Dependency of human and murine LKB1-inactivated lung cancer on aberrant CRTC-CREB activation
Figures
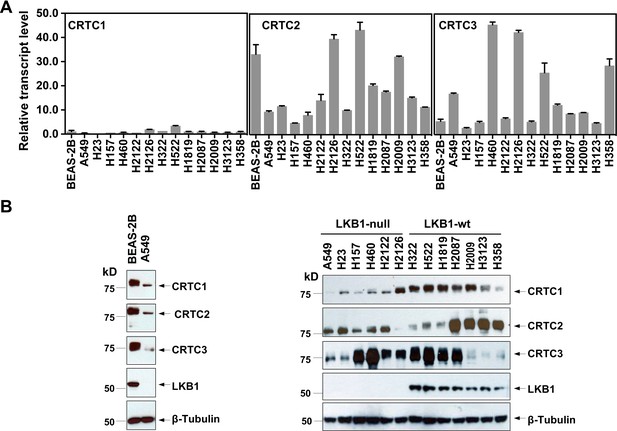
Three members of the CRTC co-activator family, CRTC1, CRTC2, and CRTC3, are expressed at varying levels in human lung epithelial and cancer cell lines.
(A) The transcript levels of the three CRTC genes were determined by RT-qPCR assays. All three CRTC transcript levels were normalized against the level of the housekeeping gene GAPDH individually. The expression level of CRTC1 in BEAS-2B was then assigned as 1, and the expression levels for the three CRTCs in various cell lines were presented as relative values to that of CRTC1 in BEAS-2B cells. (B) The protein levels of three CRTCs and LKB1 were detected by western blotting. Blotting with anti-β-Tubulin was used as a loading control.
-
Figure 1—source data 1
Numerical data for A.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig1-data1-v2.xlsx
-
Figure 1—source data 2
Unedited immunoblots in B.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig1-data2-v2.pdf
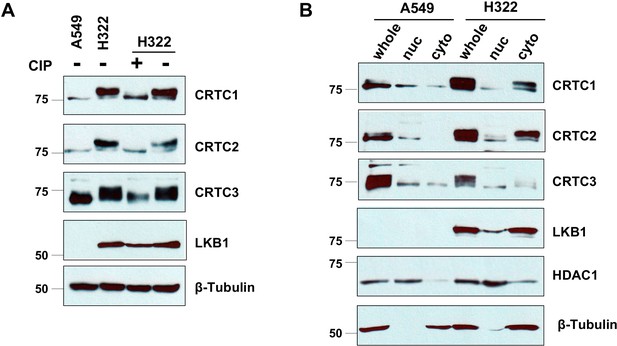
CRTC1, CRTC2, and CRTC3 showed predominantly de-phosphorylated, nuclear forms in LKB1-null cells (A549), and phosphorylated, cytoplasmic forms in LKB1-expressing cells (H322).
(A) H322 cell lysates were treated with or without alkaline calf intestinal phosphatase (CIP, one unit per ug protein) for 60 min at 37°C. Samples were loaded together with untreated A549 and H322 cell lysates in 6% gels. Phosphorylated (upper bands) and unphosphorylated (lower bands) CRTCs were then detected by western blotting. Blotting with anti-β-Tubulin was used as a loading control. (B) Nuclear and cytoplasmic fractions of A549 and H322 cells were isolated using a NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific). The whole lysates were extracted from the same number of cells using a cell lysis buffer. Protein levels of LKB1 and three CRTCs in whole-cell lysates and different fractions were detected. HDAC1 and β-Tubulin were detected as nuclear or cytoplasmic controls, respectively.
-
Figure 1—figure supplement 1—source data 1
Unedited immunoblots in A, B.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig1-figsupp1-data1-v2.pdf
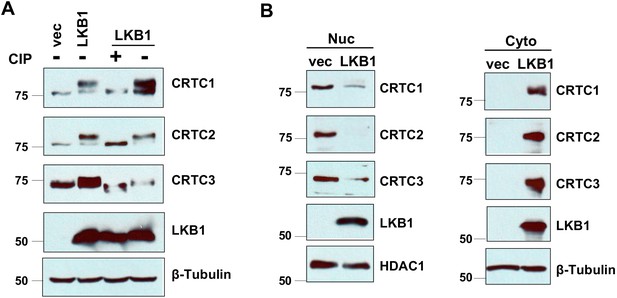
LKB1 re-introduction to LKB1-null lung cancer cells (A549) induced the phosphorylation and cytoplasmic retention of CRTCs.
A549 cells were transduced with retroviruses generated from a pBabe-LKB1 construct or pBabe vector and then selected with puromycin (1.5 µg/ml) for 48 hr. (A) A549-LKB1 cell lysates were treated with or without alkaline calf intestinal phosphatase (CIP, one unit per µg protein) for 60 min at 37°C . The samples were loaded together with untreated lysates in 6% gels for immunoblotting for CRTC1, CRTC2, CRTC3, and LKB1. Blotting with anti-β-Tubulin was used as a loading control. (B) Nuclear and cytoplasmic fractions of LKB1-expressing and vector control A549 cells were isolated using the NE-PER Nuclear and Cytoplasmic Extraction kit, and were subjected to immunoblotting for three CRTCs and LKB1. HDAC1 and β-Tubulin were detected as nuclear or cytoplasmic controls, respectively.
-
Figure 1—figure supplement 2—source data 1
Unedited immunoblots in A, B.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig1-figsupp2-data1-v2.pdf
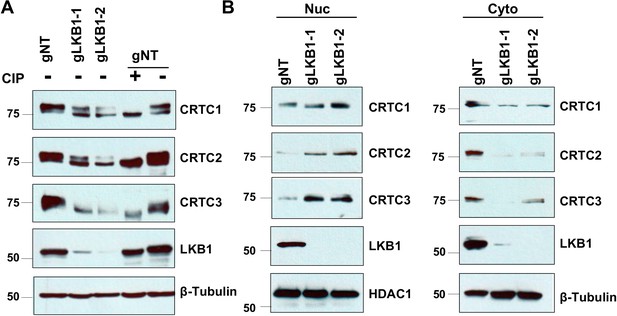
CRISPR/Cas9-mediated LKB1 knockout in LKB1-expressing lung cancer cells (H322) resulted in the dephosphorylation and nuclear translocation of CRTCs.
(A) Cas9-expressing H322 cells (H322-Cas9) were transduced with lentiviruses containing non-targeting gRNA (gNT) or two gRNAs targeting LKB1 (gLKB1-1, gLKB1-2). H322 gNT cell lysates were treated with or without alkaline calf intestinal phosphatase (CIP, one unit per ug protein) for 60 min at 37°C. The samples were loaded together with untreated cell lysates in 6% gels for immunoblotting for CRTC1, CRTC2, CRTC3, and LKB1. Blotting with anti-β-Tubulin was used as a loading control. (B) Nuclear and cytoplasmic fractions of H322-Cas9 containing gNT, gLKB1-1, or gLKB1-2 cells were isolated for immunoblotting to detect three CRTCs and LKB1. HDAC1 and β-Tubulin were detected as nuclear or cytoplasmic controls, respectively.
-
Figure 1—figure supplement 3—source data 1
Unedited immunoblots in A, B.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig1-figsupp3-data1-v2.pdf
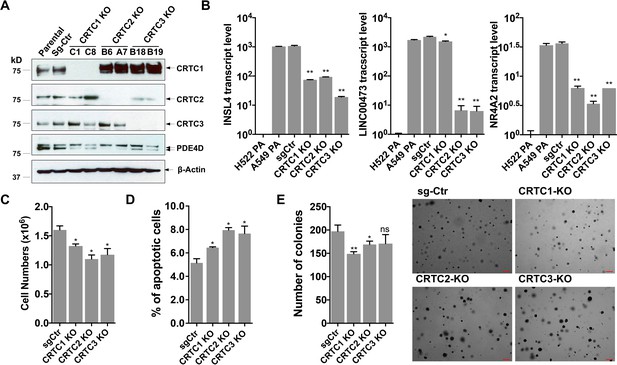
Individual knockouts of the CRTC family members in human LKB1-null lung cancer cells inhibit the CREB-mediated target gene expression and moderately affect cell viability and anchorage-independent growth.
(A) Western blot analysis of endogenous CRTC proteins in parental A549 cells, A549 cells stably transduced with non-targeting sgRNA, and two independent single knockout clones for each CRTC1, CRTC2, or CRTC3. The protein level of a CREB target gene, PDE4D was also detected. Blotting with anti-β-ACTIN was used as a loading control. (B) The transcript levels of CREB-mediated target genes (INSL4, LINC00473 and NR4A2) were determined by RT-qPCR assays (n = 2). The LKB1-wt cells, H522 parental (PA) cells, were also analyzed. (C,D) Individual CRTC knockout or control cells were cultured at 3 × 105 cells/well in the 6-well plates for 96 hr. The viable cells were quantified by trypan blue exclusion assay (C), and the number of apoptotic cells was determined by staining with annexin V/propidium iodide (PI) followed by flow cytometry (D). (E) Control and CRTC knockout cells were cultured in soft agar for 14 days, and the resulting colonies were stained by crystal violet and photographed under microscope. The number of colonies was counted using ImageJ. Assays were performed in triplicate. One-way ANOVA test was used to calculate the p values (*p<0.05, **p<0.01, ns p>0.05).
-
Figure 2—source data 1
Unedited immunoblots in A.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig2-data1-v2.pdf
-
Figure 2—source data 2
Numerical data for B, C, D, E.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig2-data2-v2.xlsx
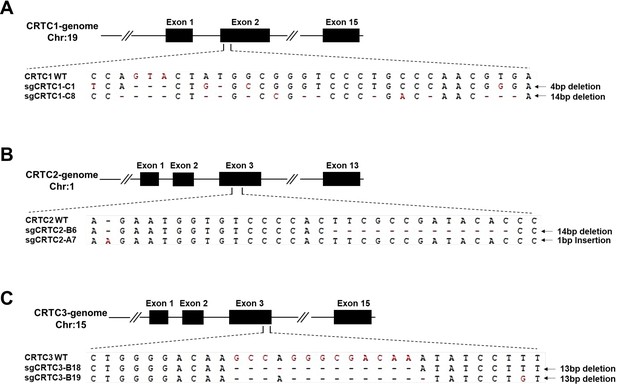
CRISPR/Cas9-edited alleles in two independent single knockout clones for each CRTC gene were validated by genomic DNA sequencing.
The altered sequences of CRTC1 (a), CRTC2 (b), and CRTC3 (c) genes in two independent single knockout clones were shown.
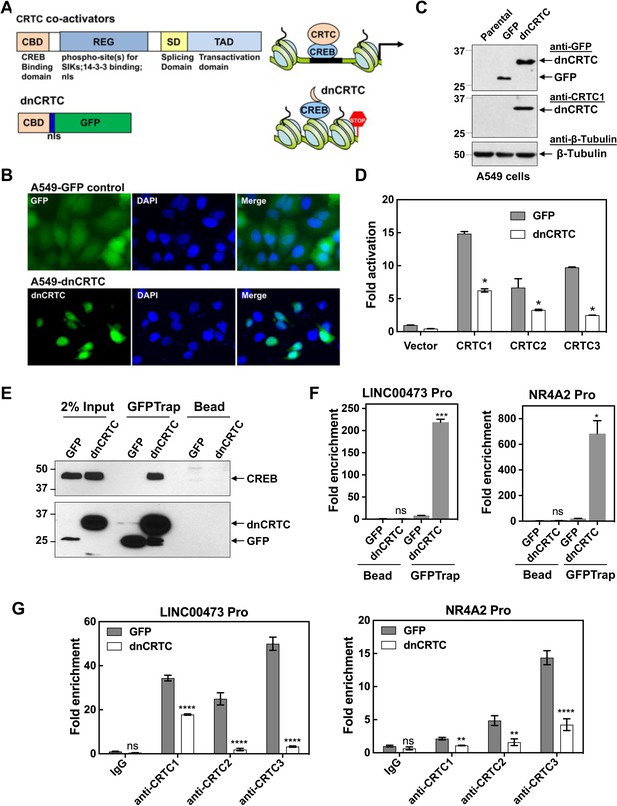
A dominant negative CRTC mutant (dnCRTC) interacted with CREB on the target gene promoters and blocked CRTC co-activation of CREB transcription.
(A) A diagram of CRTC co-activator and dnCRTC was shown. The dnCRTC consists of CRTC1 (1-55aa) followed by a nuclear localization signal (nls) and GFP, cloned into the retroviral pMSCV vector. (B) A549 cells transduced with pMSCV-dnCRTC retroviruses showed that dnCRTC was predominantly localized in the nuclear compartment (lower), while A549 control cells transduced with pMSCV-GFP retroviruses showed both cytoplasmic and nuclear GFP signals (upper). DAPI stained for the nuclei. (C) Western blotting validated the expression of dnCRTC in transduced A549 cells. (D) Expression of dnCRTC blocked the abilities of CRTC1-3 to activate the pCRE-luc reporter in 293 T cells (n = 2). (E) dnCRTC interacts with CREB in the chromatin complex. Cells (GFP-expressing control and dnCRTC-GFP expressing cells) were crosslinked and chromatins were sonicated. GFP-Trap_A (anti-GFP VHH nano body coupled to agarose beads) were used for immunoprecipitation of dnCRTC-GFP proteins which were then blotted with anti-CREB and anti-GFP antibodies. Uncoupled agarose beads were used as control. (F) dnCRTC was enriched on the CRE regions of the LINC00473 and NR4A2 promoters. (G) dnCRTC reduced the enrichment of endogenous CRTC1, CRTC2, and CRTC3 proteins on the CRE regions of the LINC00473 and NR4A2 promoters. Two-tailed student’s t-test was used to calculate the p values (*p<0.05, **p<0.01, ns p>0.05).
-
Figure 3—source data 1
Unedited immunoblots in C, E.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig3-data1-v2.pdf
-
Figure 3—source data 2
Numerical data for D, F, G.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig3-data2-v2.xlsx
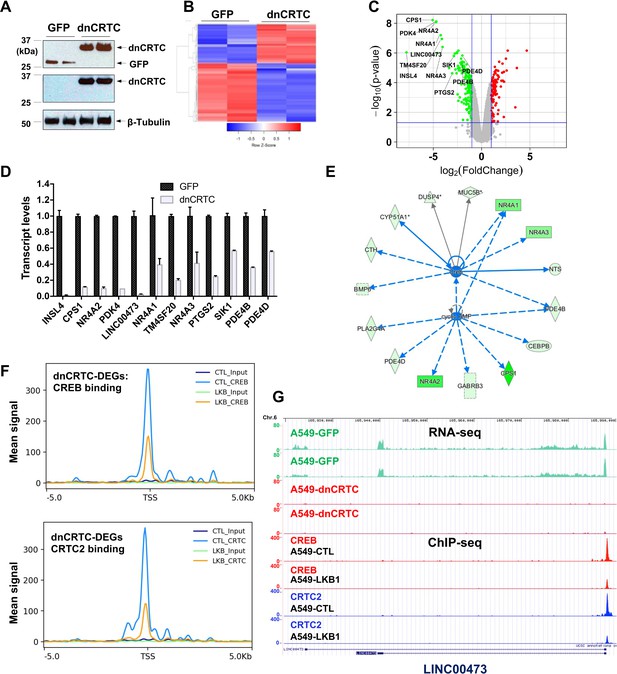
Gene expression profiling revealed dnCRTC repressed CRTC-CREB target gene expression.
(A) Western blotting confirmed dnCRTC-expressing and GFP-expressing control cells. (B, C) The heatmap and volcano plots showed gene expression changes in dnCRTC-expressing and GFP-expressing cells. (D) The RT-qPCR analysis validated differential expressed genes (DEGs) in dnCRTC-expressing A549 cells. (E) IPA analysis identified CREB and cAMP as upstream regulators for gene signature changes due to dnCRTC expression. (F) Analysis of CREB and CRTC2 binding of dnCRTC-DEGs in a ChIP-seq dataset. (G) CREB and CRTC2 binding peaks were shown in the LINC00473 target gene locus from the ChIP-seq analysis (lower panel). The mapped peaks of sequence reads from RNA-seq of A549-GFP and -dnCRTC cells was also shown (upper panel).
-
Figure 4—source data 1
Unedited immunoblots in A.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig4-data1-v2.pdf
-
Figure 4—source data 2
Numerical data for D.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig4-data2-v2.xlsx
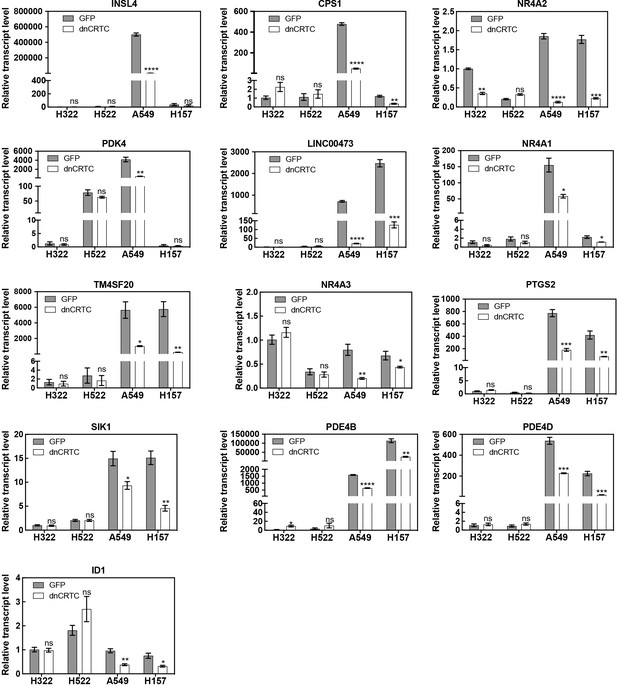
Effects of dnCRTC expression on gene expression in LKB1- expressing and LKB1-null lung cancer cells.
Two LKB1-null (A549 and H157) and two LKB1-positive (H322 and H522) NSCLC cells were transduced with dnCRTC or control GFP retroviruses. Expression of the top dnCRTC-downregulated gene candidates identified from our gene expression profiling experiment and ID1 in these cells were evaluated by RT-qPCR. Assays were performed in triplicate. The p values between dnCRTC-expressing and GFP-expressing groups were calculated based on two-tailed unpaired Student’s t-tests. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns p>0.05.
-
Figure 4—figure supplement 1—source data 1
Numerical data for bar graphs.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig4-figsupp1-data1-v2.xlsx
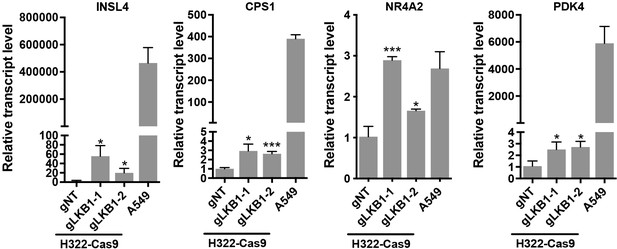
CRISPR/Cas9-mediated LKB1 knockout in in LKB1-expressing lung cancer cells led to enhanced expression of multiple dnCRTC-regulated targets.
The Cas9-expressing H322 cells (H322-Cas9) were first generated and then transduced with lentiviruses containing non-targeting gRNA (gNT) or two gRNAs targeting LKB1 (gLKB1-1, gLKB1-2). The transcript levels of several top dnCRTC-regulated genes (INSL4, CPS1, NR4A2, and PDK4) in these cells were determined by RT-qPCR. The LKB1-null NSCLC A549 cells were also analyzed. It is noted that the upregulation of these genes was moderate as compared to their corresponding levels in LKB1-null NSCLC A549 cells. Assays were performed in triplicate. The p values show one-way ANOVA tests between LKB1 knockout samples and control samples. *p<0.05, ***p<0.001, ns p>0.05.
-
Figure 4—figure supplement 2—source data 1
Numerical data for bar graphs.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig4-figsupp2-data1-v2.xlsx
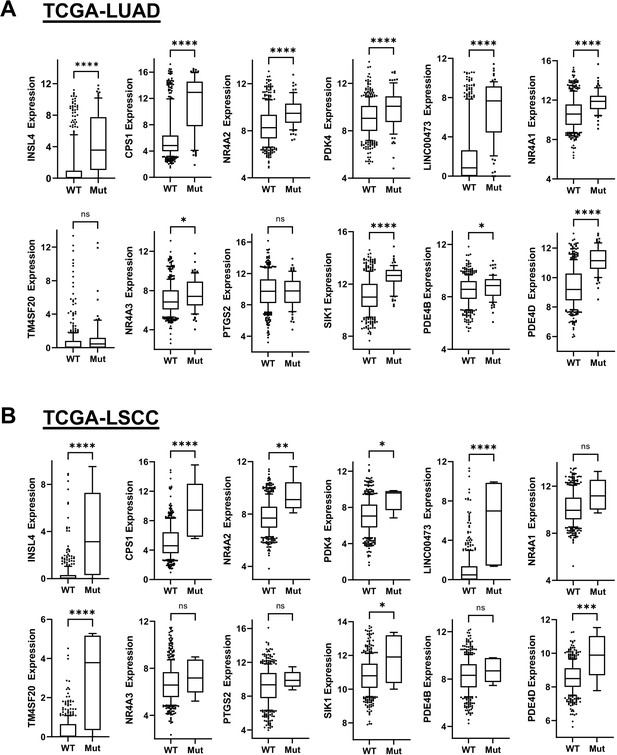
The Box and whisker plots show gene expression levels in LKB1 mutant (Mut) and wildtype (Wt) groups of lung adenocarcinoma (TCGA-LUAD, PanCancer Atlas) and lung squamous cell carcinoma (TCGA-LSCC, PanCancer Atlas).
The RNA-seq gene expression data from the TCGA-LUAD cohort (n = 503 cases with 428 WT, 75 mut) and the TCGA-LSCC cohort (n = 466 cases with 461 WT, five mut) were downloaded from cBioportal. The top 12 dnCRTC-regulated genes identified from the gene expression profiling of dnCRTC-expressing A549 vs control cells were analyzed and compared between LKB1-Mut and LKB1-WT groups. The p-values were generated by a two-tail, unpaired t test. (ns >0.05, *<0.05, **<0.01, ***<0.001, ****<0.0001). The data indicate that the overall high expression of many top dnCRTC target genes, such as INSL4, CPS1, NR4A2, LINC00473, NR4A1, PTGS2, SIK1, and PDE4D, was associated with mutations in LKB1 in both human lung adenocarcinoma and lung squamous cell carcinoma cohorts.
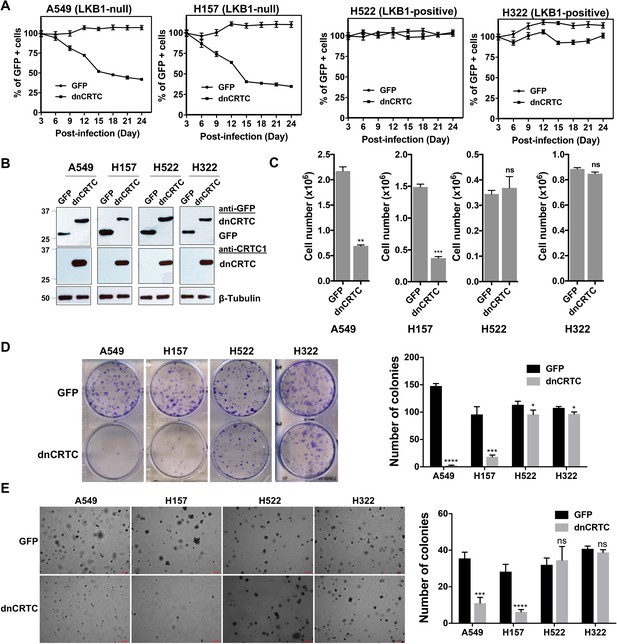
dnCRTC expression suppressed the growth of LKB1-null but not LKB1-positive lung cancer cells.
(A) Two LKB1-null (A549 and H157) and two LKB1-positive (H322 and H522) NSCLC cells were transduced with dnCRTC or control GFP retroviruses. The MOI was optimized to obtain an infection rate of 40–60%, and then the percentage of GFP-positive cells was determined by FACS analysis every 3 days for a total of 24 days starting at day 3 post-infection. The percentage of GFP-positive cells at day three post-infection was considered as 100%, and the remaining data were normalized (n = 3). (B, C) The GFP-positive cells for dnCRTC- and GFP-transduced cells were sorted and confirmed for dnCRTC and GFP expression by western blotting (B). Sorted cells were also cultured at 2 × 105 (for H322 and H522) or 3 × 105 (for A549 and H157) cells/well in the six-well plates for 96 hr and viable cells were counted using trypan blue exclusion test (C) (n = 3). (D) Transduced cells were cultured at 400 cells/well in six-well plates for 14 days and colonies were stained by crystal violet and photographed. The number of colonies in each well was counted using ImageJ. Assays were performed in triplicate. (E) Transduced cells were cultured in soft agar gels and colonies were stained by crystal violets, photographed and counted. The number of colonies from each image was counted using ImageJ. Assays were performed in triplicate. Scale bars, 200μM. Only colonies with a diameter higher than 50 μm were counted (n = 3). Two-tailed student’s t-test was used to calculate the p values (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns p>0.05).
-
Figure 5—source data 1
Numerical data for A, C, D, E.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig5-data1-v2.xlsx
-
Figure 5—source data 2
Unedited immunoblots in B.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig5-data2-v2.pdf
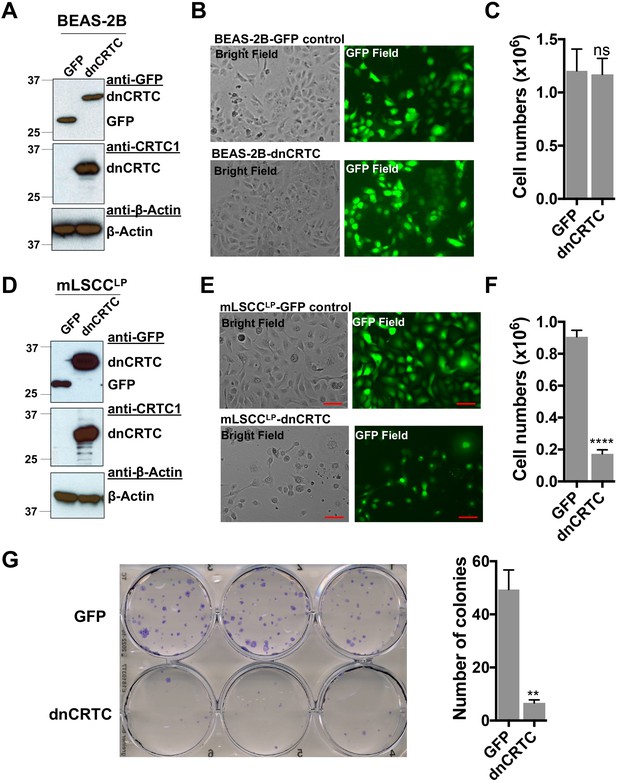
Effects of dnCRTC expression on the growth of human immortalized lung bronchial epithelial BEAS-2B cells and mouse LKB1-null NSCLC mLSCCLP cells.
(a) BEAS-2B cells were transduced with dnCRTC or GFP retroviruses and the dnCRTC and GFP expression were confirmed by western blotting. (b) The transduced dnCRTC- or GFP BEAS-2B cells were photographed under a microscope, and cell images under both the bright field and GFP field were presented. (c) The BEAS-2B cells transduced with dnCRTC or GFP retroviruses were cultured at 3 × 105 cell per well in six-well plates for 96 hr. The number of viable cells were determined by trypan blue exclusion assay (n = 3). (d) Mouse NSCLC mLSCCLP cells with LKB1 and PTEN deficiency were transduced with dnCRTC or GFP retrovirus. The dnCRTC and GFP expression were confirmed by western blotting. (e) The transduced dnCRTC- or GFP mLSCCLP cells were photographed and cell images under both the bright field and GFP field were presented. (f) mLSCCLP cells transduced with dnCRTC or GFP-control retroviruses were cultured at 2 × 105 cell per well in six-well plates for 96 hr. The number of viable cells were determined by trypan blue exclusion assay (n = 3). (g) The transduced mLSCCLP cells were cultured at 400 cells per well in six-well plates for 14 days and colonies were stained by crystal violet and photographed. The number of colonies in each image were counted using ImageJ. Assays were performed in triplicate. The p values were calculated by two tailed student’s t-test (**p<0.01, ****p<0.0001, ns p>0.05).
-
Figure 5—figure supplement 1—source data 1
Unedited immunoblots in A, D.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig5-figsupp1-data1-v2.pdf
-
Figure 5—figure supplement 1—source data 2
Numerical data for C, F, G.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig5-figsupp1-data2-v2.xlsx
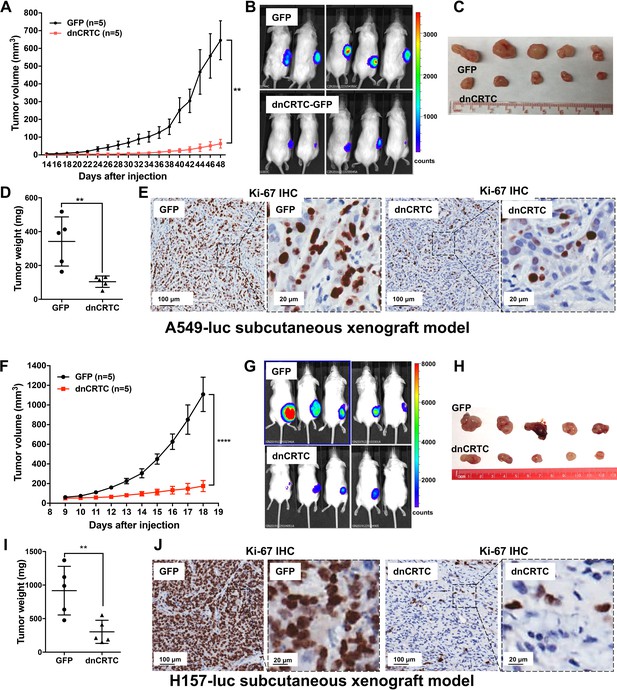
Expression of dnCRTC significantly inhibited the growth of LKB1-null NSCLC xenograft tumors.
(A–E) A549-luc were transduced with GFP control or dnCRTC for 72 hr and the transduced cells (1 × 106 per mouse) were injected subcutaneously to the right flanks of NOD/SCID mice. Tumor volumes of two cohorts (n = 5 each) were measured every two days starting from day 14 until day 48 (A). The bioluminescent images of mice (B), excised tumors (C) and tumor weights (D) as well as Ki-67 immunohistochemical staining of xenograft tumor sections (E) were shown. (F–J) H157-luc were transduced with GFP control or dnCRTC for 72 hr and the transduced cells (1 × 106 per mouse) were injected subcutaneously to the right flanks of NOD/SCID mice. Tumor volumes of two cohorts (n = 5 each) were measured daily from day 9 to day 18 (F). The bioluminescent images of mice (G), excised tumors (H), tumor weights (I) and Ki-67 immunohistochemical staining (J) were shown. Scale bars: 100 μm (left panels), 20 μm (right panels). Two-tailed student’s t-test was used to calculate the p values (**p<0.01, ****p<0.0001).
-
Figure 6—source data 1
Numerical data for A, D, F, I.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig6-data1-v2.xlsx
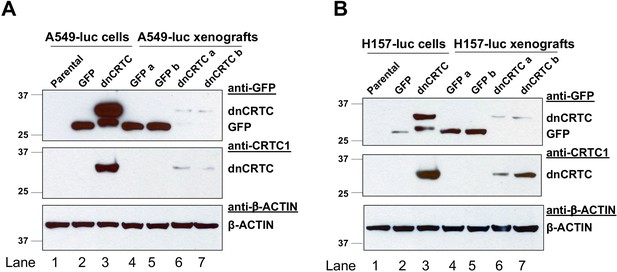
Expression of GFP and dnCRTC in the transduced human LKB1-null lung cancer cells at the time of tumor cell injection and in the resulting xenograft tumors.
(A) Western blot analysis of GFP and dnCRTC proteins in luciferase-expressing A549 (A549-luc) cells and xenograft tumors. Lane 1: A549-luc (parental); Lane 2: A549-luc transduced with GFP control retroviruses for 72 hr; Lane 3: A549-luc transduced with dnCRTC retroviruses for 72 hr; Lane 4–5: two xenograft tumors derived from two mice implanted with GFP-expressing A549-luc cells; Lane 6–7: two xenograft tumors derived from two mice implanted with dnCRTC-expressing A549-luc cells. (B) Similar western blotting analysis was performed as (a) except that H157-luc cells were used.
-
Figure 6—figure supplement 1—source data 1
Unedited immunoblots in A, B.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig6-figsupp1-data1-v2.pdf
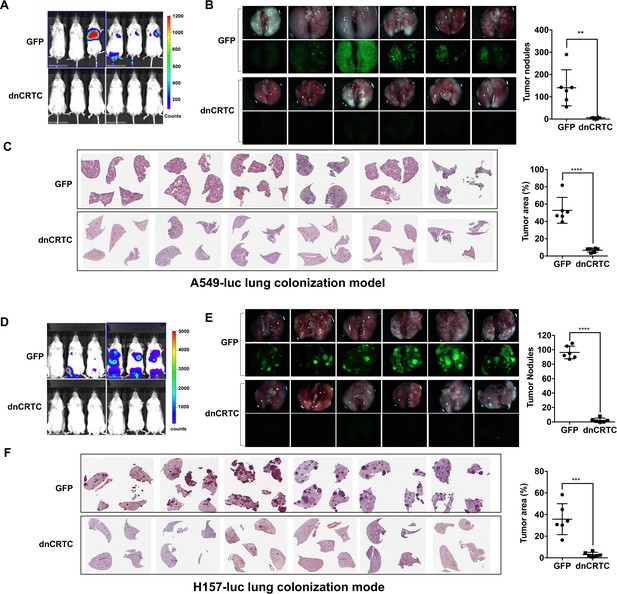
Expression of dnCRTC reduced lung colonization of LKB1-null lung cancer cells.
(A–C) Luciferase-expressing LKB1-null A549 cells (A549-luc) were transduced with retroviruses expressing GFP control or dnCRTC for 72 hr, and transduced cells (2 × 106 cells per mouse) were intravenously injected to NOD/SCID mice (n = 6 each). Eight weeks after injection, lung colonization was assessed by bioluminescent imaging (A). Lungs were dissected and bright field and GFP fluorescence images were shown (B). The number of surface tumor nodules with visible GFP signal per lung of each mouse was quantified and presented (right panel). Representative H and E staining of lung sections were shown (C). Tumor area was calculated from multiple H and E-stained lung sections from each mouse and presented as a percentage of tumor area to total lung area (right panel). (D–F) Luciferase-expressing LKB1-null H157 lung cancer cells (H157-luc) were transduced with retrovirus expressing GFP control or dnCRTC for 72 hr, and transduced cells (2 × 106 cells per mouse) were intravenously injected to NOD/SCID mice (n = 6 each). Four weeks after injection, lung colonization was assessed by bioluminescent imaging (D). Lungs were dissected and bright field and GFP fluorescence images were shown (E). The number of tumor nodules with visible GFP signal per lung of each mouse was quantified (right panel). Representative H and E staining images of lung sections were shown (F). Tumor area was calculated from multiple H and E-stained lung sections from each mouse and presented as a percentage of tumor area to total lung area (right panel). The p values were calculated by two-tailed student’s t-test (**p<0.01, ***p<0.001, ****p<0.0001).
-
Figure 7—source data 1
Numerical data for B, C, E, F.
- https://cdn.elifesciences.org/articles/66095/elife-66095-fig7-data1-v2.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Homo sapiens) | STK11 | GenBank | Gene ID: 6794 | This gene is commonly known as LKB1 in the field |
| Gene (Homo sapiens) | CRTC1 | GenBank | Gene ID: 23373 | |
| Gene (Homo sapiens) | CRTC2 | GenBank | Gene ID: 200186 | |
| Gene (Homo sapiens) | CRTC3 | GenBank | Gene ID: 64784 | |
| Gene (Homo sapiens) | NR4A2 | GenBank | Gene ID: 4929 | |
| Gene (Homo sapiens) | INSL4 | GenBank | Gene ID: 3641 | |
| Gene (Homo sapiens) | LINC00473 | GenBank | Gene ID: 90632 | |
| Gene (Homo sapiens) | PDE4D | GenBank | Gene ID: 5144 | |
| Cell line (Homo-sapiens) | A549 | ATCC | CCL-185 | |
| Cell line (Homo-sapiens) | H157 | ATCC | CRL-5802 | |
| Cell line (Homo-sapiens) | H322 | ATCC | CRL-5806 | |
| Cell line (Homo-sapiens) | H522 | ATCC | CRL-5810 | |
| Cell line (Homo-sapiens) | H2126 | ATCC | CCL-256 | |
| Cell line (Homo-sapiens) | H1819 | ATCC | CRL-5897 | |
| Cell line (Homo-sapiens) | H2087 | ATCC | CRL-5922 | |
| Cell line (Homo-sapiens) | H2009 | ATCC | CRL-5911 | |
| Cell line (Homo-sapiens) | H3123 | Frederic J Kaye lab | CVCL_Y295 PMID:11030152 | |
| Cell line (Homo-sapiens) | H23 | ATCC | CRL-5800 | |
| Cell line (Homo-sapiens) | H460 | ATCC | HTB-177 | |
| Cell line (Homo-sapiens) | H2122 | ATCC | CRL-5985 | |
| Cell line (Homo-sapiens) | H358 | ATCC | CRL-5807 | |
| Cell line (Homo-sapiens) | BEAS-2B | ATCC | CRL-9609 | |
| Cell line (M. musculus) | mLSCCLP | Francesco J DeMayo lab | PMID:31089135 | |
| Antibody | anti-CRTC1 (Rabbit Polyclonal) | Rockland Immunochemicals Inc | Cat: #600-401-936 | WB 1:1000 |
| Antibody | anti-CRTC1 (Rabbit Polyclonal) | Bethyl Laboratories | Cat: #A300-769A | ChIP 3 ug/ml |
| Antibody | anti-CRTC2 (Rabbit Polyclonal) | Bethyl Laboratories | Cat: #A300-637A | WB 1:1000 ChIP 3 ug/ml |
| Antibody | anti-CRTC3 (Rabbit Polyclonal) | Bethyl Laboratories | Cat: #A302-703A, | ChIP 3 ug/ml |
| Antibody | anti-CRTC3 (Rabbit monoclonal) | Cell Signaling Technology | Cat: #2720 | WB 1:1000 |
| Antibody | anti-LKB1 (Rabbit monoclonal) | Cell Signaling Technology | Cat: #3050 | WB 1:1000 |
| Antibody | anti-β-TUBULIN (Rabbit monoclonal) | Epitomics | Cat: #1878 | WB 1:2000 |
| Antibody | anti-HDCA1 (Rabbit Polyclonal) | Santa Cruz Biotechnology | Cat: #sc7872 | WB 1:2000 |
| Antibody | anti-β-ACTIN (Mouse monoclonal) | Sigma-Aldrich | Cat: #A5316 | WB 1:2000 |
| Recombinant DNA reagent | lentiCRISPR v2 (plasmid) | Addgene | Plasmid #52961 | |
| Recombinant DNA reagent | sgCtr- LentiCRISPRv2 (plasmid) | Addgene | Plasmid #107402 | |
| Recombinant DNA reagent | sgCRTC1- lentiCRISPR v2 (plasmid) | This paper | sgRNA sequence cloned into lentiCRISPR v2 | |
| Recombinant DNA reagent | sgCRTC2- lentiCRISPR v2 (plasmid) | This paper | sgRNA sequence clone into lentiCRISPR v2 | |
| Recombinant DNA reagent | sgCRTC3- lentiCRISPR v2 (plasmid) | This paper | sgRNA sequence clone into lentiCRISPR v2 | |
| Recombinant DNA reagent | pMSCV-GFP (plasmid) | Addgene | Plasmid #86537 | |
| Recombinant DNA reagent | pMSCV-dnCRTC (plasmid) | This paper | dnCRTC sequence cloned into pMSCV-GFP | |
| Recombinant DNA reagent | pcDNA FLAG TORC1 (plasmid) | Addgene | Plasmid #25718 | |
| Recombinant DNA reagent | pcDNA FLAG TORC2 (plasmid) | Addgene | Plasmid #22975 | |
| Recombinant DNA reagent | pcDNA FLAG TORC3 (plasmid) | Addgene | Plasmid #22976 | |
| Recombinant DNA reagent | lentiCas9-Blast (plasmid) | Addgene | Plasmid #52962 | |
| Recombinant DNA reagent | non-targeting control gRNA (plasmid) | Addgene | Plasmid #80180 | |
| Recombinant DNA reagent | STK11 gRNA-1 (plasmid) | Addgene | Plasmid #75912 | |
| Recombinant DNA reagent | STK11 gRNA-2 (plasmid) | Addgene | Plasmid #75913 | |
| Recombinant DNA reagent | pMD2.G (plasmid) | Addgene | Plasmid #12259 | Lentiviral Envelope |
| Recombinant DNA reagent | psPAX2 (plasmid) | Addgene | Plasmid #12260 | Lentiviral Packaging |
| Sequence-based reagent | CRTC1-qRT-F | This paper | qPCR primers | TGTCTCTCTGACCCCCTTCCAATCC |
| Sequence-based reagent | CRTC1-qRT-R | This paper | qPCR primers | GTCCGCGGGTGGTGAGAGGTA |
| Sequence-based reagent | CRTC2-qRT-F | This paper | qPCR primers | AGCCCCCTGAGTTTGCTCGC |
| Sequence-based reagent | CRTC2-qRT-R | This paper | qPCR primers | TGGGGGTAACCGCTGGTCAGT |
| Sequence-based reagent | CRTC3-qRT-F | This paper | qPCR primers | TGACCAGCAGTCCATGAGGCCA |
| Sequence-based reagent | CRTC3-qRT-R | This paper | qPCR primers | GGTCTTTGAACAGGCTGGTGCTGG |
| Sequence-based reagent | LINC00473-qRT-F | This paper | qPCR primers | AAACGCGAACGTGAGCCCCG |
| Sequence-based reagent | LINC00473-qRT-R | This paper | qPCR primers | CGCCATGCTCTGGCGCAGTT |
| Sequence-based reagent | FOS-qRT-F | This paper | qPCR primers | CACTCCAAGCGGAGACAG |
| Sequence-based reagent | FOS-qRT-R | This paper | qPCR primers | AGGTCATCAGGGATCTTGCAG |
| Sequence-based reagent | NR4A2-qRT-F | This paper | qPCR primers | GCCGGAGAGGTCGTTTGCCC |
| Sequence-based reagent | NR4A2-qRT-R | This paper | qPCR primers | AGGGTTCGCCTGGAACCTGGAA |
| Sequence-based reagent | INSL4-qRT-F | This paper | qPCR primers | GATGTGGTCCCCGATTTGGA |
| Sequence-based reagent | INSL4-qRT-R | This paper | qPCR primers | AGGTTGACACCATTTCTTTGGG |
| Sequence-based reagent | CPS1-qRT-F | This paper | qPCR primers | CTGATGCTGCCCACACAAAC |
| Sequence-based reagent | CPS1-qRT-R | This paper | qPCR primers | AGGGGAAGGATCGAGAAGCT |
| Sequence-based reagent | PDK4-qRT-F | This paper | qPCR primers | ACAGACAGGAAACCCAAGCC |
| Sequence-based reagent | PDK4-qRT-R | This paper | qPCR primers | GTTCAACTGTTGCCCGCATT |
| Sequence-based reagent | NR4A1-qRT-F | This paper | qPCR primers | GAGTCCCAGTGGCGGAGGCT |
| Sequence-based reagent | NR4A1-qRT-R | This paper | qPCR primers | CAGGCTGCACCCTACCCGGC |
| Sequence-based reagent | TM4SF20-qRT-F | This paper | qPCR primers | TCCAGGCTCTCTTAAAAGGTCC |
| Sequence-based reagent | TM4SF20-qRT-R | This paper | qPCR primers | ATGGTGTCGTTACTGGTGGG |
| Sequence-based reagent | NR4A3-qRT-F | This paper | qPCR primers | GAAGAGGGCAGCCCGGCAAG |
| Sequence-based reagent | NR4A3-qRT-R | This paper | qPCR primers | ACGCAGGGCATATCTGGAGGGT |
| Sequence-based reagent | PTGS2-qRT-F | This paper | qPCR primers | GTTCCCACCCATGTCAAAAC |
| Sequence-based reagent | PTGS2-qRT-R | This paper | qPCR primers | CCGGTGTTGAGCAGTTTTCT |
| Sequence-based reagent | SIK1-qRT-F | This paper | qPCR primers | AGCTTCTGAACCATCCACACA |
| Sequence-based reagent | SIK1-qRT-R | This paper | qPCR primers | TTTGCCAGAACTTCTTCCGC |
| Sequence-based reagent | PDE4B-qRT-F | This paper | qPCR primers | CCGATCGCATTCAGGTCCTTCGC |
| Sequence-based reagent | PDE4B-qRT-R | This paper | qPCR primers | TGCGGTCTGTCCATTGCCGA |
| Sequence-based reagent | PDE4D-qRT-F | This paper | qPCR primers | AACACATGAATCTACTGGCTGA |
| Sequence-based reagent | PDE4D-qRT-R | This paper | qPCR primers | TCACACATGGGGCTTATCTCC |
| Sequence-based reagent | GAPDH-qRT-F | This paper | qPCR primers | CAATGACCCCTTCATTGACC |
| Sequence-based reagent | GAPDH-qRT-R | This paper | qPCR primers | GACAAGCTTCCCGTTCTCAG |
| Sequence-based reagent | ID1-qRT-F | This paper | qPCR primers | TTCTCCAGCACGTCATCGAC |
| Sequence-based reagent | ID1-qRT-R | This paper | qPCR primers | CTTCAGCGACACAAGATGCG |
| Sequence-based reagent | LINC00473 promotor-qRT-F | This paper | qPCR primers | CTACAGACGTCATCGCCTCC |
| Sequence-based reagent | LINC00473 promotor-qRT-R | This paper | qPCR primers | CACATTTGGGGGTGCTTGTG |
| Sequence-based reagent | NR4A2 promoter-qRT-F | This paper | qPCR primers | GGGGAAAGTGAAGTGTCG |
| Sequence-based reagent | NR4A2 promoter-qRT-R | This paper | qPCR primers | CCGCGCTCGCTTTGGTAT |
| Sequence-based reagent | sgCRTC1-A | This paper | gRNA targets | TGGCGACTTCGAACAATCCG |
| Sequence-based reagent | sgCRTC1-B | This paper | gRNA targets | TTACCCGCGCGGCCCGCGTC |
| Sequence-based reagent | sgCRTC1-C | This paper | gRNA targets | CCCAGCCGAGGCCAGTACTA |
| Sequence-based reagent | sgCRTC2-A | This paper | gRNA targets | GCAGCGAGATCCTCGAAGAA |
| Sequence-based reagent | sgCRTC2-B | This paper | gRNA targets | AGGATATGTGGCGGGTGTAT |
| Sequence-based reagent | sgCRTC2-C | This paper | gRNA targets | ACAGGCCCAAAAACTGCGAC |
| Sequence-based reagent | sgCRTC3-A | This paper | gRNA targets | CTGACGCACTGCTCCGCAGC |
| Sequence-based reagent | sgCRTC3-B | This paper | gRNA targets | AAAAAGGATATTTGTCGCCC |
| Sequence-based reagent | sgCRTC3-C | This paper | gRNA targets | AACCCGCCATCACGGGCTGG |
| Sequence-based reagent | sg-Ctr | This paper | gRNA targets | CTTCCGCGGCCCGTTCAA |
| Commercial assay or kit | Bronchial Epithelial Cell Growth Medium kit | Lonza | Cat: # CC-4175 | BEAS-2B cell culture |
| Commercial assay or kit | Effectene Transfection Reagent | QIAGEN | Cat: #301425 | Transfection |
| Commercial assay or kit | RNeasy Mini Kit | QIAGEN | Cat: #74106 | RNA extraction |
| Commercial assay or kit | cDNA Reverse Transcription Kit | Applied Biosystems | Cat: #4368814 | |
| Commercial assay or kit | SYBR Green Supermix | Bio-Rad | Cat: #1725120 | |
| Commercial assay or kit | Alkaline Phosphatase, Calf Intestinal | New England BioLabs | Cat: #M0290 | |
| Commercial assay or kit | Nuclear and Cytoplasmic Extraction Reagents | Thermo Scientific | Cat: #78833 | |
| Commercial assay or kit | West Dura Extended Duration Substrate | Thermo Scientific | Cat: # 34076 | |
| Commercial assay or kit | GFP-Trap Magnetic Agarose | ChromoTek | Cat:# #gtma-10 | |
| Commercial assay or kit | VeriBlot for IP Detection Reagent | abcam | Cat:# ab131366 | |
| Commercial assay or kit | Dual-Luciferase Reporter Assay System | Promega | Cat:# E1910 | |
| Commercial assay or kit | FITC Annexin V Apoptosis Detection Kit | BD Bioscience | Cat: #556547 | |
| Chemical compound, drug | Hexadimethrine bromide | Sigma-Aldrich | Cat: # H9268 | polybrene |
| Chemical compound, drug | Puromycin Dihydrochloride | Gibco | Cat: #A1113803 | |
| Chemical compound, drug | Matrigel | Corning | Cat: #356231 | |
| Chemical compound, drug | D-Luciferin | PerkinElmer | Cat: #122799 | |
| Software, algorithm | GraphPad Prism 7 | GraphPad Prism | ||
| Software, algorithm | ImageJ software | ImageJ |
Additional files
-
Supplementary file 1
dnCRTC-reguated gene analysis and oligo sequences used in this study.
(a) Differentially expressed genes in dnCRTC-expressing A549 lung cancer cells in comparison with GFP-expressing control cells were shown. (b) GSEA analysis revealed that multiple oncogenic signatures were negatively associated with the dnCRTC-regulated target genes. (c) Primer and sgRNA sequences used in this study.
- https://cdn.elifesciences.org/articles/66095/elife-66095-supp1-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/66095/elife-66095-transrepform-v2.pdf





