the first occurrence of menstruation, age at first reproduction, number of offspring per litter, number of births per year, interval between births and life span.
Mating system: The distribution of matings among sexually active individuals within a social unit. Primate species can be monogamous (mating occurs mostly between pair partners), polyandrous (one female mates with multiple males), polygynous (one male mates with multiple females), or polygynandrous (males and females have multiple mating partners).
Mitochondrial and nuclear lineages: Mitochondria, organelles of almost all eukaryotic cells, carry their own genome. In contrast to nuclear genomes, recombination of the mitochondrial genome is a rare event and, since mitochondria are almost exclusively inherited via the maternal lineage, nuclear and mitochondrial genetic lineages can experience independent evolutionary histories. This often results in discordant phylogenies when using sex chromosomes (gonosomal), non-sex chromosomes (autosomal) or mitochondrial markers. Even phylogenies based on different nuclear genes or parts of the nuclear genome can lead to some discordances. Nevertheless, one can use nuclear and mitochondrial lineages to infer different evolutionary events within the evolutionary history of a species.
Phylogenetic species concept: This concept defines a species as an irreducible group or cluster whose members are descendants from a common ancestor and who all possess a combination of certain defining derived traits known as apomorphies. Such groups are monophyletic (contrasted with paraphyletic or polyphyletic groups). Reproductive isolation is not a precondition for the definition of species. Since monophyletic groups are often nested, ranking a particular group as a species can be problematic.
Social organization: The number of individuals and the composition of a group, including when and where those individuals are distributed. Groups may for instance be stable or reveal a fission-fusion system where the group temporarily splits into smaller sub-groups. Baboon societies may be uni-level (individuals live in a stable group and generally roam together) or multi-level (groups consist of predictable sub-groups, which may in turn consist of smaller sub-groups). An important aspect of the social organization is the dispersal behavior, that is, which sex typically remains in the group into which is was born (i.e. its natal group) and which sex leaves the natal group to breed. In most mammals, females stay (female philopatry) and males leave (male dispersal), which is considered the ancestral state. The timing and type of dispersal has important consequences for the genetic structure of groups.
Social style: The degree of aggressiveness among group members in a species. In societies that exhibit steep dominance hierarchies ("high despotism"), aggression is extremely asymmetrical, while in tolerant species, aggression is mild and frequently bi-directional. A further important component is the degree of nepotism within the species, that is, how kin-biased affiliation is. The social style of a species is associated with variation in relationship quality, which in turn characterizes the social structure of a species.
Social system: A primate species’ social system encompasses its social organization, social style, mating patterns and parental care system (Kappeler, 2019).
https://doi.org/10.7554/eLife.50989.002Systematic classification and distribution
Within the primate order, all extant baboons belong to the genus Papio. The genus is part of the tribe Papionini, within the family Cercopithecidae. The fossil record and phylogeographic analyses indicate that baboons originated in southern Africa. Nuclear and mitochondrial estimates put the date of initial divergence of baboon lineages at 1.5–2.1 million years ago (Newman et al., 2004; Rogers et al., 2019; Zinner et al., 2013). At about the same time, during the Pleistocene epoch, baboons started to expand their range across sub-Saharan Africa into both northern and southern savannas.
Presently, six species are recognized: the chacma baboon (Papio ursinus), which is found in southern Africa; the yellow baboon (Papio cynocephalus), which inhabits large parts of eastern Africa; the Kinda baboon (Papio kindae), which is found in Zambia, eastern Angola, and southern DR Congo; the olive baboon (Papio anubis), whose distribution ranges from northern DR Congo to parts of Kenya and Tanzania across to Sierra Leone in the west and to Eritrea in the east; the Guinea baboon (Papio papio), which is found from Sierra Leone to Mauritania and Senegal; and the hamadryas baboon (Papio hamadryas), which inhabits parts of Eritrea, Ethiopia, Somalia and the south-western part of the Arabian peninsula (Figure 1). Hybrid zones are found where species' distributions come into contact.
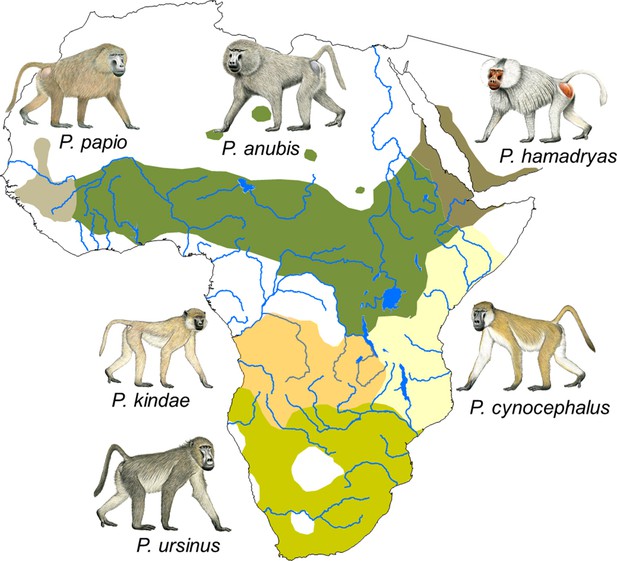
Distribution of the six Papio species.
Species distributions are modified from Zinner et al. (2013). Male baboon drawings by Stephen Nash. Reprinted with permission from Fischer et al. (2017).
While the systematic grouping into taxa within the genus Papio is well accepted on both phenotypic and genetic evidence, the taxonomic ranking is disputed. According to the biological species concept (Mayr, 1963), all taxa would be considered one polytypic species (Papio hamadryas) because where they meet in the wild they interbreed freely, producing viable and fertile hybrid offspring. Given that the different taxa vary substantially in appearance, behavior, and the characteristics of their society, we follow the phylogenetic species concept (Cracraft, 1983), and refer to the different taxa as "species".
Morphology
All baboon species are anatomically and morphologically well adapted to a quadrupedal terrestrial lifestyle (Fleagle, 2013). They have dog-like muzzles and males have large canine teeth. Depending on the species, body mass varies between 17 and 30 kg for adult males, and between 10 and 15 kg for females, resulting in a sexual dimorphism in mass ranging between 1.55 and 2.20 (Anandam et al., 2013; Fischer et al., 2017; Swedell, 2011).
The species differ notably in their fur color, body size and sexually selected characteristics, such as the distinct capes which are most pronounced in Guinea and hamadryas baboons but also present in olive baboons. Females of all species develop sexual swellings of the anogenital region when they are fertile. These swelling change throughout the menstrual cycle, such that maximum swelling typically coincides with ovulation (Higham et al., 2008). The size and shape of the swellings varies considerably among species (Figure 2; Petersdorf et al., 2019).

Illustration of key traits across baboon species.
(A) Phenotypic variation between species. Pictures show adult males and females. (B) Crania of male baboons. (C) Sexual swellings of female baboon during peak estrus. Species are grouped by social organization (uni- and multi-level) and dispersal behavior (male- or female-biased dispersal). Images from Alexis Amann, Andrea Cardini, Sarah Elton, Julia Fischer, Courtney Fitzpatrick, James Higham, Megan Petersdorf, Joan Silk and Larissa Swedell.
Ecology
All baboon species are largely terrestrial during the day but retreat to sleeping trees or cliffs during the night. They exhibit great ecological flexibility, allowing them to occupy habitats including semi-deserts grasslands, woodland savannas, humid forests, and Afroalpine grasslands over 3,000 meters above sea level (Chala et al., 2019; Fuchs et al., 2018). Baboons eat a broad range of foods, although their diet mainly consists of plants, including fruit, seeds, leaves, and roots. They also eat insects and other arthropods and, occasionally, kill small antelopes, hares, rodents, birds and smaller monkeys (Goffe and Fischer, 2016; Swedell, 2011).
Phylogeography
Phenotypic differences between species are well recognized (Jolly, 1993), and based on their molecular phylogeny, baboons are generally split in two major groups: north and south (Dunn et al., 2013; Frost et al., 2003). However, genetic evidence reveals a complex evolutionary history of the genus Papio. Analysis of mitochondrial DNA yields a phylogeny that includes several major haplogroups or clades – groups of individuals who belong to a specific mitochondrial lineage. These haplogroups reflect the geographic origin of the respective specimens better than their external phenotypes or taxonomic classification, making species appear to be paraphyletic and polyphyletic when mapped onto the mitochondrial phylogeny (Zinner et al., 2009; Zinner et al., 2011).
Comparisons of whole genome sequences confirm the six baboon species taxonomy and suggest that the initial evolutionary divergence separated a southern lineage that ultimately produced Kinda, chacma and yellow baboons, from a northern lineage that produced olive, hamadryas and Guinea baboons (Rogers et al., 2019). Ancient hybridization events appear to have affected the genetic makeup of all species. For instance, Guinea baboons most likely experienced genetic admixture with a "ghost lineage" that is probably extinct, or that is at least not represented in the sample of genomes analyzed to date (Rogers et al., 2019).
Multiple episodes of admixture and introgression have been linked to climate change and range expansion (Rogers et al., 2019; Walker et al., 2017; Wall et al., 2016; Zinner et al., 2013). Similar evolutionary mechanisms, including gene transfer by introgressive hybridization, are now recognized to have influenced the evolution of Neanderthals, Denisovans and modern humans (Green et al., 2010; Prüfer et al., 2017; Prüfer et al., 2014; Reich et al., 2010; Ackermann et al., 2019). However, the absence of genomic data from non-sapiens African hominins presently hinders our ability to ask questions about ancestral African hominin hybridization (Scerri et al., 2018; Stringer, 2016). Baboons allow us to study the impact of gene flow in an extant model.
Of particular interest for understanding baboon evolution is how changes in population density and spatial structure, such as the opening and closing of forest and other barriers, gave rise to different social systems (Jolly, 2019). The range expansion of the genus appears to be of particular relevance. Given a southern African origin, modern baboons experienced a tremendous expansion of their range, possibly linked to changes to the habitats, animal communities and climate that occurred during the Pleistocene and that gave baboons the chance to disperse into the savanna belt north of the tropical forest zone (Dolotovskaya et al., 2017; Zinner et al., 2011). Like humans and other savanna species, baboons have thus been subject of recurrent range shifts, fragmentation, and isolation and reconnection of populations (Zinner et al., 2011) – dynamics that affected baboon genetic structure and speciation (Rogers et al., 2019).
In summary, baboons can serve as a valuable model for evolutionary divergence and hybridization, triggered by climatic changes and the expansion and fragmentation of populations in the African savanna. Such analyses are also highly relevant for a better understanding of early hominins.
Variation in social organization and behavior
The six baboon species vary substantially in their social characteristics, including social organization, social style and mating patterns. Group size varies within and among species. In chacma, olive and yellow baboons, group size ranges from about a dozen up to roughly one hundred animals (Markham et al., 2015; Swedell, 2011), while hamadryas and Guinea baboons temporarily aggregate into groups of several hundreds of individuals (Patzelt et al., 2011; Swedell, 2013). Sex ratios in baboons vary too; some groups are fairly balanced, while adult females in other groups can outnumber adult males by about 10 to 1 (Swedell, 2011).
Chacma, olive, Kinda and yellow baboons – recently dubbed "COKY" baboons (Jolly, 2019) – live in multi-male-multi-female groups, in which related females constitute the stable core, while males leave the group they were born into and join another. Clear rank hierarchies among males and females can be discerned based on aggressive interactions, including threats, chases and physical aggression, as well as signals of submission. In females, related individuals (known as matrilines) typically occupy adjacent ranks. For female chacma, olive and yellow baboons, female kin constitute the most important social partners (Silk et al., 2017; Silk et al., 2010; Silk, 2003). In Kinda baboons, however, males are the most significant grooming partners for females (Petersdorf et al., 2019).
Females of all COKY species interact and mate with several males in the group. High-ranking males generally experience higher mating success than lower-ranking ones, though this rank-related mating skew is more pronounced in chacma baboons than in olive or yellow baboons (Bulger, 1993; Henzi and Barrett, 1999; Städele et al., 2019). During female receptive periods, males aggressively guard their female mating partner, resulting in sexual "consortships" (Noë and Sluijter, 1990; Smuts, 1985). Consorts may last from several hours up to several days. Consort success (and thus mating success) is often related to male dominance status (Gesquiere et al., 2011). In yellow and olive baboons, however, male coalitions may be able to take the female away from a dominant male (Noë and Sluijter, 1990; Smuts, 1985).
Male competition and aggressiveness vary considerably between species. Infanticide is frequent in some populations of chacma baboons (Palombit et al., 2001), but rare in olive, yellow and hamadryas baboons (Lemasson et al., 2008; Swedell, 2011). Lactating females often form close ties to specific males, which are often the sires of their infants (Huchard et al., 2010; Moscovice et al., 2010; Nguyen et al., 2009; Städele et al., 2019). These relationships appear to be an adaptation against infanticide by recent immigrant males (Palombit et al., 1997) and harassment by other group members (Alberts et al., 2003) as well as a form of paternal investment (Buchan et al., 2003; Huchard et al., 2013). Male alliances are absent in chacma baboons, while common in yellow and olive baboons (Noë and Sluijter, 1995). These differences in male competitive regimes are reflected in their dispersal behavior: male chacma baboons in the Okavango delta, for instance, do not emigrate from their natal group until after they are fully grown (Beehner et al., 2009), while male olive and yellow baboons often emigrate during adolescence (Alberts and Altmann, 1995; Packer et al., 1995).
Over the past decade, studies of Kinda baboons have broadened our knowledge of morphological and behavioral variation within the genus. Kinda baboons are smaller in body size, have reduced sexual dimorphism in body and canine size, and have larger relative testis volume, compared to other baboon species (Jolly, 2019). Kinda females exhibit small sexual swellings (Figure 2) and give inconspicuous calls (Petersdorf et al., 2019). Chacma females, in contrast, give loud copulation calls, which function to incite male-male competition (O'Connell and Cowlishaw, 1994).
Hamadryas baboons – in contrast to the COKY baboon species described above – live in a multi-level society with reproductive units, called "one male units" comprising one sexually active leader male, a variable number of females, and sometimes a follower male (Kummer, 1968). Associations between several one-male units constitute a clan (Abegglen, 1984); several clans and unaffiliated bachelor males form a band, the main ecological unit, and multiple bands coalesce at resources, especially sleeping sites, to form troops (Schreier and Swedell, 2009). Recent behavioral and genetic studies of hamadryas baboons show that leader and follower males tend to be maternally related, in line with the fact that they disperse only rarely. Females within a unit are also more likely to be related than expected by chance (Städele et al., 2016).
Guinea baboons also live in a multi-level society. Several units comprising a primary male, 1–6 females, young, and occasional secondary males make up parties, and 2 to 3 parties constitute a gang within a larger community (Fischer et al., 2017). Male Guinea baboons maintain strong bonds and a high degree of spatial tolerance (Fischer et al., 2017). Some, but not all males with strong bonds are highly related, suggesting that the existence of kin in the group promotes male tolerance (Patzelt et al., 2014). In striking contrast to most other baboon species, aggression between males is so rare that it is not possible to discern a dominance hierarchy with certainty (Dal Pesco and Fischer, 2018). Males engage in extended ritualized greetings that apparently function to reinforce delineations between parties and to test bonds between males (Dal Pesco and Fischer, 2018). Females freely transfer between units, parties and gangs. Female tenure with a given male may vary between weeks and years (Goffe et al., 2016). Both Guinea and hamadryas baboons exhibit female-biased dispersal (Kopp et al., 2015; Städele et al., 2015).
Note that many of the most significant differences in social behavior between species have been observed across different populations in multiple African sites, as well as in captivity. Thus, there is good evidence that the variation we describe here reflects true species differences and not just variation between populations. Yet, characterizing the variation within species in greater detail would be extremely valuable.
Despite the variation in social organization and aggressiveness between the different baboon species, there is very little variation in the vocal repertoires and call types within the genus (Hammerschmidt and Fischer, 2019). This suggests that the structure of vocal patterns is highly conserved. Because species vary in their aggressiveness and their propensity to affiliate, they also differ in the frequency with which they use signals that either relate to fighting ability or "benign intent", respectively (Faraut et al., 2019; Fischer et al., 2017).
Variation in social cognition
Variation in social organization and in the nature and extent of competition over resources between baboon species is thought to result in differential selective pressure on social cognition (Amici et al., 2008; Aureli et al., 2008). To date, most of the work on baboon social knowledge has been done on chacma baboons that exhibit steep dominance hierarchies (known as despotism). A suite of studies by the American primatologists Dorothy Cheney and Robert Seyfarth and colleagues revealed that chacma baboons have sophisticated social knowledge (reviewed in Cheney and Seyfarth, 2008). For instance, the animals represent the nested hierarchical rank relationships of their group members (Bergman, 2003), track the consortship status of pairs in their group (Crockford et al., 2007), and selective deploy aid to unrelated individuals that were former grooming partners (Cheney et al., 2010).
Field playback experiments revealed that baboon species respond differently to social information. While the territorial chacma baboons respond strongly to apparent intruders (Kitchen et al., 2013), the spatially tolerant Guinea baboons paid more attention to vocalizations from co-resident group members compared to neighbors or strangers (Maciej et al., 2013). Similarly, chacma baboons respond strongly to simulated rank reversals (Bergman, 2003) or break-ups of existing consortships (Crockford et al., 2007), while Guinea baboon males were more interested in social information consistent with current social association patterns (Faraut and Fischer, 2019). The somewhat surprising responses of the Guinea baboons may be a result of the high gregariousness of the species, where deviant interaction patterns may initially be classified as "social noise" (Faraut and Fischer, 2019). In summary, these findings suggest that the content of what is represented, namely the associations between different individuals or their group memberships, appears to be relatively similar across the two species, while the value of different types of social information may vary substantially in relation to the type of society.
Sociality, health, aging and fitness
Over the past decade, baboon research has provided ground-breaking insights into the relationships between social status, social relationships, health and fitness measures such as offspring survival and longevity. Data from two long-term studies of baboon behavior and life history suggest that sociality enhances the fitness of females. For example, infants born to yellow baboon females who are more socially integrated have higher survival than infants of less social mothers (Archie et al., 2014; Silk, 2003); similar patterns are also seen in chacma baboons (Silk et al., 2010). As in many other primates, higher-ranking male baboons sire more offspring than other males (Altmann et al., 1996). Higher-ranking females have shorter periods before they resume menstrual cycling following birth (Gesquiere et al., 2018; Johnson, 2003; Packer et al., 1995; Smuts and Nicolson, 1989; Wasser et al., 2004), which may be linked to quicker restoration of positive energy balance (Gesquiere et al., 2018). Consistent with this, feeding on crops in olive baboons (Higham et al., 2009), or discarded human food for yellow baboons (Altmann et al., 1977), also leads to a quicker return to menstrual cycling and increases reproductive output.
A number of studies have investigated the proximate mediators of the relationship between behavior and fitness. In particular, many researchers have taken advantage of non-invasive ways to measure glucocorticoid hormones, a class of hormones known to mediate the energetic demands that accompany social and ecological challenges. Concentrations of glucocorticoid hormones increase during specific challenges that are known to threaten an individual's fitness. For example, lactating chacma females that were at risk for infanticide because a new male immigrated into the group exhibited elevated glucocorticoid hormones compared to female counterparts that were not at risk (Beehner et al., 2005). Additionally, loss of a close female relative increases glucocorticoid concentrations, and this increase may be responsible for initiating a compensatory broadening and strengthening of female grooming networks (Engh et al., 2006).
Several studies have investigated the relationship between glucocorticoid concentrations, rank and social stability in male baboons. In a long term-study of yellow baboons, high-ranking males had lower glucocorticoid concentrations, regardless of hierarchy stability, while alpha males may experience higher concentrations than expected for their rank (Gesquiere et al., 2011). Nonetheless, high-ranking yellow baboon males get sick less often and heal from wounds faster, suggesting that these high-ranking males are in better health and do not suffer trade-offs from these extra demands (Archie et al., 2012). Higher-ranking chacma baboon males also had higher glucocorticoid concentrations (Bergman et al., 2005; Kalbitzer et al., 2015).
More recently, baboons were also established as a promising model for studying the impact of sexually transmitted diseases on mating behavior. Female olive baboons in a population infected with the bacterium Treponema pallidum, a substrain of which causes syphilis in humans, copulated less with males showing clinical signs of infection (Paciência et al., 2019). These findings highlight how pathogens may impose important selective pressures in mate choice and ultimately social evolution.
Functional genomics
The addition of data on Kinda and Guinea baboons increases the value of the baboon as a model, as we begin to have data available for all baboon species. While one aim of future analyses will be to understand the sources of variation between species, documenting similarities is equally valuable. Technological developments in genomic sequencing (Robinson et al., 2019; Rogers et al., 2019), including from non-invasively collected samples such as feces (Perry et al., 2010; Snyder-Mackler et al., 2016), have brought genomics to the forefront of baboon behavioral studies (Tung et al., 2010). Given the close phylogenetic relatedness of the six baboon species, variation in key aspects of social behavior, and the presence of hybrids displaying intermediate phenotypes within hybrid zones, investigation of causal pathways from genotype to phenotype seems particularly promising within the baboon model (Bergey et al., 2016; Jolly et al., 2008).
Formerly, research into primate behavioral genetics focused on identifying a few small specific functional polymorphisms in sequence or length, and on linking these to phenotypic variation (e.g., Kalbitzer et al., 2016). However, such studies are likely to overestimate the effect of one single aspect of genetic variation. With genotyping of single nucleotide polymorphisms (SNPs), and whole-genome sequencing, primate field studies are beginning to explore the wider genomic architecture that underlies variation in social behavior (Rogers, 2018). As well as whole genome sequence data, researchers now have access to annotations for protein coding genes and transcriptomes (Robinson et al., 2019; Rogers et al., 2019; Vilgalys et al., 2019). We therefore expect an exponential increase in the number and diversity of available genomes, which will facilitate the investigation of the basis of baboon adaptations and adaptive flexibility. In conjunction with research on other model organisms, such as deer mice (Bedford and Hoekstra, 2015), such studies provide fundamental insights into the foundation of natural variation and adaptation in socially living mammals.
Baboons in the Anthropocene
Baboons allow us to study the effects of accelerating anthropogenic fragmentation, loss of natural habitats and climate change in a highly adaptable primate system. For example, baboons may rapidly change how long they allocate time and energy to different behaviors or where they range, in response to human-related activities and habitat changes (Fehlmann et al., 2017; Ferreira da Silva et al., 2018). Studies of individual baboon behavior can use sophisticated GPS tracking and non-invasive genetic tools to make broad-scale inferences about movements and processes at the population level (Kopp et al., 2014; Strandburg-Peshkin et al., 2015). These inferences can then be applied to questions of how other large populations of mammals will respond to changes in their environment.
Baboons are not considered a global priority in conservation, with the exception of Guinea baboons which are categorized as Near Threatened by the IUCN (Oates et al., 2008). However, populations geographically overlapping with human communities often damage crops and infrastructure and are persecuted as pests. In some locations, people consume substantial number of baboons and sell their meat in bushmeat markets (e.g., Minhós et al., 2013). Humans and baboons often compete for space and hunting of specific individuals or even entire groups is increasingly frequent, leading to fragmented populations and local extinctions (Ferreira da Silva et al., 2018). Non-monitored populations living outside protected areas may be at a higher risk of silently disappearing. The challenge is to assess the risk of different populations and develop appropriate conservation plans.
The long-term nature of many baboon field studies has provided great insight into how populations may rise and fall rapidly in response to changes in the environment. The Amboseli Baboon Project, for example, has been running continuously for 50 years, and has documented numerous periods of relative drought or rainfall abundance (Alberts et al., 2005; Alberts and Altmann, 2011). This variation in precipitation has been linked to variation in fecundity and survival (Beehner et al., 2006; Lea et al., 2015) and to subsequent changes in population structure (Altmann et al., 1985). Periods of environmental change, and consequent boom and bust cycles in populations, are driven by both natural phenomena, such as natural aging of woodland, and anthropogenic influences, such as overgrazing by pastoralists (Alberts and Altmann, 2011). Many long-term baboon field sites also carefully collect detailed data on temperature and rainfall, as well as food availability and diseases. They also monitor the habitats in addition to the baboon populations. The breadth and scope of such data ensure that the baboon represents an outstanding model of both individual-level and population-level responses to environmental change.
Conclusion
Baboons constitute a fascinating and informative analog model for hominin evolution in savanna habitats, with their ongoing patterns of range expansions and contractions, and regular occurrences of hybridization where two species meet. Given the availability of long-term data and the variation in the types of societies baboons live in, they constitute an excellent test case to study the link between sociality, health, longevity and reproductive success, as well as the emergence and spread of diseases. Such studies are extremely important to put biomedical data from captive baboon studies into natural context. For future research, we propose an approach that integrates field observations and carefully designed field experiments with cutting-edge measures of genomic variation, gene expression, non-invasive endocrinology and immunology. The fact that baboons have been studied in a wide range of habitats at sites across Africa for several decades also make them an informative example to investigate how populations of large mammals respond to environmental diversity and change (see Box 2 for suggested future research questions).
Box 2.
Outstanding questions about the natural history of baboons.
How have changes in population density and environmental conditions (e.g., opening and closing of forest and other barriers) affected dispersal and mating patterns, and ultimately given rise to different social systems?
What is the genetic architecture of baboon social behavior (including social style, patterns of dispersal, and degree of reproductive skew according to social status)? How does that architecture promote or restrict evolutionary flexibility in social systems?
Does the link between sociality and reproductive success vary among species or even local populations?
Do the different species vary in the way they represent the social relationships around them and how they attend to social information?
How responsive are baboons to changes in temperature patterns due to global warming, as well as to associated changes in aridity or habitat type?
Data availability
This is a review article; there are no data sets associated with this article.
References
-
Hybridization in human evolution: insights from other organismsEvolutionary Anthropology: Issues, News, and Reviews 28:189–209.https://doi.org/10.1002/evan.21787
-
BookSeasonality and long-term change in a savanna environmentIn: Brockmann D. K, van Schaik C. P, editors. Seasonality in Primates. Cambridge University Press. pp. 157–195.https://doi.org/10.1017/CBO9780511542343.007
-
Balancing costs and opportunities: dispersal in male baboonsThe American Naturalist 145:279–306.https://doi.org/10.1086/285740
-
BookThe Amboseli Baboon Research Project: 40 Years of Continuity and ChangeIn: Kappeler P, Watts D, editors. Long-Term Field Studies of Primates. Springer Science & Business Media. pp. 261–287.https://doi.org/10.1007/978-3-642-22514-7_12
-
Demography of amboseli baboons, 1963-1983American Journal of Primatology 8:113–125.https://doi.org/10.1002/ajp.1350080204
-
BookSpecies accounts of CercopithecidaeIn: Mittermeier R, Rylands A, Wilson D, editors. Handbook of the Mammals of the World, 3. Barcelona, Primates: Lynx. pp. 628–753.
-
Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboonsProceedings of the Royal Society B: Biological Sciences 281:20141261.https://doi.org/10.1098/rspb.2014.1261
-
The effect of new alpha males on female stress in free-ranging baboonsAnimal Behaviour 69:1211–1221.https://doi.org/10.1016/j.anbehav.2004.08.014
-
The ecology of conception and pregnancy failure in wild baboonsBehavioral Ecology 17:741–750.https://doi.org/10.1093/beheco/arl006
-
Testosterone related to age and life-history stages in male baboons and geladasHormones and Behavior 56:472–480.https://doi.org/10.1016/j.yhbeh.2009.08.005
-
BookAdult Male Savanna Baboon Mating Activity and Interactions with InfantsSpringer.
-
BookBaboon Metaphysics: The Evolution of a Social MindUniversity of Chicago Press.https://doi.org/10.7208/chicago/9780226102429.001.0001
-
Species concepts and speciation analysisCurrent Ornithology pp. 159–187.https://doi.org/10.1007/978-1-4615-6781-3_6
-
Baboons eavesdrop to deduce mating opportunitiesAnimal Behaviour 73:885–890.https://doi.org/10.1016/j.anbehav.2006.10.016
-
Greetings in male guinea baboons and the function of rituals in complex social groupsJournal of Human Evolution 125:87–98.https://doi.org/10.1016/j.jhevol.2018.10.007
-
Notebook M: [Metaphysics on morals and speculations on expression (1838)] Darwin OnlineNotebook M: [Metaphysics on morals and speculations on expression (1838)] Darwin Online, http://darwin-online.org.uk.
-
Comparing mitogenomic timetrees for two african savannah primate genera (Chlorocebus and Papio)Zoological Journal of the Linnean Society 67:471–483.https://doi.org/10.1093/zoolinnean/zlx001
-
Biogeographic variation in the baboon: dissecting the clineJournal of Anatomy 223:337–352.https://doi.org/10.1111/joa.12085
-
Charting the neglected west: the social system of Guinea baboonsAmerican Journal of Physical Anthropology 162:15–31.https://doi.org/10.1002/ajpa.23144
-
Primate Adaptation and Evolution119–150, Old World monkeys, Primate Adaptation and Evolution, 3rd Edition, London, Academic Press, 10.1016/C2009-0-01979-5.
-
Ecological niche modeling of the genus PapioAmerican Journal of Physical Anthropology 166:812–823.https://doi.org/10.1002/ajpa.23470
-
Interbirth intervals in wild baboons: environmental predictors and hormonal correlatesAmerican Journal of Physical Anthropology 166:107–126.https://doi.org/10.1002/ajpa.23407
-
Sex and friendship in a multilevel society: behavioural patterns and associations between female and male Guinea baboonsBehavioral Ecology and Sociobiology 70:323–336.https://doi.org/10.1007/s00265-015-2050-6
-
Baboon vocal repertoires and the evolution of primate vocal diversityJournal of Human Evolution 126:1–13.https://doi.org/10.1016/j.jhevol.2018.10.010
-
The historical socioecology of savanna baboons (Papio hamadryas)Journal of Zoology 265:215–226.https://doi.org/10.1017/S0952836904006399
-
Baboon sexual swellings: information content of size and colorHormones and Behavior 53:452–462.https://doi.org/10.1016/j.yhbeh.2007.11.019
-
Living on the edge: life-history of olive baboons at Gashaka-Gumti National Park, NigeriaAmerican Journal of Primatology 71:293–304.https://doi.org/10.1002/ajp.20651
-
More than friends? Behavioural and genetic aspects of heterosexual associations in wild chacma baboonsBehavioral Ecology and Sociobiology 64:769–781.https://doi.org/10.1007/s00265-009-0894-3
-
Paternal effects on access to resources in a promiscuous primate societyBehavioral Ecology 24:229–236.https://doi.org/10.1093/beheco/ars158
-
Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboonsAmerican Journal of Physical Anthropology 120:83–98.https://doi.org/10.1002/ajpa.10139
-
BookSpecies, subspecies, and baboon systematicsIn: Kimbel WH MLB, editors. Species, Species Concepts and Primate Evolution. Boston, MA: Springer. pp. 67–107.https://doi.org/10.1007/978-1-4899-3745-2_4
-
Cerebrospinal fluid monoaminergic metabolites in wild Papio anubis and P. hamadryas are concordant with Taxon-specific behavioral ontogenyInternational Journal of Primatology 29:1549–1566.https://doi.org/10.1007/s10764-008-9318-x
-
Philopatry at the frontier: a demographically-driven scenario for the evolution of multi-level societies in baboons (Papio)Journal of Human Evolution In press.
-
A framework for studying social complexityBehavioral Ecology and Sociobiology 73:13.https://doi.org/10.1007/s00265-018-2601-8
-
Male baboon responses to experimental manipulations of loud “wahoo calls”: testing an honest signal of fighting abilityBehavioral Ecology and Sociobiology 67:1825–1835.https://doi.org/10.1007/s00265-013-1592-8
-
The influence of social systems on patterns of mitochondrial DNA variation in baboonsInternational Journal of Primatology 35:210–225.https://doi.org/10.1007/s10764-013-9725-5
-
Population genetic insights into the social organization of Guinea baboons (Papio papio): Evidence for female-biased dispersalAmerican Journal of Primatology 77:878–889.https://doi.org/10.1002/ajp.22415
-
Developmental constraints in a wild primateThe American Naturalist 185:809–821.https://doi.org/10.1086/681016
-
Friendships between males and lactating females in a free-ranging group of olive baboons (Papio hamadryas anubis): evidence from playback experimentsBehavioral Ecology and Sociobiology 62:1027–1035.https://doi.org/10.1007/s00265-007-0530-z
-
Social monitoring in a multilevel society: a playback study with male Guinea baboonsBehavioral Ecology and Sociobiology 67:61–68.https://doi.org/10.1007/s00265-012-1425-1
-
Mitochondrial phylogeny and systematics of baboons (Papio)American Journal of Physical Anthropology 124:17–27.https://doi.org/10.1002/ajpa.10340
-
“Friendships” between new mothers and adult males: adaptive benefits and determinants in wild baboons (Papio cynocephalus)Behavioral Ecology and Sociobiology 63:1331–1344.https://doi.org/10.1007/s00265-009-0786-6
-
Reproductive tactics of male savanna baboonsBehaviour 113:117–169.https://doi.org/10.1163/156853990X00455
-
Which adult male savanna baboons form coalitions?International Journal of Primatology 16:77–105.https://doi.org/10.1007/BF02700154
-
ReportPapio Papio, Guinea BaboonIUCN Red List of Threatened Species.https://doi.org/10.2305/IUCN.UK.2008.RLTS.T16018A5354225.en
-
Mating avoidance in female olive baboons (Papio anubis) infected by Treponema pallidumScience Advances In press.
-
Group composition of guinea baboons (Papio papio) at a water place suggests a fluid social organizationInternational Journal of Primatology 32:652–668.https://doi.org/10.1007/s10764-011-9493-z
-
Genomic-scale capture and sequencing of endogenous DNA from fecesMolecular Ecology 19:5332–5344.https://doi.org/10.1111/j.1365-294X.2010.04888.x
-
Sexual selection in the Kinda baboonJournal of Human Evolution 135:102635.https://doi.org/10.1016/j.jhevol.2019.06.006
-
The behavioral genetics of nonhuman primates: status and prospectsAmerican Journal of Physical Anthropology 165:23–36.https://doi.org/10.1002/ajpa.23384
-
Did our species evolve in subdivided populations across Africa, and why does it matter?Trends in Ecology & Evolution 33:582–594.https://doi.org/10.1016/j.tree.2018.05.005
-
The fourth level of social structure in a multi-level society: ecological and social functions of clans in hamadryas baboonsAmerican Journal of Primatology 71:948–955.https://doi.org/10.1002/ajp.20736
-
Reproduction in wild female olive baboonsAmerican Journal of Primatology 19:229–246.https://doi.org/10.1002/ajp.1350190405
-
Fine-scale genetic assessment of sex-specific dispersal patterns in a multilevel primate societyJournal of Human Evolution 78:103–113.https://doi.org/10.1016/j.jhevol.2014.10.019
-
The ties that bind: maternal kin bias in a multilevel primate society despite natal dispersal by both sexesAmerican Journal of Primatology 78:731–744.https://doi.org/10.1002/ajp.22537
-
Male–female relationships in olive baboons (Papio anubis): Parenting or mating effort?Journal of Human Evolution 127:81–92.https://doi.org/10.1016/j.jhevol.2018.09.003
-
The origin and evolution of Homo sapiensPhilosophical Transactions of the Royal Society B: Biological Sciences 371:20150237.https://doi.org/10.1098/rstb.2015.0237
-
BookAfrican Papionins: Diversity of Social Organization and Ecological FlexibilityIn: Campbell C, Fuentes A, MacKinnon K, Bearder S, Stumpf R, editors. Primates in Perspective. New York: Oxford University Press. pp. 241–277.
-
BookHamadryas Baboon (Papio hamadryas)In: Butynski T, Kingdon J, editors. The Mammals of Africa. Primates. London: Bloomsbury Publishing. pp. 221–224.https://doi.org/10.5040/9781472926920.0038
-
BookIntroductionIn: Vandeberg J. L, Williams-Blangero S, Tardif S. D, editors. The Baboon in Biomedical Research. Springer Science & Business Media. pp. i–xxiii.https://doi.org/10.1007/978-0-387-75991-3
-
Evolution of DNA methylation in Papio baboonsMolecular Biology and Evolution 36:527–540.https://doi.org/10.1093/molbev/msy227
-
The social life of baboonsScientific American 204:62–72.https://doi.org/10.1038/scientificamerican0661-62
-
Papio baboon species indicative alu elementsGenome Biology and Evolution 9:1788–1796.https://doi.org/10.1093/gbe/evx130
-
Population trend alters the effects of maternal dominance rank on lifetime reproductive success in yellow baboons (Papio cynocephalus)Behavioral Ecology and Sociobiology 56:338–345.https://doi.org/10.1007/s00265-004-0797-2
-
The strange blood: natural hybridization in primatesEvolutionary Anthropology: Issues, News, and Reviews 20:96–103.https://doi.org/10.1002/evan.20301
-
Baboon phylogeny as inferred from complete mitochondrial genomesAmerican Journal of Physical Anthropology 150:133–140.https://doi.org/10.1002/ajpa.22185
Decision letter
-
Stuart RF KingReviewing Editor; eLife, United Kingdom
-
Peter RodgersSenior Editor; eLife, United Kingdom
-
Jason KamilarReviewer; University of Massachusetts
In the interests of transparency, eLife includes the editorial decision letter and accompanying author responses. A lightly edited version of the letter sent to the authors after peer review is shown, indicating the most substantive concerns; minor comments are not usually included.
Thank you for submitting your article "The Natural History of Model Organisms: Insights into the evolution of social systems and species from baboon studies" for consideration by eLife. Your article has been reviewed by two peer reviewers, and the evaluation has been overseen by two Features Editors at eLife (Stuart King and Peter Rodgers). The following individual involved in review of your submission has agreed to reveal their identity: Jason Kamilar.
The reviewers have discussed the reviews with one another and the Associate Features Editor has drafted this decision to help you prepare a revised submission.
Summary:
This essay is being considered as part of a series of articles on "The Natural History of Model Organisms": https://elifesciences.org/collections/8de90445/the-natural-history-of-model-organisms. Each article should explain how our knowledge of the natural history of a model organism has informed recent advances in biology, and how understanding its natural history can influence/advance future studies.
Overall, it was a pleasure to read this clear summary of current knowledge about the natural history of baboons. The manuscript is a wide-ranging, authoritative, and interesting read. The authors have done an excellent job of concisely presenting an overview of baboon biology and its relevance for being considered a model organism in ecology and evolution. The reviewers expect this paper to be highly cited and look forward to seeing it published after a few revisions are made. The hope is that the revisions will provide some more context for readers who are less familiar with baboon research, and help condense the article slightly to make it more focused, which could help to increase its ultimate impact.
Essential revisions:
1) This manuscript could be considered to be an interesting outlier for the "Natural History of Model Organisms" series, which has thus far focused on species that have largely been studied in the lab or under domestication (or are close relatives of a lab model organism). In contrast, this paper focuses exclusively on research in the wild, on a set of species that are unusually well studied in their natural context. For the benefit of non-baboon researchers, it would be helpful if the Introduction acknowledged that baboons may seem to be an unusual choice for a model organism, but then explain what it is that makes them such a good model for certain questions (i.e. better than other species that are routinely studied in the lab). A mention of the rather extensive work on baboons in captivity, where they are among the most intensively studied non-human primates in biomedical science, would also be appreciated here. Work in the wild extends the scope of what the community can learn from baboons beyond captive research, and it would be interesting if the authors shared their thoughts as to whether it also helps to motivate the research done with captive baboons, or to interpret those results?
2) The sections on systematics and phylogeography could be combined with some text currently in the Introduction to give a more general introduction to the "Natural history of baboons". Restructuring this text may also present opportunities to cut some text, because, for example, geographic ranges are currently discussed in three separate places. Breaking the new section into short, clearly defined sub-sections (i.e. Systematics, Morphology, Habitats, Diet etc.) may also help the reader to navigate this background information.
3) The reviewers felt that the discussion of allele surfing (subsection "Population genetics and range expansion", third paragraph) could probably be removed. While this could be interesting and relevant to baboon evolution, none of the citations in this paragraph are actually about baboons. As such, it was unclear how baboons have contributed thus far to this question or even whether they would be better models than, say, humans or classical lab model organisms. Removing the text would help to keep the article focused.
4) Given that they touch upon related topics, the section on "Baboons in the Anthropocene" could be moved to follow the section on "Population and range expansion". This change could help this later section to feel more integrated in the article, and would mean that the article's structure more closely follows the order of the research areas that are very nicely articulated in the Conclusion.
5) The sections on "Variation in social organization and behavior" and "Previously understudied species reveal hidden diversity" should also be integrated into one and condensed, since the hidden diversity discussed is also about social organization and behavior. The Social Cognition section could also likely be condensed by a few sentences.
6) The section on "Functional genomics" seems to be more tool or method-driven that the previous sections and is almost all future-oriented. It would be good if it could give more concrete examples of how, when applied to baboons, these methods could advance broad biological knowledge. If the section on "Baboons in the Anthropocene" is moved earlier in the text, this section would be the penultimate in the article. As such, one approach would be to use this section to explain how/why functional genomic approaches could be leveraged to advance the questions laid out in the previous sections, especially if the authors can include examples from the recent literature. Alternatively, the authors should explicitly note that, compared to the other areas highlighted, there's much more to be done in this area of research.
7) Throughout the article there are a few places where the paper may assume too much about domain/disciplinary knowledge on the part of readers. The Glossary can help with this, if the definitions provided are written to be as accessible as possibly. Most importantly, the reviewers suggest explaining what "rank" is (it first appears in the subsection "Variation in social organization and behavior"), whether it's sex-specific, and why it's important in baboons. Other cases include the use of the term "despotic" (likely to be familiar to primatologists, but not to many others), mention of copulation calls (why is it meaningful that they might differ in chacma baboons?), and citing "the African multi-regionalism" hypothesis (a reader may know why it's important that baboons are a model for this hypothesis if they don't know what it is).
https://doi.org/10.7554/eLife.50989.008Author response
Essential revisions:
1) This manuscript could be considered to be an interesting outlier for the "Natural History of Model Organisms" series, which has thus far focused on species that have largely been studied in the lab or under domestication (or are close relatives of a lab model organism). In contrast, this paper focuses exclusively on research in the wild, on a set of species that are unusually well studied in their natural context. For the benefit of non-baboon researchers, it would be helpful if the Introduction acknowledged that baboons may seem to be an unusual choice for a model organism, but then explain what it is that makes them such a good model for certain questions (i.e. better than other species that are routinely studied in the lab). A mention of the rather extensive work on baboons in captivity, where they are among the most intensively studied non-human primates in biomedical science, would also be appreciated here. Work in the wild extends the scope of what the community can learn from baboons beyond captive research, and it would be interesting if the authors shared their thoughts as to whether it also helps to motivate the research done with captive baboons, or to interpret those results?
We have followed this suggestion, and tried to be more explicit why this paper might be relevant for scientists who use baboons in biomedical research. In the Introduction, we now write: "In the 1950s, baboons became the subject of more systematic scientific enquiry, both in the field, and in captivity. […] An understanding of the evolution and natural life-history also allows assessment of the validity of results derived from captive populations." We also return to this theme briefly in the Conclusion.
2) The sections on systematics and phylogeography could be combined with some text currently in the Introduction to give a more general introduction to the "Natural history of baboons". Restructuring this text may also present opportunities to cut some text, because, for example, geographic ranges are currently discussed in three separate places. Breaking the new section into short, clearly defined sub-sections (i.e. Systematics, Morphology, Habitats, Diet etc.) may also help the reader to navigate this background information.
We thought a lot about this suggestion, and have partly implemented it. Our reasoning was that we first introduce the taxon and then talk about the different species. We feel that we need to introduce this variation first before we go on to present species differences in Morphology etc. There are only two sentences on Diet, so we did not establish a new subsection. We did, however, cut text in a number of places to avoid the highlighted redundancy.
3) The reviewers felt that the discussion of allele surfing (subsection "Population genetics and range expansion", third paragraph) could probably be removed. While this could be interesting and relevant to baboon evolution, none of the citations in this paragraph are actually about baboons. As such, it was unclear how baboons have contributed thus far to this question or even whether they would be better models than, say, humans or classical lab model organisms. Removing the text would help to keep the article focused.
We agree, and have removed this section.
4) Given that they touch upon related topics, the section on "Baboons in the Anthropocene" could be moved to follow the section on "Population and range expansion". This change could help this later section to feel more integrated in the article, and would mean that the article's structure more closely follows the order of the research areas that are very nicely articulated in the Conclusion.
We understand this suggestion, but we believe that the "Baboons in the Anthropocene" section belongs as the final section, as it is prospective, and about the future of baboons. We have, however, edited the Conclusion such that the order there accurately reflects the order of the content.
5) The sections on "Variation in social organization and behavior" and "Previously understudied species reveal hidden diversity" should also be integrated into one and condensed, since the hidden diversity discussed is also about social organization and behavior. The Social Cognition section could also likely be condensed by a few sentences.
We have followed this suggestion and have both integrated and shortened these two sections.
6) The section on "Functional genomics" seems to be more tool or method-driven that the previous sections and is almost all future-oriented. It would be good if it could give more concrete examples of how, when applied to baboons, these methods could advance broad biological knowledge. If the section on "Baboons in the Anthropocene" is moved earlier in the text, this section would be the penultimate in the article. As such, one approach would be to use this section to explain how/why functional genomic approaches could be leveraged to advance the questions laid out in the previous sections, especially if the authors can include examples from the recent literature. Alternatively, the authors should explicitly note that, compared to the other areas highlighted, there's much more to be done in this area of research.
Thank you for this suggestion. We now explicitly state that this is a research area on the cusp of blooming; we now state: "Researchers now have access to whole genome sequence data and other genomic information such as annotations for protein coding genes and transcriptome information (Robinson et al., 2019; Rogers et al., 2019; Vilgalys et al., 2019). We therefore expect an exponential increase in the number and diversity of available genomes, which will facilitate the genomic investigation of the molecular and cellular basis of baboon adaptations and adaptive flexibility."
7) Throughout the article there are a few places where the paper may assume too much about domain/disciplinary knowledge on the part of readers. The Glossary can help with this, if the definitions provided are written to be as accessible as possibly. Most importantly, the reviewers suggest explaining what "rank" is (it first appears in the subsection "Variation in social organization and behavior"), whether it's sex-specific, and why it's important in baboons. Other cases include the use of the term "despotic" (likely to be familiar to primatologists, but not to many others), mention of copulation calls (why is it meaningful that they might differ in chacma baboons?), and citing "the African multi-regionalism" hypothesis (a reader may know why it's important that baboons are a model for this hypothesis if they don't know what it is).
We are sorry that we sometimes used too much jargon. We now explain the concept of rank and why it is important. We also explain despotism, and have used more general terms whenever we felt we could (e.g., perineal --> anogenital; shorter periods of post-partum amenorrhea --> shorter periods before they resume menstrual cycling following birth). We cut the African multi-regionalism hypothesis and simply refer to different hypotheses regarding human origins. We appreciate any further suggestions where we might remove further jargon that we may have overlooked.
https://doi.org/10.7554/eLife.50989.009Article and author information
Author details
Funding
Deutsche Forschungsgemeinschaft (FI 707/21-1)
- Julia Fischer
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
This paper is dedicated to the memory of Dorothy L Cheney who loved baboons. We thank the funders of the Frontiers in Baboon Research Symposium, namely the Deutsche Forschungsgemeinschaft (FI 707/21–1), the Leibniz ScienceCampus Primate Cognition and the University of Göttingen. We thank PJ Perry and the editors and reviewers, for valuable comments and suggestions.
Publication history
- Received: August 12, 2019
- Accepted: October 16, 2019
- Version of Record published: November 12, 2019 (version 1)
Copyright
© 2019, Fischer et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 9,869
- Page views
-
- 615
- Downloads
-
- 36
- Citations
Article citation count generated by polling the highest count across the following sources: Crossref, Scopus, PubMed Central.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Evolutionary Biology
Understanding how plants adapt to changing environments and the potential contribution of transposable elements (TEs) to this process is a key question in evolutionary genomics. While TEs have recently been put forward as active players in the context of adaptation, few studies have thoroughly investigated their precise role in plant evolution. Here, we used the wild Mediterranean grass Brachypodium distachyon as a model species to identify and quantify the forces acting on TEs during the adaptation of this species to various conditions, across its entire geographic range. Using sequencing data from more than 320 natural B. distachyon accessions and a suite of population genomics approaches, we reveal that putatively adaptive TE polymorphisms are rare in wild B. distachyon populations. After accounting for changes in past TE activity, we show that only a small proportion of TE polymorphisms evolved neutrally (<10%), while the vast majority of them are under moderate purifying selection regardless of their distance to genes. TE polymorphisms should not be ignored when conducting evolutionary studies, as they can be linked to adaptation. However, our study clearly shows that while they have a large potential to cause phenotypic variation in B. distachyon, they are not favored during evolution and adaptation over other types of mutations (such as point mutations) in this species.
-
- Evolutionary Biology
Biologically-controlled mineralization producing organic-inorganic composites (hard skeletons) by metazoan biomineralizers has been an evolutionary innovation since the earliest Cambrian. Among them, linguliform brachiopods are one of the key invertebrates that secrete calcium phosphate minerals to build their shells. One of the most distinct shell structures is the organo-phosphatic cylindrical column exclusive to phosphatic-shelled brachiopods, including both crown and stem groups. However, the complexity, diversity, and biomineralization processes of these microscopic columns are far from clear in brachiopod ancestors. Here, exquisitely well-preserved columnar shell ultrastructures are reported for the first time in the earliest eoobolids Latusobolus xiaoyangbaensis gen. et sp. nov. and Eoobolus acutulus sp. nov. from the Cambrian Series 2 Shuijingtuo Formation of South China. The hierarchical shell architectures, epithelial cell moulds, and the shape and size of cylindrical columns are scrutinised in these new species. Their calcium phosphate-based biomineralized shells are mainly composed of stacked sandwich columnar units. The secretion and construction of the stacked sandwich model of columnar architecture, which played a significant role in the evolution of linguliforms, is highly biologically controlled and organic-matrix mediated. Furthermore, a continuous transformation of anatomic features resulting from the growth of diverse columnar shells is revealed between Eoobolidae, Lingulellotretidae, and Acrotretida, shedding new light on the evolutionary growth and adaptive innovation of biomineralized columnar architecture among early phosphatic-shelled brachiopods during the Cambrian explosion.







