A role for CIM6P/IGF2 receptor in memory consolidation and enhancement
Figures
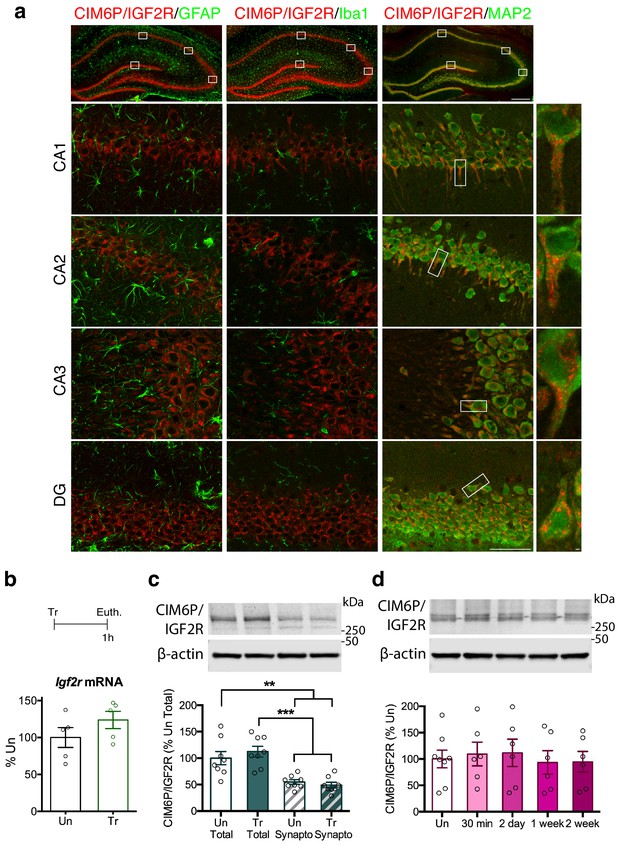
CIM6P/IGF2R is expressed in rat hippocampal neurons and mostly localizes to the somatic compartment.
(a) Immunofluorescence co-staining of CIM6P/IGF2R and GFAP, Iba1, or MAP2. Upper panels: representative composite tile scans of whole hippocampus (scale bar, 500 μm). Lower panels: CA1, CA2, CA3, and DG (scale bar, 50 μm). Far right panels: zoomed images showing co-localization of MAP2 with CIM6P/IGF2R (scale bar, 1 μm). (b) Rats were trained on IA (Tr) or remained in their home cages (untrained, Un) and euthanized 1 hr after training. Igf2r mRNA levels (n = 5, two independent experiments). (c) Western blot analyses comparing total and synaptoneurosomal extracts (n = 8, two independent experiments). (d) Total extracts from rats euthanized at various time points after training (30 min, 2 days, 1 week, and 2 weeks) (n = 6–8, four independent experiments). Two-tailed Student t-test or one-way ANOVA followed by Tukey’s post-hoc tests. **p<0.01 and ***p<0.001; see Source data one for detailed statistical information.
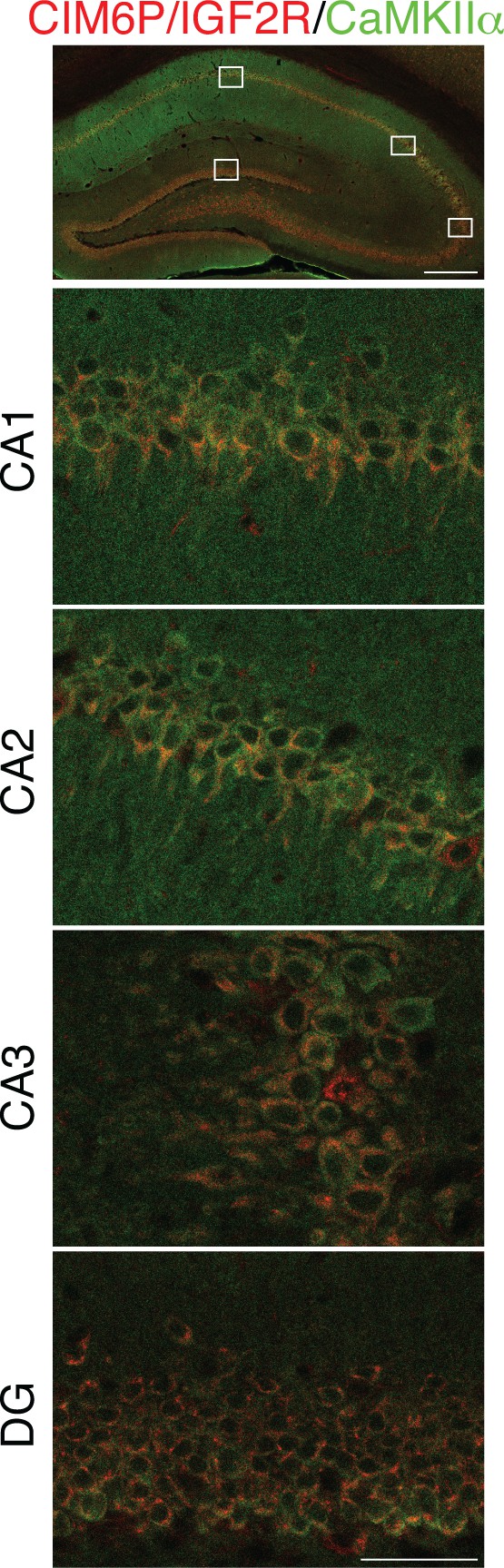
CIM6P/IGF2R is expressed in CaMKIIα neurons of rat hippocampus.
Immunofluorescence co-staining of CIM6P/IGF2R and CaMKIIα. Upper panels: representative composite tile scans of whole hippocampus (scale bar, 500 μm). Lower panels: CA1, CA2, CA3, and DG (scale bar, 50 μm).
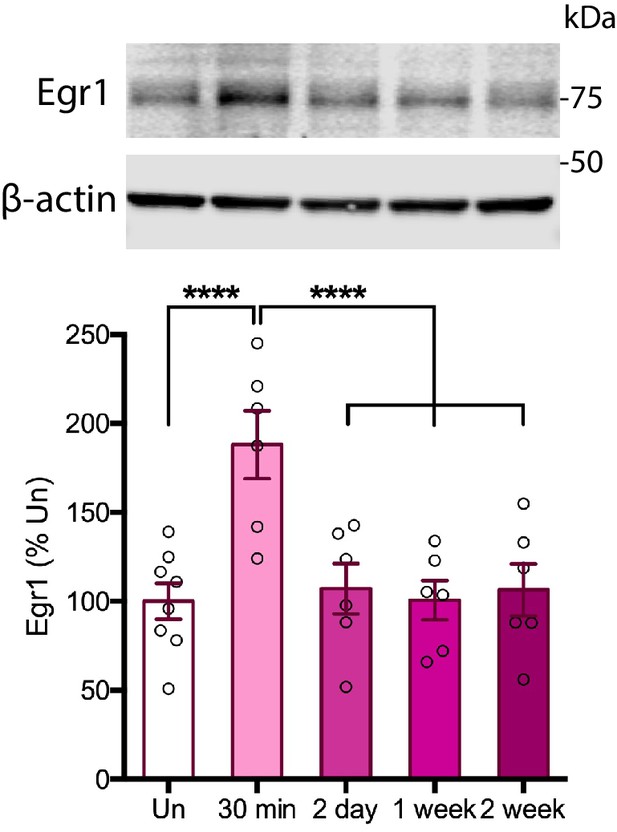
Time course of Egr1 protein induction following IA training in rats.
Cohorts of rats were trained on IA, and euthanized 30 min, 2 days, 1 week, or 2 weeks later. Total homogenates were analyzed by western blot for Egr1 protein levels. Egr1 was induced at 30 min after training (Tr), as compared to untrained controls (Un) (n = 6–8, four independent experiments). One-way ANOVA followed by Tukey’s post hoc test. ****p<0.0001; see Source data one for detailed statistical information.
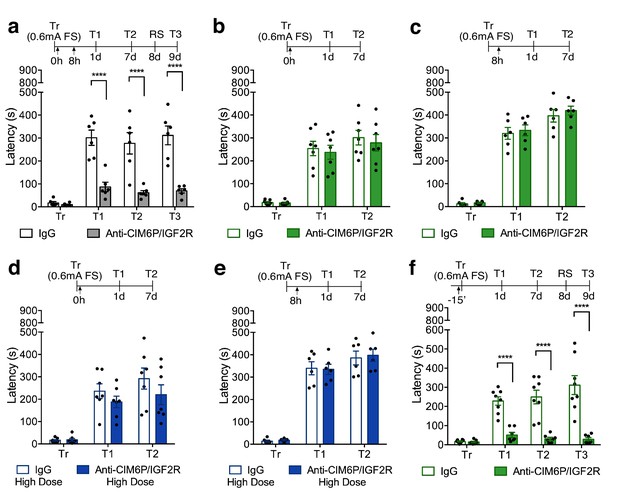
In rats, CIM6P/IGF2R is rapidly recruited by learning and required for memory consolidation within a limited temporal window.
Experimental timelines are shown above graphs. Rats were injected with IgG or anti-CIM6P/IGF2R antibody before or after IA training (↑). IA acquisition (Tr) and memory retention are expressed as mean latency ± SEM (in seconds). (a) The effect of two injections of either IgG control or anti-CIM6P/IGF2R, given immediately and 8 hr after training (Tr), on IA memory tested 1 day (1 d) and 1 week after training, as well as after a reminder shock (RS; n = 6, two independent experiments). (b and c) The effect of a single injection of anti-CIM6P/IGF2R, given immediately or 8 hr after training, on memory tested 1 day and 1 week after training (n = 6–7, two independent experiments). (d and e) The effect of a single 10-fold higher dose of IgG or anti-CIM6P/IGF2R, given at the same time points, on memory tested 1 day and 1 week later (n = 6–7, two independent experiments). (f) The effect of a single injection, given 15 min before training, on memory tested 1 day and 1 week after training, as well as after a reminder shock (n = 7–8, two independent experiments). Two-way repeated measures ANOVA followed by Sidak’s post-hoc tests. ****p<0.0001; see Source data one for detailed statistical information.
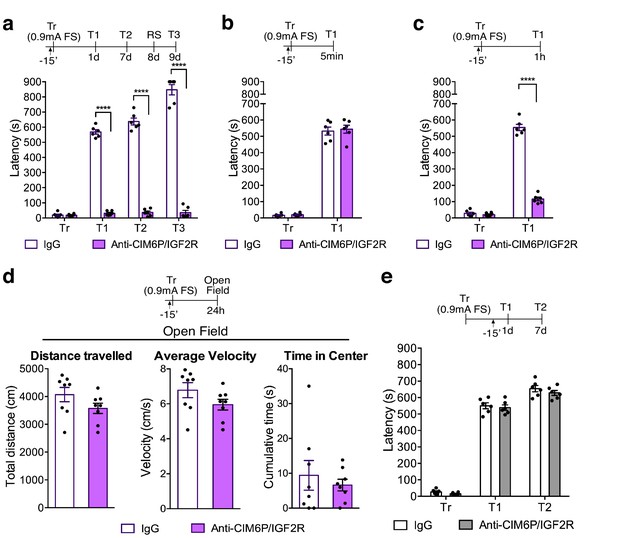
In rats, CIM6P/IGF2R is required for memory consolidation, but not learning or memory retrieval.
Experimental timelines are shown above graphs. Rats were injected with IgG or anti-CIM6P/IGF2R antibody 15 min before IA training or testing (↑). Inhibitory avoidance training and memory retention are expressed as mean latency ± SEM (in seconds). (a) The effect of a single injection of anti-CIM6P/IGF2R on memory retention in animals trained (Tr) with a stronger, (0.9 mA) shock intensity (n = 6, two independent experiments). (b) The effect of anti-CIM6P/IGF2R on memory tested 5 min after training (n = 6, two independent experiments), or 1 hr after training (c) n = 6, two independent experiments). (d) Open field test was conducted 24 hr after IA training; total distance travelled, average travel velocity, and cumulative time spent in the center of the arena were recorded (n = 8, two independent experiments). (e) The effect of anti-CIM6P/IGF2R on memory retrieval: anti-CIM6P/IGF2R was injected 15 min before memory test given 1 day after IA training (n = 6, two independent experiments). Two-way repeated measures ANOVA followed by Sidak’s post hoc tests. ****p<0.0001; see Source data one for detailed statistical information.
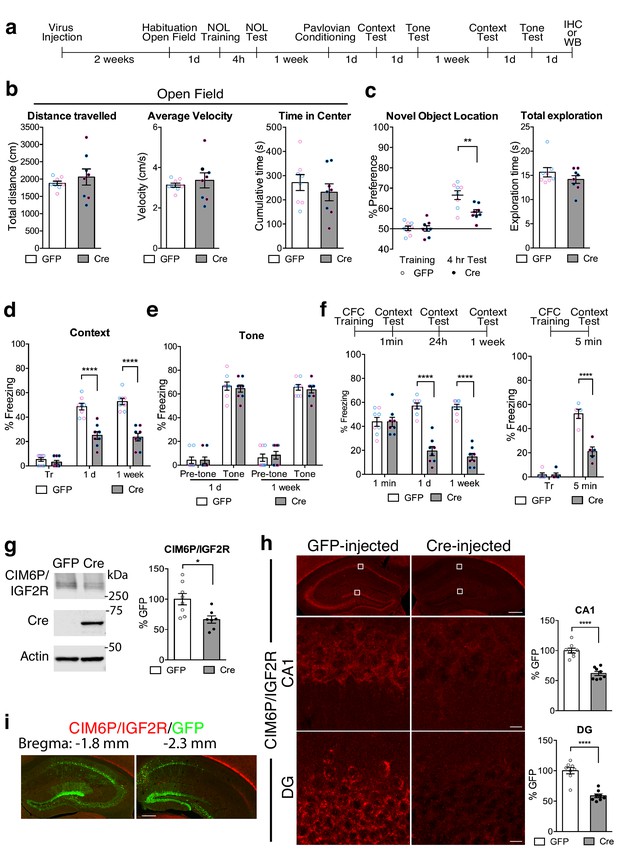
Hippocampal neuronal knockdown of CIM6P/IGF2R in mice selectively impairs hippocampus-dependent memories.
(a) Experimental timeline for b–e and g–i. Data are expressed as mean ± SEM, with blue datapoints representing male mice, and pink datapoints representing female mice. Igf2r-floxed mice received bilateral hippocampal injections of AAV-hSyn-Cre-eGFP (Cre) or control AAV-hSyn-eGFP (GFP), 2 weeks prior to behavioral experiments. (b) Open field of GFP- and Cre-injected mice measured total distance travelled, average travel velocity, and cumulative time spent in the center of the area (n = 8, four independent experiments). (c) Novel objection location memory and total exploration time, tested 4 hr after training, (n = 8, four independent experiments). (d) Percent (%) of time spent freezing in mice tested in the training context 1 day (1 d) and 1 week after Pavlovian conditioning (n = 8, four independent experiments). (e) Percent (%) of time spent freezing to a new context prior to onset of tone, and during the tone (n = 8, four independent experiments). (f) Contextual fear conditioning expressed as percent (%) of time spent freezing. Left: % of time spent freezing in mice tested 1 min, 1 day, and 1 week after training (n = 8, four independent experiments). Right: % of time spent freezing in mice tested 5 min after training. (g) Representative western blot and quantification obtained from dorsal hippocampi homogenates from Cre- or GFP-injected mice stained for CIM6P/IGF2R, Cre recombinase (Cre), and actin. Actin-normalized values were expressed as mean percentage ± SEM (n = 7–8, four independent experiments). (h) Upper panels: representative dorsal hippocampus composite tile scans in GFP-injected or Cre-injected mice immunostained for CIM6P/IGF2R (scale bar, 500 μm). Middle and lower panels: CA1 and DG (scale bar, 10 μm) are shown. Bar graphs on the right report immunostaining intensity quantifications for each sub-region (n = 8, four independent experiments). (i) Representative images of GFP- or Cre-injected mice, composite tile scans of dorsal hippocampus double staining with anti-GFP and anti-CIM6P/IGF2R antibodies (scale bar 500 μm). Two-tailed Student’s t-test or two-way repeated measures ANOVA followed by Sidak’s post-hoc tests. *p<0.05, ****p<0.0001; see Source data one for detailed statistical information.
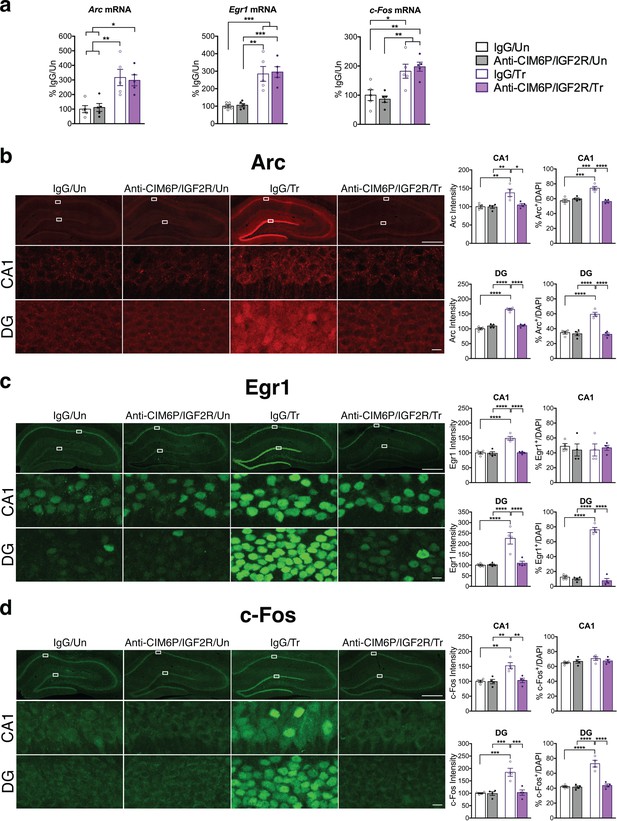
CIM6P/IGF2R is required for learning-induced IEG protein induction in rats.
Rats were injected with IgG or anti-CIM6P/IGF2R, and then were either trained on IA 15 min later (Tr) or remained Untrained (Un). To examine mRNA levels, rats were euthanized 1 hr after training and hippocampi flash-frozen. (a) qPCR of Arc, Egr1, and c-Fos performed on dorsal hippocampal extracts obtained from rats injected with either IgG or anti-CIM6P/IGF2R and euthanized 1 hr after IA training (n = 5, two independent experiments). (b–d) Rats underwent IA training and were perfused 1 hr later; coronal brain sections were immunostained for Arc (b), Egr1 (c), and c-Fos (d). Upper panels: representative composite tile scan of dorsal hippocampus (scale bar, 500 μm). Middle and lower panels: CA1 and DG (scale bar, 10 μm). Bar graphs shown at right show quantifications for normalized intensity and percentage of cells positive for IEGs, for each sub-region (n = 4, two independent experiments). Two-way ANOVA followed by Tukey’s post hoc tests. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; see Source data one for detailed statistical information.
Total number of cells assessed by DAPI staining in the rat dorsal hippocampus fields used in the IEGs immunofluorescent analyses.
The number of total cells detected in our fields of analyses (DAPI-positive cells) used for (a) Arc, (b) Egr1, and (c) c-Fos, for each sub-region (n = 4, two independent experiments). Two-way ANOVA followed by Tukey’s post hoc tests. See Source data one for detailed statistical information.
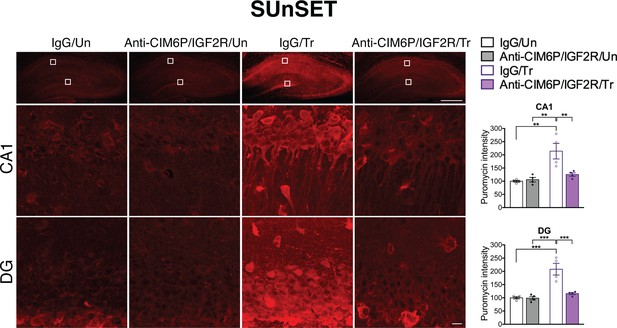
CIM6P/IGF2R is required for learning-induced de novo protein synthesis in rats.
SUnSET was employed to quantify de novo protein synthesis in the rat hippocampus. Puromycin was co-injected with IgG or anti-CIM6P/IGF2R prior to training, and rats were perfused 2 hr later. Upper panels: representative images of anti-puromycin immunostaining, composite tile scans of whole hippocampus (scale bar, 500 μm). Middle and lower panels: CA1 and DG (scale bar 10 μm). Bar graphs at right show immunostaining intensity quantifications for each sub-region (n = 4, two independent experiments). Two-way ANOVA followed by Tukey’s post hoc tests. **p<0.01, ***p<0.001; see Source data one for detailed statistical information.
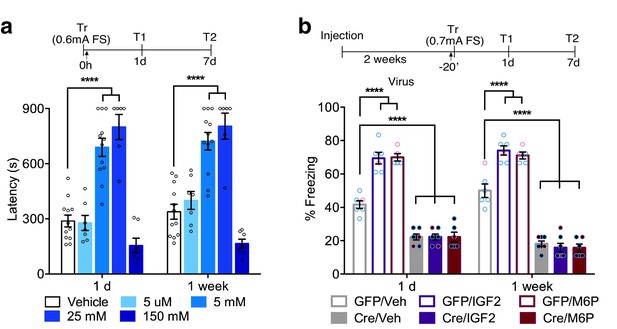
In rats and mice, M6P, like IGF2, enhances memories via CIM6P/IGF2R in hippocampal neurons.
Experimental timelines are shown above graphs. (a) Rats were injected bilaterally into the dorsal hippocampus with vehicle or the indicated concentrations of mannose-6-phosphate (M6P) immediately after IA training (↑), and memory was tested 1 day and 1 week later (n = 6–12, five independent experiments). (b) Vehicle, IGF2 (30 μg/kg), or M6P (850 μg/kg) was administered systemically (s.c., ↑) 20 min before contextual fear conditioning training to GFP- or Cre-injected mice. Percent (%) of time spent freezing were measured 1 day or 1 week after training (n = 5–7, five independent experiments). Two-way repeated measures ANOVA followed by Sidak’s post hoc tests. ****p<0.0001; see Source data one for detailed statistical information.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (R. norvegicus, male) | BluHsd:LE Long-Evans (blue spruce) | Envigo | RRID:RGD_5508398 | |
| Strain, strain background (M. musculus, male and female) | Igf2r-floxed mice | Dr. David Skaar (NC State University | MGI Cat# 3795370, RRID:MGI:3795370 | C57Bl/6J background, homozygotes used in experiments |
| Antibody | anti-Human IGF-II R (goat polyclonal) | R and D Systems | Cat# AF2447, RRID:AB_442153 | 5 ng/µL or 50 ng/µL |
| Antibody | anti-GFAP (chicken polyclonal) | Abcam | Cat# ab4674, RRID:AB_304558 | IF (1:5000) |
| Antibody | anti-Iba1 (rabbit polyclonal) | Wako | Cat# 019–19741, RRID:AB_839504 | IF (1:5000) |
| Antibody | anti-CaMKIIα (mouse monoclonal) | Millipore | Cat# 05–532, RRID:AB_309787 | IF (1:1000) |
| Antibody | anti-IGF2R (rabbit monoclonal) | Abcam | Cat# ab124767, RRID:AB_10974087 | IF (1:1000), WB (1:1000) |
| Antibody | anti-GFP (chicken polyclonal) | Aves Labs | Cat# GFP-1020, RRID:AB_10000240 | IF (1:1000) |
| Antibody | anti-Arc (rabbit polyclonal) | Synaptic systems | Cat# 156 003, RRID:AB_887694 | IF (1:2000) |
| Antibody | anti-Egr1 (rabbit monoclonal) | Cell Signaling Technology | Cat# 4153, RRID:AB_2097038 | IF (1:1000), WB (1:1000) |
| Antibody | anti-c-Fos (rabbit monoclonal) | Cell Signaling Technology | Cat# 2250, RRID:AB_2247211 | IF (1:500) |
| Antibody | anti-Cre (rabbit monoclonal) | Cell Signaling Technology | Cat# 15036, RRID:AB_2798694 | WB (1:1000) |
| Antibody | anti-β-actin (mouse monoclonal) | Santa Cruz Biotechnology | Cat# sc-47778 HRP, RRID:AB_2714189 | WB (1:10000) |
| Sequence-based reagent | Igf2r forward | NM_012756.2 | Primers | TTGCCCTCCAGAAACGGAAG |
| Sequence-based reagent | Igf2r reverse | NM_012756.2 | Primers | TACACCACAGTTTCGCTCGT |
| Sequence-based reagent | Arc forward | NM_019361.1 | Primers | CCCTGCAGCCCAAGTTCAAG |
| Sequence-based reagent | Arc reverse | NM_019361.1 | Primers | GAAGGCTCAGCTGCCTGCTC |
| Sequence-based reagent | c-Fos forward | NM_022197.2 | Primers | CCCTGCAGCCCAAGTTCAAG |
| Sequence-based reagent | c-Fos reverse | NM_022197.2 | Primers | GAAGGCTCAGCTGCCTGCTC |
| Sequence-based reagent | Egr1 forward | NM_012551.2 | Primers | ACCTACCAGTCCCAACTCATC |
| Sequence-based reagent | Egr1 reverse | NM_012551.2 | Primers | GACTCAACAGGGCAAGCATAC |
| Sequence-based reagent | Gapdh forward | NM_017008.4 | Primers | GAACATCATCCCTGCATCCA |
| Sequence-based reagent | Gapdh reverse | NM_017008.4 | Primers | CCAGTGAGCTTCCCGTTCA |
| Peptide, recombinant protein | Recombinant mouse IGF-II | R and D Systems | Cat# 792 MG | |
| Commercial assay or kit | RNeasy Plus Universal Mini Kit | Qiagen | Cat# 73404 | |
| Commercial assay or kit | QuantiTect Reverse Transcription Kit | Qiagen | Cat# 205311 | |
| Commercial assay or kit | iQ SYBR Green Supermix | Bio-Rad | Cat# 107–8882 | |
| Software, algorithm | ImageJ | National Institutes of Health | RRID:SCR_003070 | |
| Software, algorithm | Leica Application Suite X | Leica | RRID:SCR_013673 |
Additional files
-
Source data 1
Detailed information and statistical analyses related to data presented in the manuscript.
- https://cdn.elifesciences.org/articles/54781/elife-54781-data1-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/54781/elife-54781-transrepform-v1.pdf





