Ion Channels: Taking a close look at a large-pore channel
A cell relies on proteins called ion channels and transporters to allow various ions and molecules to enter and leave the cell. These proteins are embedded in the plasma membrane of the cell, and some of them form dynamic pores that can close and open, allowing both ions and molecules to pass through. To date, researchers have largely focused on ion channels with relatively selective and narrow pores through which only specific types of atomic ions can pass. However, channel proteins with pores that are large enough for molecules to pass through are attracting more attention. These large-pore channels participate in a variety of physiological functions because they allow molecules with important signaling functions, such as ATP and glutamate, to pass through them.
Pannexin 1 is a large-pore channel that has important roles in inflammation, pain, infertility, cancer progression and epilepsy. It shows selectivity for anions, but it may also allow the passage of molecules as large as ~1 kilodalton in molecular weight. However, a lack of structural information has limited our understanding of how this and other large-pore channels work at the molecular level. Now, in eLife, Toshimitsu Kawate (Cornell University), Hiro Furukawa (Cold Spring Harbor Laboratory; CSHL) and colleagues – including Kevin Michalski (Cornell) and Johanna Syrjanen (CSHL) as joint first authors – report the first high-resolution structure of the pannexin 1 channel, obtained using cryo-electron microscopy (Michalski et al., 2020).
Michalski et al. show that the pannexin 1 channel has a unique architecture amongst eukaryotic channels, with seven subunits arranged around a large central pore (Figure 1). This contradicts previous studies that suggested that the pannexin 1 channel would be hexameric. The pore has three constriction sites, with the one in the extracellular region of the protein being the narrowest. This detail makes it likely that this constriction site acts as the main size-exclusion barrier, since its width could stop larger molecules from entering the pore. In this narrow extracellular region, the side chains of the tryptophan at position 74 of each subunit interact with the arginine at position 75 of the adjacent subunit, lining the pore. Arginine’s positive charge could repulse other positively charged molecules, potentially giving the channel its anion selectivity.
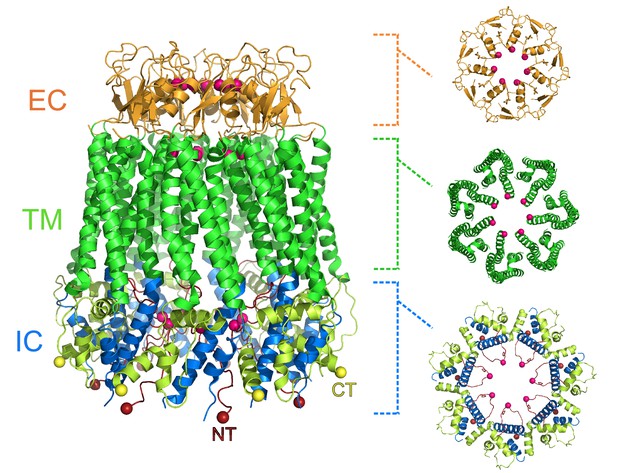
Heptameric structure of the pannexin 1 channel.
Side view (left) of the pannexin 1 structure resolved by Michalski et al., and top views of the extracellular region (EC; top right), the transmembrane region (TM; middle right), and the intracellular region (IC; bottom right). The arrangement of seven subunits to form the channel is clearly visible in the structure. Each of the three regions shown in the top views contains a constriction site in the pore that runs through the center of the protein, and the amino acid residues involved in the constriction sites are represented as pink spheres. Protein data bank ID: 6VD7. CT: C-terminus (yellow); NT: N-terminus (red).
Mutating these arginine and tryptophan residues in all the subunits of the channel shows that their interaction, and particularly the presence of the arginine, are required for anion selectivity. These results are consistent with previous findings obtained by functional approaches (Ma et al., 2012). Both amino acids are highly conserved in different species, suggesting that selectivity for atomic anions could play an essential role in cell physiology, in addition to molecular transport.
Despite pannexin 1 being different in its amino acid sequence to other large-pore channels, including innexins and connexins, their topologies are quite similar: all have four transmembrane segments, two extracellular loops and one intracellular loop. Additionally, both their N-terminal and C-terminal regions are inside the cell. Consistent with this, the transmembrane segments of pannexin 1 almost overlap with the transmembrane segments of other large-pores channels. However, the structure of pannexin 1 shows substantial differences in the spatial conformation of the extracellular loops. This conformation may underlie specificity for two mechanisms that determine a channel’s activity. The first is gating, or how a channel changes its conformation to open and close the pore to allow atomic ions and other molecules through. The second is permeation, which determines how easily these molecules flow through the open pore.
Michalski et al. used their structural data to investigate the mechanisms through which carbenoxolone, one of the most widely used pannexin 1 inhibitors, blocks the channel. The amino acid residues involved in carbenoxolone sensitivity (identified in Michalski and Kawate, 2016) were located in a groove where the two extracellular loops interact, near the narrowest part of the pore. These structural insights could lead to the rational development of new, more specific, and potentially therapeutic drugs.
As with previous structural studies of large-pore channels, Michalski et al. could not resolve the full N- and C-terminal domains of pannexin 1, which suggests that these regions are flexible (Oshima et al., 2016; Kasuya et al., 2018; Michalski et al., 2020; Syrjanen et al., 2020). The importance of the N- and C-terminals has been shown through functional studies by either mutating the N-terminus, which alters gating and permeability (Michalski et al., 2018); or truncating the C-terminus using caspases, leading to a constitutively open channel (Chekeni et al., 2010; Sandilos et al., 2012).
This new evidence supports the need to obtain the full-length channel structures in open and closed conformations to understand both gating and permeability in large-pore channels. For example, the N-terminus is likely to further narrow the pore, making it difficult to envision how molecules such as ATP permeate pannexin 1 and other large-pore channels based on the partially solved structures reported to date. These issues are probably the next major challenges for structural biologists working on these channels.
Nonetheless, the structure resolved by Michalski et al. provides new insights into the biophysical properties of pannexin 1 channels. It can also serve as the basis for studies using complementary methodologies, including simulations of molecular dynamics and electrophysiology. In this view, the future of pannexin 1 research is auspicious, and several questions about the role of pannexin 1 in health and disease may be answered by integrating structural biology, biophysics and physiology.
References
-
Cryo-EM structures of the human volume-regulated anion channel LRRC8Nature Structural & Molecular Biology 25:797–804.https://doi.org/10.1038/s41594-018-0109-6
-
Pannexin 1 forms an anion-selective channelPflügers Archiv - European Journal of Physiology 463:585–592.https://doi.org/10.1007/s00424-012-1077-z
-
The weak voltage dependence of pannexin 1 channels can be tuned by N-terminal modificationsJournal of General Physiology 150:1758–1768.https://doi.org/10.1085/jgp.201711804
-
Carbenoxolone inhibits Pannexin1 channels through interactions in the first extracellular loopJournal of General Physiology 147:165–174.https://doi.org/10.1085/jgp.201511505
-
Atomic structure of the innexin-6 gap junction channel determined by cryo-EMNature Communications 7:13681.https://doi.org/10.1038/ncomms13681
-
Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory regionJournal of Biological Chemistry 287:11303–11311.https://doi.org/10.1074/jbc.M111.323378
-
Structure and assembly of calcium homeostasis modulator proteinsNature Structural & Molecular Biology 27:150–159.https://doi.org/10.1038/s41594-019-0369-9
Article and author information
Author details
Publication history
- Version of Record published: March 31, 2020 (version 1)
Copyright
© 2020, Gaete and Contreras
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,070
- views
-
- 139
- downloads
-
- 3
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
Obstructive sleep apnea (OSA) is a prevalent sleep-related breathing disorder that results in multiple bouts of intermittent hypoxia. OSA has many neurological and systemic comorbidities, including dysphagia, or disordered swallow, and discoordination with breathing. However, the mechanism in which chronic intermittent hypoxia (CIH) causes dysphagia is unknown. Recently, we showed the postinspiratory complex (PiCo) acts as an interface between the swallow pattern generator (SPG) and the inspiratory rhythm generator, the preBötzinger complex, to regulate proper swallow-breathing coordination (Huff et al., 2023). PiCo is characterized by interneurons co-expressing transporters for glutamate (Vglut2) and acetylcholine (ChAT). Here we show that optogenetic stimulation of ChATcre:Ai32, Vglut2cre:Ai32, and ChATcre:Vglut2FlpO:ChR2 mice exposed to CIH does not alter swallow-breathing coordination, but unexpectedly disrupts swallow behavior via triggering variable swallow motor patterns. This suggests that glutamatergic–cholinergic neurons in PiCo are not only critical for the regulation of swallow-breathing coordination, but also play an important role in the modulation of swallow motor patterning. Our study also suggests that swallow disruption, as seen in OSA, involves central nervous mechanisms interfering with swallow motor patterning and laryngeal activation. These findings are crucial for understanding the mechanisms underlying dysphagia, both in OSA and other breathing and neurological disorders.
-
- Neuroscience
The central tendency bias, or contraction bias, is a phenomenon where the judgment of the magnitude of items held in working memory appears to be biased toward the average of past observations. It is assumed to be an optimal strategy by the brain and commonly thought of as an expression of the brain’s ability to learn the statistical structure of sensory input. On the other hand, recency biases such as serial dependence are also commonly observed and are thought to reflect the content of working memory. Recent results from an auditory delayed comparison task in rats suggest that both biases may be more related than previously thought: when the posterior parietal cortex (PPC) was silenced, both short-term and contraction biases were reduced. By proposing a model of the circuit that may be involved in generating the behavior, we show that a volatile working memory content susceptible to shifting to the past sensory experience – producing short-term sensory history biases – naturally leads to contraction bias. The errors, occurring at the level of individual trials, are sampled from the full distribution of the stimuli and are not due to a gradual shift of the memory toward the sensory distribution’s mean. Our results are consistent with a broad set of behavioral findings and provide predictions of performance across different stimulus distributions and timings, delay intervals, as well as neuronal dynamics in putative working memory areas. Finally, we validate our model by performing a set of human psychophysics experiments of an auditory parametric working memory task.






