Long-range migration of centrioles to the apical surface of the olfactory epithelium
Figures
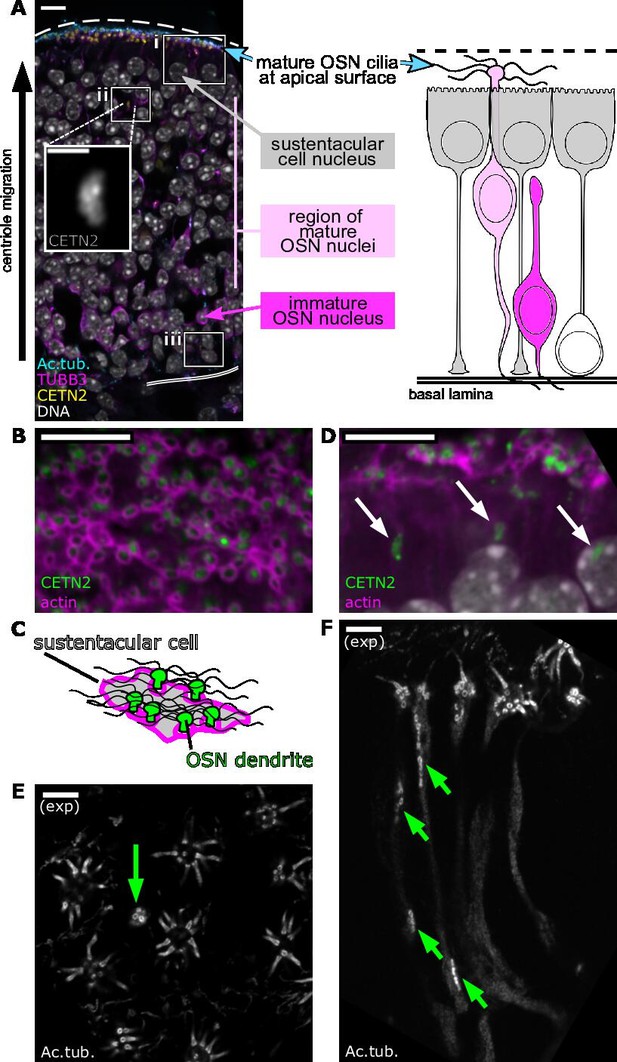
Centrioles in olfactory sensory neurons (OSNs) migrate tens of micrometers to the apical surface.
(A) Overview of the olfactory epithelium. Single-plane fluorescence image of a side view of the olfactory epithelium (left), corresponding to a schematic of cell types in the olfactory epithelium (right). Yellow: eGFP-centrin2; cyan: staining for acetylated tubulin, strongly marking olfactory cilia and, faintly, neuronal microtubules; magenta: staining for β-tubulin III, marking neuronal microtubules; white: DAPI, marking DNA. The apical surface is oriented at the top of this image and all subsequent side-view images. Dashed line: apical surface; double solid line: basal lamina. Boxes show the relative positions of critical stages of OSN differentiation: (i) the subapical compartment of the olfactory epithelium, defined as the space between the bottom of the sustentacular cell nuclei and the apical surface with olfactory cilia. (ii and inset) A group of centrioles migrating through the middle of the olfactory epithelium, below the sustentacular cell nuclei. (iii) A progenitor cell near the basal lamina. Scale bar = 10 μm. Inset scale bar = 2 μm. (B) Mature olfactory sensory neurons. Single-plane, en face fluorescence image of the apical surface of the olfactory epithelium. Green: eGFP-centrin2; magenta: dye-conjugated phalloidin, marking an enrichment of F-actin at the apical borders of sustentacular cells. Scale bar = 10 μm. (C) Schematic of (B) depicting a sustentacular cell wrapping around the dendrites of nearby OSNs. Green: OSN dendrite; gray: sustentacular cell cytoplasm; magenta: F-actin; wavy black lines: multiple OSN cilia. (D) Centriole migration in the olfactory epithelium. Single-plane fluorescence image of a side view of the subapical compartment of the olfactory epithelium. Green: eGFP-centrin2; magenta: dye-conjugated phalloidin; arrows: groups of migrating centrioles. Scale bar = 10 μm. (E) Mature olfactory sensory neurons, as imaged by expansion microscopy. Single-plane fluorescence image of the en face apical surface. White: staining for acetylated tubulin, marking centrioles, cilia, and, faintly, neuronal microtubules. Multiple cilia can be seen protruding from mature OSNs. A green arrow marks a dendrite with a group of migrating centrioles arriving at the apical tip. Scale bar = 2 μm. (F) Centriole migration, as imaged by expansion microscopy. Single-plane fluorescence image of a side view of the subapical compartment. White: staining for acetylated tubulin, marking centrioles, cilia, and, faintly, neuronal microtubules. Cilia can be seen at the apical surface, and green arrows mark groups of migrating centrioles. Scale bar = 2 μm.
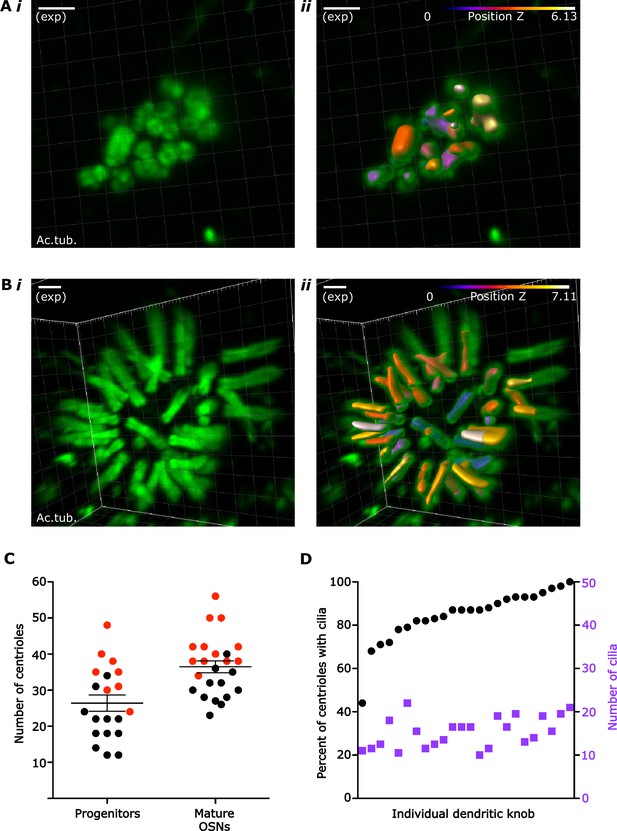
Centriole and cilium numbers in olfactory sensory neuron (OSN) progenitors and mature OSNs.
(A) Example of Imaris segmentation of centrioles in progenitor cells. (i) 3D image of centrioles in a progenitor cell. Green: staining for acetylated tubulin. (ii) The same image after segmentation in Imaris. Segmented objects are color-coded with a LUT to encode z-depth. After automated counting, each image was visually inspected, and counts were corrected where objects and centrioles or cilia did not correspond. Scale bar = 2 µm. (B) Example of Imaris segmentation of centrioles and cilia in mature OSNs. (i) 3D image of a mature OSN dendritic knob. Green: staining for acetylated tubulin. (ii) The same image after segmentation in Imaris. Segmented objects are color-coded with a LUT to encode z-depth. After automated counting, each image was visually inspected, and counts were corrected where objects and centrioles or cilia did not correspond. Scale bar = 2 µm. (C) Plot of the number of centrioles per cell in progenitor cells and mature OSNs. Red dots: sample 1, 3-month-old female. Black dots: sample 2, 1-month-old male. Mean and standard error of the mean (SEM) are graphed. In progenitors: mean = 26.40 centrioles, SEM = 2.234 and standard deviation = 9.992. In mature OSNs: mean = 36.46 centrioles, SEM = 1.665, and standard deviation = 8.156. (D) Plot of centriole and cilium numbers in mature OSNs. Black dots: percentage of centrioles that have cilia. Purple squares: number of cilia in each cell. Each dot and square in the same column belong to the same dendritic knob. Measurements were arranged along the x-axis in order of increasing percentage of centrioles with cilia. Mean: 85% of centrioles at a dendritic knob nucleated cilia (SEM = 2.47; standard deviation = 12.10). Mean cilia number per dendritic knob: 30.54 cilia (SEM = 1.455; standard deviation = 7.126).
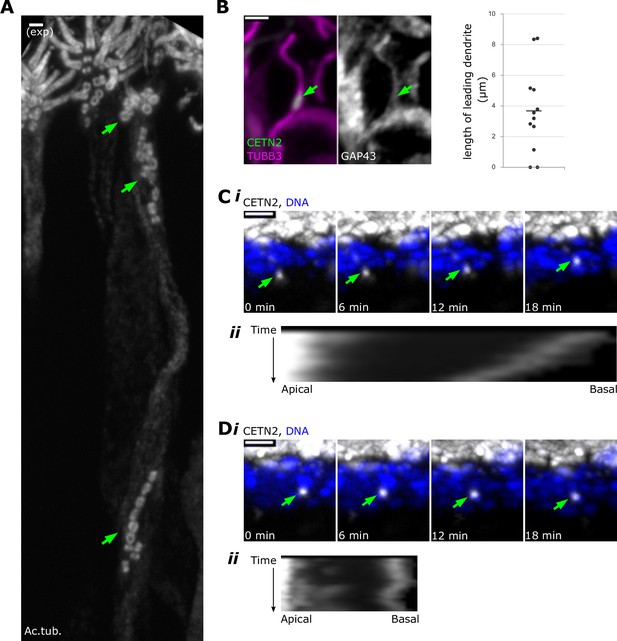
Centrioles migrate in multiple groups during dendrite elongation.
(A) Within a single dendrite, centrioles migrate in multiple groups. Expansion microscopy – side view of expanded olfactory epithelium. In all side-view images, apical is oriented toward the top of the image. White: staining for acetylated tubulin. Maximum z-projection of confocal stack. Mature olfactory sensory neurons (OSNs) have multiple cilia, which are visible at the apical surface. Green arrows: groups of centrioles migrating separately within the dendrite of a single OSN. Scale bar = 2 μm. (B) Centriole migration occurs concomitantly with dendrite elongation. Fluorescence image of a side view of the olfactory epithelium, maximum z-projection of confocal stack. In the leftmost image, magenta: staining for β-tubulin III; green: eGFP-centrin2. Middle image: staining for GAP43 in the same cell; arrows: a group of migrating centrioles. Scale bar = 2 μm. Rightmost image: a plot of lengths reflecting the distance from the centriole group to the end of the dendrite. (C) A centriole group migrates toward the apical surface. (i) Live time-lapse imaging of olfactory epithelium. Maximum x-projection image, showing a side view. White: eGFP-centrin2; blue: Hoechst, marking DNA in the most apical layer of nuclei, which are mostly sustentacular cells; green arrows: a group of centrioles moving toward the apical surface at 0.18 μm/min (fastest rate of all observed groups). See Figure 2—source data 1 for migration rates. See Figure 2—video 1 for original video. (ii) Kymograph illustrating migration of the centriole group. Apical and basal direction labels indicate orientation of the sample in the kymograph. (D) A centriole group with no net movement. (i) Live time-lapse imaging of olfactory epithelium, highlighting a different centriole group from the same acquisition as (C). Maximum x-projection image, showing a side view. White: eGFP-centrin2; blue: Hoechst; green arrows: a group of centrioles that have no net movement. See Figure 2—source data 1 for migration rates. See Figure 2—video 2 for original video. (ii) Kymograph illustrating a lack of total migration of the centriole group. Apical and basal direction labels indicate orientation of the sample in the kymograph.
-
Figure 2—source data 1
Migration rates of centriole groups from time-lapse imaging.
- https://cdn.elifesciences.org/articles/74399/elife-74399-fig2-data1-v2.xlsx
A centriole group migrates toward the apical surface.
Movie of the same centriole group as depicted in Figure 2C. Maximum x-projection image, showing a side view. White: eGFP-centrin2; blue: Hoechst, marking DNA in the most apical layer of nuclei, which are mostly sustentacular cells. Time between frames = 2 min.
A centriole group with no net movement.
Movie of the same centriole group as depicted in Figure 2D. Maximum x-projection image, showing a side view. White: eGFP-centrin2; blue: Hoechst. Time between frames = 2 min.
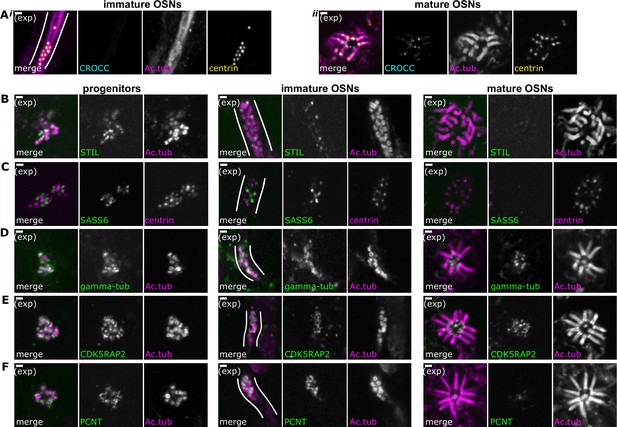
Composition of centriole groups during olfactory sensory neuron (OSN) differentiation.
(A) The cohesion fiber and striated rootlet protein rootletin is absent during centriole migration but is gained at the mature dendritic knob. Expansion microscopy, maximum z-projection of confocal stack. In all side-view images, apical is oriented toward the top of the image. Cyan: staining for Rootletin (CROCC). Magenta: staining for acetylated tubulin; yellow: staining for centrin. (i) Side view of migrating centrioles. Centrioles below the apical surface, in the dendrite of an immature OSN. (ii) Centrioles at the apical surface in the same sample as those shown in (Ai). Scale bars = 2 μm. (B–F) Expansion microscopy – single-plane fluorescence images of centrioles in progenitor cells (left column), immature OSNs with migrating centrioles (middle column, white lines outline an OSN dendrite), and mature OSNs imaged en face (right column). Scale bars = 2 μm. (B) The immature centriole protein STIL is present in progenitors and immature OSNs. Green: staining for STIL; magenta: staining for acetylated tubulin. (C) The immature centriole protein SASS6 is present in progenitors and immature OSNs. Green: staining for SASS6; magenta: staining for centrin. (D) The pericentriolar material protein gamma-tubulin is present throughout OSN differentiation. Green: staining for gamma-tubulin; magenta: staining for acetylated tubulin. (E) The pericentriolar material protein CDK5RAP2 is present throughout OSN differentiation. Green: staining for CDK5RAP2; magenta: staining for acetylated tubulin. (F) The pericentriolar material protein pericentrin (PCNT) is present in progenitors and immature OSNs. Green: staining for pericentrin (PCNT); magenta: staining for acetylated tubulin.
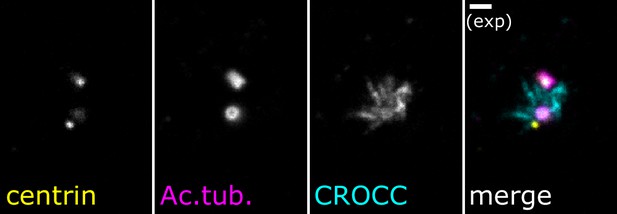
Expansion microscopy staining of rootletin in cycling cells.
Expansion microscopy – single-plane fluorescence image of a centrosome in expanded tissue culture cells (retinal pigment epithelium cells, RPE-1). Staining for rootletin (CROCC, cyan) demonstrates the appearance of cohesion fibers by expansion microscopy. Acetylated tubulin (magenta) marks the centriole barrel, and centrin (yellow) marks the centriole lumen. Scale bar = 2 µm.
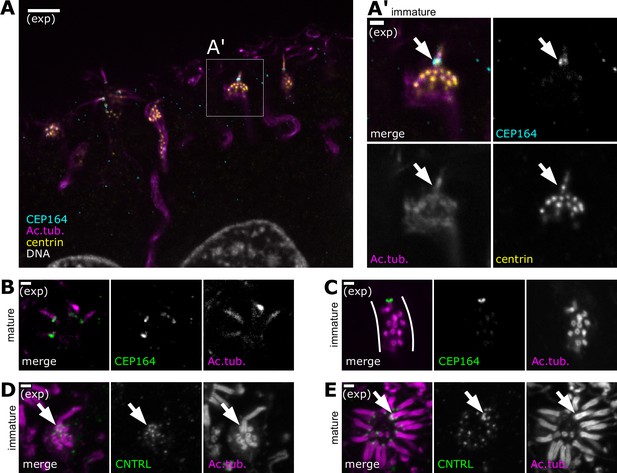
A single cilium forms prior to formation of multiple cilia.
(A) An olfactory sensory neuron (OSN) bearing a single cilium in the subapical compartment of the olfactory epithelium. Expansion microscopy – single-plane fluorescence image of a side view of the subapical compartment. In all side-view images, apical is oriented toward the top of the image. Cyan: staining for CEP164, marking the location of distal appendages; magenta: staining for acetylated tubulin, marking centrioles, cilia, and, faintly, neuron microtubules; yellow: staining for centrin, marking centrioles; white: DAPI, marking DNA. A box marks the location of the inset (A′). Scale bar = 10 μm. (A′) Immature OSN with a single cilium, inset from (A). Magenta: staining for acetylated tubulin; yellow: staining for centrin; cyan: staining for CEP164, marking distal appendages at the base of the cilium; arrow: Cep164 is only found at the base of the cilium, and not present on other centrioles within the dendrite. Scale bar = 2 μm. (B) During centriole migration in immature OSNs, a single centriole bears Cep164. Expansion microscopy – single-plane fluorescence image of migrating centrioles in a side view of the olfactory epithelium. Green: staining for CEP164; magenta: staining for acetylated tubulin. Scale bar = 2 μm. (C) In mature OSNs, multiple cilia bear Cep164. Expansion microscopy – single-plane fluorescence image of a mature OSN with multiple cilia. Green: staining for CEP164, marking distal appendages at the base of cilia; magenta: staining for acetylated tubulin. Scale bar = 2 μm. (D) In OSNs bearing a single cilium, the centriole at the base of the cilium is surrounded by the subdistal appendage/basal foot marker centriolin. Expansion microscopy – maximum z-projection of a confocal stack. Magenta: staining for acetylated tubulin; green: staining for centriolin, marking subdistal appendages on the mother centriole at the base of the cilium. Scale bar = 2 μm. (E) In mature OSNs, a single cilium is surrounded by centriolin. Expansion microscopy – mature OSN imaged en face, maximum z-projection of a confocal stack. Magenta: staining for acetylated tubulin; green: staining for centriolin; arrow: centriolin surrounds the base of one cilium. Other cilia of the same dendrite are only associated with one centriolin punctum. Scale bar = 2 μm.
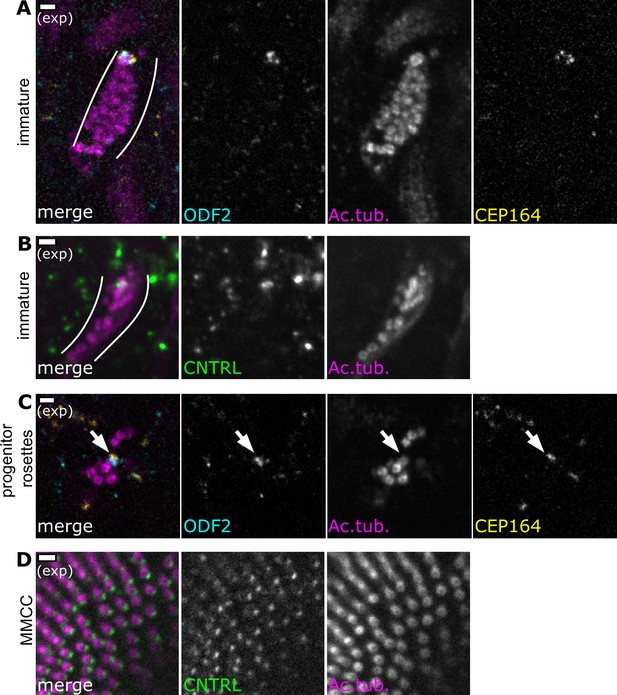
Centriole maturation in progenitors and migrating centriole groups.
(A) During centriole migration in immature olfactory sensory neurons (OSNs), a single centriole bears distal and subdistal appendages. Expansion microscopy – single-plane fluorescence image of centrioles below the apical surface in a side view of the expanded olfactory epithelium. Cyan: staining for ODF2, marking the base of subdistal and distal appendages; yellow: staining for Cep164, marking distal appendages; magenta: staining for acetylated tubulin. Within the group, a single centriole bears both ODF2 and Cep164. Scale bar = 2 μm. (B) During centriole migration in immature OSNs, a single centriole bears centriolin. Expansion microscopy – single-plane fluorescence image of centrioles below the apical surface in expanded olfactory epithelium. Green: staining for centriolin; magenta: staining for acetylated tubulin. Scale bar = 2 μm. (C) In progenitor cells, a single centriole bears distal and subdistal appendages. Expansion microscopy – single-plane fluorescence image of centriole rosettes in expanded olfactory epithelium. Cyan: staining for ODF2; yellow: staining for CEP164; magenta: staining for acetylated tubulin; arrow: subdistal appendages and distal appendages are found on a single centriole. Scale bar = 2 μm. (D) In cells with multiple motile cilia (MMCCs), centriolin is found in a single puncta corresponding to the basal foot. Expansion microscopy – single-plane fluorescence image of an MMCC imaged en face in expanded nasal epithelium. Green: staining for centriolin, marking a basal foot on each centriole; magenta: staining for acetylated tubulin. Scale bars = 2 µm.
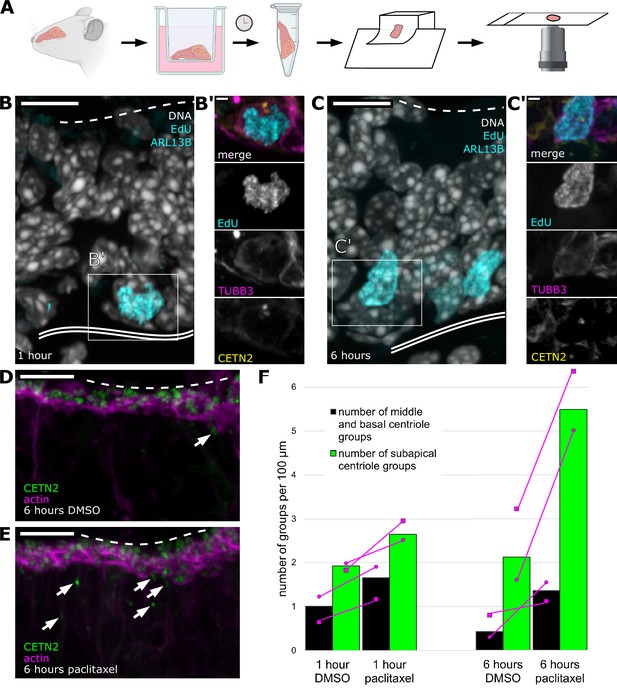
Centriole progression toward the apical surface can be altered by stabilizing microtubules.
(A) Schematic: workflow from sample collection through imaging of olfactory epithelium explants. Septal olfactory epithelium was taken from adult mice expressing eGFP-centrin2 and Arl13b-mCherry and plated on transwell filters with an air-liquid interface. After 1 or 6 hr, samples were fixed and processed for sectioning. Stained sections were analyzed by confocal microscopy. Figure created with BioRender.com. (B) Progenitor cells synthesize DNA by 1 hr ex vivo. Fluorescence image of an olfactory epithelium explant grown in EdU for 1 hr, maximum intensity projection. Gray: DAPI, marking all nuclei; cyan: EdU, conjugated to dye after fixation, marks cells that synthesized DNA ex vivo; dashed line: the apical surface; double solid line: basal lamina; box: location of the inset (B′). Scale bar = 10 μm. (B′) Inset from (B) showing a progenitor cell positive for EdU. Magenta: staining for β-tubulin III, showing that the cell is neuronally fated; yellow: eGFP-centrin 2, showing signal consistent with centriole amplification; cyan: EdU. Scale bar = 2 μm. (C) More cells synthesize DNA by 6 hr ex vivo. Fluorescence image of an olfactory epithelium explant grown in EdU for 6 hr, maximum intensity projection. Tissue was taken from the same animal as that shown in (B). Gray: DAPI, marking all nuclei; cyan: EdU, conjugated to dye after fixation, shows an increased number of cells that have synthesized DNA ex vivo, compared to 1 hr treatment. Box: location of the inset (C′). Scale bar = 10 μm. (C′) Inset from (C) showing a progenitor cell positive for EdU. Magenta: staining for β-tubulin III, showing that the cell is neuronally fated; yellow: eGFP-centrin 2 shows signal consistent with centriole amplification; cyan: EdU. Scale bar = 2 μm. (D) Control image of centriole group position in explants treated with DMSO for 6 hr. Single-plane fluorescence image. Green: eGFP-centrin2; magenta: dye-conjugated phalloidin; dashed line: apical surface; arrows: migrating centriole groups. Scale bar = 10 μm. (E) Centriole group position in explants treated with paclitaxel for 6 hr. Single-plane fluorescence image. Tissue was taken from the same animal as that shown in (D). Green: eGFP-centrin 2; magenta: dye-conjugated phalloidin; arrows: migrating centriole groups. Compared to (D), many centrioles are found below the apical surface. Dashed line: apical surface. Scale bar = 10 μm. (F) Bar plot summarizing centriole group position in explants grown for 1 or 6 hr in the presence of DMSO or paclitaxel. Green bars: migrating centriole groups in the subapical compartment of the epithelium (apical surface through sustentacular cell nuclei); black bars: centriole groups in the middle and basal regions of the epithelium (between sustentacular cell nuclei and basal lamina); magenta circles: normalized number of groups for the female sample; magenta squares: normalized number of groups for the male sample. Magenta lines connect paired samples. Counts were normalized to the lateral length of the basal lamina. Length of epithelium scored for each time point: 1 hr DMSO = 988.17 μm, 1 hr paclitaxel = 905.37 μm, 6 hr DMSO = 1126.44 μm, 6 hr paclitaxel = 1019.76 μm. Number of centriole groups counted: 1 hr DMSO = 29, 1 hr paclitaxel = 39, 6 hr DMSO = 29, 6 hr paclitaxel = 70. N = 2 animals. See Figure 5—source data 1 for values.
-
Figure 5—source data 1
Counts of centriole groups - paclitaxel treatment.
- https://cdn.elifesciences.org/articles/74399/elife-74399-fig5-data1-v2.xlsx
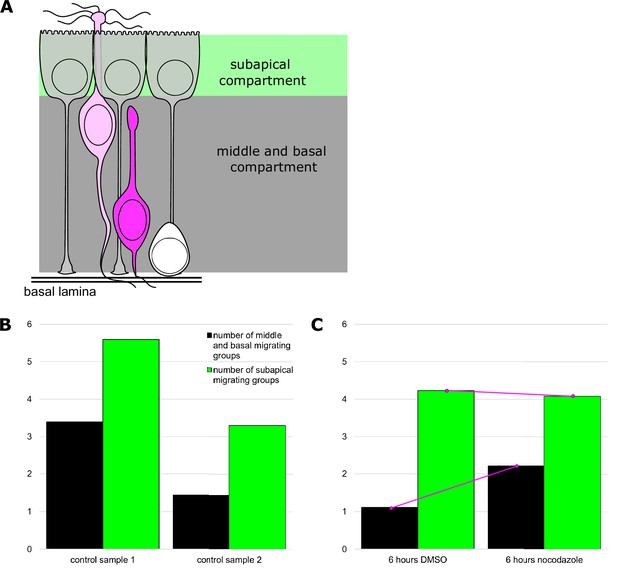
Migrating centriole groups in olfactory epithelium explants.
(A) Schematic diagram showing the locations of the subapical and middle/basal regions. Cilia are located in the apical region (above green region). The subapical compartment is located between the apical region and the bottom of the sustentacular cell nucleus (green). The middle/basal compartment is located below the sustentacular cell nucleus (gray). (B) Bar plot summarizing variability in the number of migrating centriole groups in untreated samples prior to placement on filters. Green bars: migrating centriole groups in the subapical compartment of the epithelium (apical surface through sustentacular cell nuclei); black bars: centriole groups in the middle and basal regions of the epithelium (between sustentacular cell nuclei and basal lamina). Counts were normalized to the lateral length of the basal lamina. Length of epithelium scored: sample 1 = 2413.24 μm, sample 2 = 1666.99 μm. Number of centriole groups counted: sample 1 = 217, sample 2 = 79. See Figure 5—figure supplement 1—source data 1 for values. (C) Bar plot summarizing centriole group position in explants grown for 6 hr in the presence of DMSO or nocodazole. Bar colors match those in (A). Counts were normalized to the lateral length of the basal lamina. Length of epithelium scored: 6 hr DMSO = 1984.36 μm, 6 hr nocodazole = 1619.15 μm. Number of centriole groups counted: 6 hr DMSO = 106, 6 hr nocodazole = 102. Bars are connected by lines to reflect that samples were paired, N = 1 animal. See Figure 5—figure supplement 1—source data 2 for values.
-
Figure 5—figure supplement 1—source data 1
Counts of centriole groups - explants prior to treatment.
- https://cdn.elifesciences.org/articles/74399/elife-74399-fig5-figsupp1-data1-v2.xlsx
-
Figure 5—figure supplement 1—source data 2
Counts of centriole groups - nocodazole treatment.
- https://cdn.elifesciences.org/articles/74399/elife-74399-fig5-figsupp1-data2-v2.xlsx
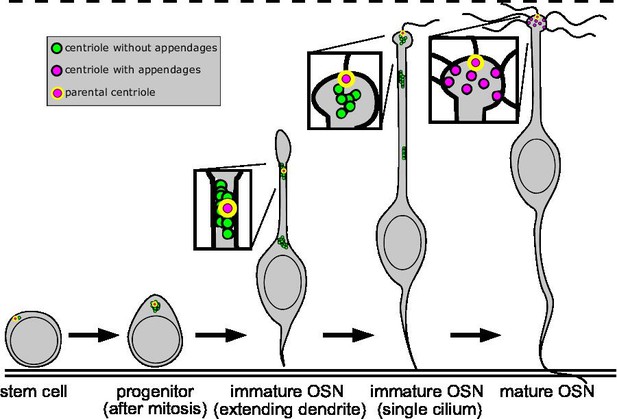
Summary of centriole migration and maturation during olfactory sensory neuron (OSN) differentiation.
Diagram summarizing the migration and maturation of centrioles over the course of OSN differentiation. A double solid line marks the basal lamina, and a dashed line is drawn above the apical surface. Newly formed centrioles lacking appendages are represented as green dots, centrioles with appendages as magenta dots, and the parental centriole as a magenta dot with a yellow outline. After centriole amplification and mitosis in the progenitor, the immature OSN extends a dendrite toward the apical surface. Centrioles migrate primarily in groups during dendrite elongation. At the apical surface, a single cilium forms from the appendage-bearing, parental centriole, before multiple cilia form in the mature OSN.
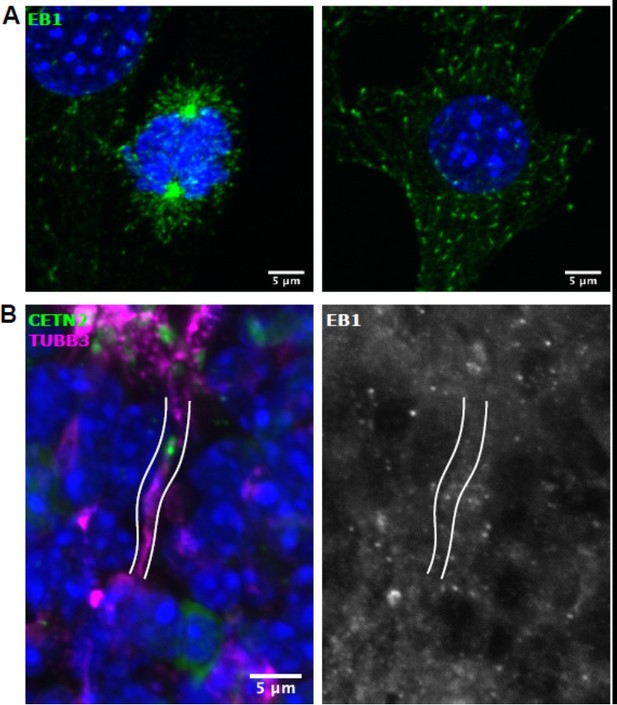
EB1 staining in cultured NIH3T3 cells and olfactory epithelium.
(A) Left: Mitotic 3T3 cell. Right: Interphase 3T3 cell. EB1 (green) and DAPI (blue). Both images are maximum z-projections of confocal stacks. (B) Olfactory epithelium. EB1 (white), β- tubulin III (magenta), eGFP-centrin2 (green), and DAPI (blue). The dendrite is outlined in white. Images are maximum projections of confocal stacks.
Tables
Antibodies used in this study.
| Target | Source | Dilution |
|---|---|---|
| Acetylated tubulin | Sigma-Aldrich, clone 6-11B-1, RRID:AB_477585 | 1:1000 |
| CDK5RAP2 | Millipore, 06-1398 rabbit polyclonal, RRID:AB_11203651 | 1:200 |
| Centrin | EMD Millipore, clone 20H5, RRID:AB_10563501 | 1:1000 |
| CEP164 | Rabbit polyclonal previously described in Lee et al., 2014 | 1:500 |
| GFP | Rabbit antibody previously described in Hatch et al., 2010 | 1:2000 |
| GAP43 | Novus Biologicals, NB300, rabbit polyclonal, RRID:AB_921392 | 1:250 (Blocking buffer: PBSBT. 2% BSA, 0.1% Triton X-100, 1x PBS) |
| Pericentrin | BD Biosciences, clone 30, RRID:AB_399294 | 1:500 |
| Centriolin | Santa Cruz Biotech, clone C-9, RRID: AB_10851483 | 1:50 |
| STIL | Abcam, rabbit polyclonal, RRID:AB_2197878 | 1:1,000 |
| SASS6 | Santa Cruz Biotech, RRID:AB_1128357 | 1:200 |
| ODF2 | Novus Biologicals, mouse IgG2a, RRID:AB_1146453 | 1:200 |
| Rootletin (CROCC) | Santa Cruz Biotech, clone C-2, RRID:AB_10918081 | 1:100–200 |
| β-Tubulin III | BioLegend, clone TuJ1, RRID:AB_2313773 | 1:1000–2000 |
| Gamma-tubulin | Sigma-Aldrich, clone GTU88, RRID:AB_532292 | 1:1000 |





