Endurance exercise ameliorates phenotypes in Drosophila models of spinocerebellar ataxias
Abstract
Endurance exercise is a potent intervention with widespread benefits proven to reduce disease incidence and impact across species. While endurance exercise supports neural plasticity, enhanced memory, and reduced neurodegeneration, less is known about the effect of chronic exercise on the progression of movement disorders such as ataxias. Here, we focused on three different types of ataxias, spinocerebellar ataxias type (SCAs) 2, 3, and 6, belonging to the polyglutamine (polyQ) family of neurodegenerative disorders. In Drosophila models of these SCAs, flies progressively lose motor function. In this study, we observe marked protection of speed and endurance in exercised SCA2 flies and modest protection in exercised SCA6 models, with no benefit to SCA3 flies. Causative protein levels are reduced in SCA2 flies after chronic exercise, but not in SCA3 models, linking protein levels to exercise-based benefits. Further mechanistic investigation indicates that the exercise-inducible protein, Sestrin (Sesn), suppresses mobility decline and improves early death in SCA2 flies, even without exercise, coincident with disease protein level reduction and increased autophagic flux. These improvements partially depend on previously established functions of Sesn that reduce oxidative damage and modulate mTOR activity. Our study suggests differential responses of polyQ SCAs to exercise, highlighting the potential for more extensive application of exercise-based therapies in the prevention of polyQ neurodegeneration. Defining the mechanisms by which endurance exercise suppresses polyQ SCAs will open the door for more effective treatment for these diseases.
Editor's evaluation
In this strong study, the investigators examine the therapeutic ability of endurance exercise to disease in Drosophila models of the spinocerebellar ataxias types (SCAs) 2, 3, and 6. Results support that exercise induced improvements rely on Sesn and perhaps its role in regulating autophagy.
https://doi.org/10.7554/eLife.75389.sa0Introduction
Nine inherited neurodegenerative disorders are caused by expansion of a CAG triplet repeat in the protein-coding region of the respective disease genes. The CAG repeat encodes the amino acid glutamine; thus, the disease protein carries a lengthened tract of polyglutamine (polyQ) residues that renders it toxic. The polyQ family includes six spinocerebellar ataxias (SCAs 1, 2, 3, 6, 7, and 17), Huntington’s disease (HD), dentatorubral-pallidoluysian atrophy, and Kennedy’s disease (also known as spinal-bulbar muscular atrophy, or SBMA) (Paulson et al., 1997; Zoghbi and Orr, 2000; La Spada and Taylor, 2003; Todi et al., 2007; Zoghbi and Orr, 2009; Paulson et al., 2017; Lieberman et al., 2019; Buijsen et al., 2019). Various pathogenic mechanisms are shared, involving primarily toxic gain-of-function acquired by the disease protein. These mutations yield aggregation of the toxic polyQ protein and, despite their wide expression throughout the body, only select central nervous system regions degenerate in each disease. Other common mechanisms underlying polyQ pathogenesis include mitochondrial, transcriptional, and autophagic dysfunction (Sullivan et al., 2019). Although the disorders share disease mechanisms, the individual host proteins have divergent functions (Zoghbi and Orr, 2000; La Spada and Taylor, 2003; Todi et al., 2007; Zoghbi and Orr, 2009; Paulson et al., 2017; Lieberman et al., 2019; Buijsen et al., 2019).
There is an unmet need for the treatment of polyQ diseases as there is currently no therapeutic solution for any of these disorders. Here, we examined the idea that daily endurance exercise might be of benefit against polyQ disorders. Endurance exercise is a low-cost, effective intervention with broad pro-healthspan effects, including reducing the incidence of obesity, heart disease, cancer, and cognitive defects (Booth et al., 2012; Wilmot et al., 2012; Strasser, 2013). Previous studies examined to a limited extent the effects of chronic exercise in patients with advanced stages of ataxia, including polyQ disorders: some types of exercise might help in certain cases (Chang et al., 2015; Schatton et al., 2017; de Oliveira et al., 2018) but not others (D’Abreu et al., 2010; Wang et al., 2018). These studies may have been limited by lack of normalized protocols, genetics, and variable disease stage among patients. Some work on the possible role of exercise was also conducted in mouse models of HD, SBMA, and SCA1. SCA1 and SBMA mice seemed to benefit from exercise (Fryer et al., 2011; Chuang et al., 2019; Chua et al., 2014; Giorgetti et al., 2016; Sujkowski et al., 2022), whereas some models of HD improved (Herbst and Holloway, 2015; Ji et al., 2015) while others did not (Corrochano et al., 2018).
Differences in these investigations could be due to variation in protocols, genetic background, age of testing, and other parameters difficult to normalize among studies. While studies in animal models have identified potential pathways by which exercise may affect polyQ phenotypes, absence of normalization may limit the applicability and extension of those results to other polyQ diseases and further up the evolutionary chain. Here, we take advantage of the Drosophila model system to systematically examine which polyQ disorders are responsive to exercise and to identify mechanisms involved.
Drosophila melanogaster has been widely and successfully utilized to understand polyQ disease mechanisms (Bonini and Fortini, 2003; Perrimon et al., 2016; Pandey and Rajamma, 2018; Ueyama and Nagai, 2018). In parallel, the fly has emerged as a highly efficient model for studying long-term effects of chronic exercise (Sujkowski and Wessells, 2018). Following a 3 week daily, gradually ramped training program, flies of diverse genotypes experience reproducible changes to speed, endurance, flight, cardiac performance, mitophagy, and mitochondrial function (Piazza et al., 2009; Sujkowski et al., 2012; Laker et al., 2014; Sujkowski et al., 2015; Damschroder et al., 2018b; Sujkowski et al., 2019). These changes track with adaptive physiological changes seen in chronically exercising humans (Sujkowski and Wessells, 2018; Wessells et al., 2018), and induce conserved changes in gene expression, including conserved mediators of exercise benefits, such as peroxisome proliferator-activated receptor γ coactivator 1-α(PGC-1α) and Sestrin (Sesn) (Tinkerhess et al., 2012b; Kim et al., 2020).
Here, we tested the effects of chronic exercise in three Drosophila models of polyQ SCAs: 2, 3, and 6. Ectopic expression of these human, polyQ-expanded disease proteins in flies is toxic (Tsou et al., 2013; Tsou et al., 2015a; Tsou et al., 2015b; Costa et al., 2016; Tsou et al., 2016; Sutton et al., 2017; Ristic et al., 2018). We find that exercise has a dramatically positive effect in SCA2 flies, with modest effects on SCA6 model flies, and no benefit to SCA3 flies, indicating a range of response in different disease models. In addition, the exercise-mimicking protein, Sesn, improves early death and rescues mobility declines in SCA2 model flies. We further find that improvement in disease phenotypes depends on functions of Sesn that reduce oxidative damage and modulate the mechanistic target of rapamycin (mTOR). Our observations identify Sesn as a mechanistic target that can be leveraged toward therapeutic options for patients unable to exercise, or to supplement the benefits of those who can.
Results
Exercise differentially impacts mobility in Drosophila polyQ SCA models
Expression of polyQ disease proteins in flies causes reduced motility and neuronal cell death, as we and others have shown in various publications (Bonini and Fortini, 2003; Perrimon et al., 2016; Pandey and Rajamma, 2018; Ueyama and Nagai, 2018; Tsou et al., 2015a; Sutton et al., 2017; Ristic et al., 2018; Jackson et al., 1998; Fernandez-Funez et al., 2000; Romero et al., 2008; Hsu et al., 2014; Rosas-Arellano et al., 2018). To test if endurance exercise reduces polyQ-dependent phenotypes in the fly, we selected three SCAs: 2, 3, and 6 (Figure 1A). Full-length, human disease protein was expressed through the binary, RU486-inducible Gal4-UAS system (Sujkowski et al., 2015; Brand et al., 1994) in all fly neurons and only during adulthood, which is toxic (Tsou et al., 2013; Tsou et al., 2015a; Tsou et al., 2015b; Costa et al., 2016; Tsou et al., 2016; Sutton et al., 2017; Ristic et al., 2018). We have established an optimized exercise protocol (Figure 1B) that drives reproducible effects that are robust across genotypes (Piazza et al., 2009). These effects are not due to non-specific stress or behavioral effect of the regimen—unexercised flies exposed to training, but prevented from running, do not experience improvements (Piazza et al., 2009).
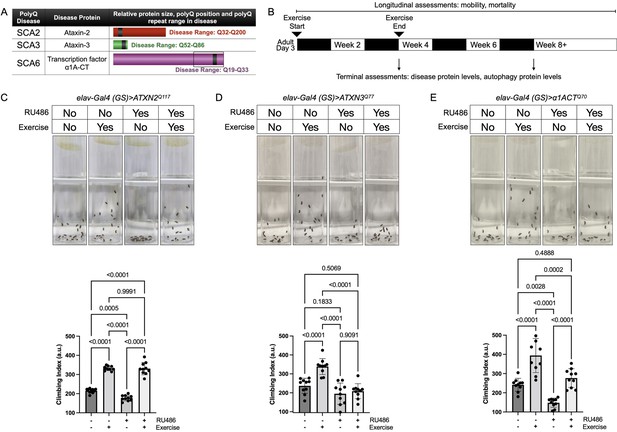
Endurance exercise differentially affects mobility in Drosophila models of spinocerebellar ataxia.
(A) Spinocerebellar ataxia (SCA) models used in this study. Box in SCA6 highlights the transcription factor, β1A-CT that is encoded through an internal ribosomal entry site of the β1A transcript. It is this transcript that we utilized for SCA6 model flies. (B) Timeline of endurance exercise program and assessment of physiology and disease protein levels. (C–E) Representative climbing speed images (upper panels) in Drosophila models of (C) SCA2, (D) SCA3 and (E) SCA6. Photos taken 2 s after inducing negative geotaxis response in 4-week-old flies, following endurance exercise completion. Bottom panels: quantification by ANOVA with Tukey’s post-hoc for significantly different groups; means ± SD. n = 10. Each individual datum depicts average week four climbing speed in arbitrary units (a.u.) for five vials of 20 flies.
Exercised wild-type flies retained faster climbing speed than age-matched, unexercised siblings (Sujkowski and Wessells, 2018). SCA2 model flies expressing polyQ-expanded ATXN2 specifically in adult neurons (elav-Gal4 (GS)> ATXN2Q117) had reduced climbing speed by 4 weeks of age, but exercise fully rescued climbing speed to the level of age-matched, uninduced control flies that complete the three-week, ramped exercise program (Figure 1C, Video 1). In contrast, four-week-old SCA3 model flies (elav-Gal4 (GS)> ATXN3Q77 RU+) did not increase climbing speed after exercise training (Figure 1D). Flies expressing polyQ-expanded β1ACT (elav-Gal4 (GS)> β1ACTQ70), the disease gene in SCA6 (Tsou et al., 2016), had reduced week-four climbing speed in comparison to uninduced, unexercised control flies, but climbing was partially rescued after exercise training (Figure 1E).
SCA2 model flies show improved motility with exercise.
Video of 4-week-old uninduced control and SCA2 model flies taken after 3 weeks of ramped endurance exercise. Order of vials depicted, left to right: Vial 1-uninduced, exercised control, Vials 2,3-SCA2 model, exercised, Vials 4,5-SCA2 model, unexercised.
Neurodegenerative severity of polyQ disorders tends to increase with age (Zoghbi and Orr, 2000; La Spada and Taylor, 2003). We have previously shown that exercise-induced improvements to climbing speed and endurance are long-lasting and persist even after the three-week exercise program is complete (Piazza et al., 2009). Neither RU486 feeding, nor expression of the empty vector used to generate transgenic flies in adult neurons, altered exercise adaptations to either climbing speed or endurance. Furthermore, expression of an isolated polyQ80 repeat (Johnson et al., 2020) in adult neurons reduced climbing speed and abrogated exercise benefits, while age-matched, uninduced control flies adapted to exercise (Figure 1—figure supplement 1).
Having confirmed that post-exercise differences in climbing speed can be discerned in polyQ model flies, we next subjected the same three SCA models from Figure 1 to our endurance exercise program and tested them longitudinally for changes to climbing speed and endurance. In contrast to the ramped exercise program, endurance is tested by placing flies on the climbing apparatus on non-training days and allowing them to climb to exhaustion. When fewer than 20% of flies in a vial no longer respond to the climbing stimulus, the time is recorded, and the vial is removed from the apparatus and scored as fatigued. These data are plotted similarly to a survival curve, with each datum representing a single vial of 20 flies (Tinkerhess et al., 2012a).
Spinocerebellar ataxias type 2 model flies had reduced climbing speed by adult week four (Figure 2A, compare RU- UN to RU+ UN), but endurance exercise fully rescued climbing speed to the level of exercised, uninduced control flies, and improvement persisted into adult week five (Figure 2A, compare RU- EX to RU+ EX). Baseline endurance in SCA2 flies was similar to uninduced, unexercised siblings (log-rank, P = 0.2018) and exercised SCA2 flies improved endurance as well as exercised, uninduced controls (Figure 2B). We next examined ATXN2 protein levels to see if improved mobility correlated with changes in toxic protein levels. Exercise markedly reduced ATXN2 levels relative to age-matched, unexercised siblings, linking improved physiology to reductions in disease protein (Figure 2C, I).
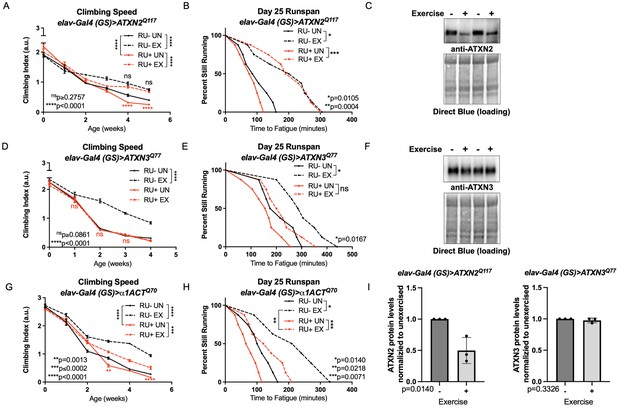
Endurance exercise differentially affects mobility and disease protein levels in Drosophila models of SCA.
(A) Flies ectopically expressing polyQ-expanded ATXN2 in adult neurons (RU+ UN) have lower climbing speed than unexercised, uninduced control flies (RU- UN) by adult week 4. Exercise fully rescues climbing speed to the level of exercised, uninduced control flies (compare RU- EX to RU+ EX). (B) Flies expressing polyQ-expanded ATXN2 in adult neurons (RU+) have similar endurance to uninduced control flies (RU-) whether exercised or not. (C) Exercise reduces ATXN2 protein levels in flies ectopically expressing polyQ-expanded ATXN2 in adult neurons, quantified in (I). (D, E) Flies ectopically expressing polyQ-expanded ATXN3 in adult neurons (RU+) have similar (D) climbing speed and endurance to uninduced, unexercised control flies and fail to improve either (D) climbing speed (E) endurance with exercise. (F) Exercise does not affect ATXN3 protein levels in flies expressing CAG-expanded ATXN3 in adult neurons, quantified in (I). (G) Flies ectopically expressing CAG-expanded β ACT in adult neurons (RU+) have lower climbing speed than unexercised, uninduced control flies (RU- UN) by adult week 3, and exercise partially rescues climbing speed, although not to the level of exercised, uninduced control flies. (H) Exercise improves endurance in flies expressing CAG-expandedα1ACT in adult neurons (RU+ EX), but not to the level of exercised, uninduced control siblings (compare RU- EX to RU+ EX). Mobility and endurance data are presented as representative experiments from triplicate biological replicates. Each individual datum depicts average climbing speed for ≥5 vials of 20 flies, analyzed by 2-way ANOVA (climbing speed), or time for 80% of flies in an individual vial (n ≥ 8 vials of 20 flies) to reach exhaustion, analyzed by log rank (endurance). Representative Western blots (five flies/lysate) from three biological repetitions, analyzed by ANOVA with Tukey’s post-hoc for significantly different groups. Error bars indicate ± SD.
-
Figure 2—source data 1
Uncropped, unedited blots from Figure 2C.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig2-data1-v4.zip
-
Figure 2—source data 2
Uncropped, unedited blots from Figure 2F.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig2-data2-v4.zip
In contrast, SCA3 model flies did not improve climbing speed or endurance (Figure 2D, E) after exercise training. Furthermore, exercised and unexercised SCA3 flies had similar levels of ATXN3, the causative, polyQ-expanded disease protein in SCA3 (D’Abreu et al., 2010; Figure 2F, I). SCA6 model flies had reduced climbing speed at weeks three and five compared to unexercised, uninduced siblings, and exercise training improved climbing speed, albeit not to the level of uninduced, exercised control flies (Figure 2G). Unexercised SCA6 flies trended toward lower endurance relative to uninduced, unexercised control flies (log-rank, P = 0.0559) and improved endurance after exercise training, but did not equal the endurance of uninduced, exercised controls (log-rank, P = 0.0218) (Figure 2H).
Sestrin expression improves phenotypes in SCA2 model flies without exercise
We have previously shown that the stress-inducible protein, Sesn, is necessary for exercise adaptations in multiple species (Kim et al., 2020), and Sesn activity has been proposed to play a protective role against neurodegeneration (Chen et al., 2019). Both endurance exercise and Sesn increase autophagy by inhibiting mTOR (Sujkowski et al., 2015; Mahalakshmi et al., 2020; Lee et al., 2010), and increased autophagy enhances ATXN2 solubility and reduces phenotypes in SCA2 patient cells (Wardman et al., 2020). Since muscle-specific dSesn overexpression is sufficient to replicate the beneficial adaptations of endurance exercise, even in sedentary Drosophila (Kim et al., 2020), we next overexpressed dSesn in SCA2 model flies.
Longevity assessment in SCA model flies expressing polyQ-expanded disease protein selectively in adult neurons confirmed previously reported lifespan reductions in both SCA3 and SCA6 model flies (Tsou et al., 2016; Johnson et al., 2019; Figure 3B, C), but neither ATXN2 expression nor exercise significantly affected longevity in SCA2 flies (Figure 3A). CAG expansion in ATXN2, a widely expressed gene, causes early mortality in SCA2 patients (Scoles and Pulst, 2018). We performed subsequent experiments in flies ubiquitously expressing two copies of polyQ-expanded ATXN2 simultaneously with dSesn overexpression (sqh> ATXN2Q117;dSesnWT;ATXN2Q117) to more closely model clinical subjects. Indeed, constitutive expression of polyQ-expanded ATXN2 in all tissues, throughout development and in adults, (sqh> ATXN2Q117;ATXN2Q117) caused early death in both female (Figure 4A) and male (Figure 4D) flies. Mobility reductions appeared earlier than with pan-neuronal expression, present upon adult eclosion from the pupal case and persisting until death (Figure 4B, E). On the other hand, flies ubiquitously expressing both ATXN2Q117 and dSesn improved survival (Figure 4A, D), although rescue was not to the level of background control flies. Mobility improvements were more pronounced, with complete rescue of climbing speed in both females (Figure 4B) and males (Figure 4E). We next examined disease protein levels in sqh> ATXN2Q117;dSesnWT;ATXN2Q117 flies and found significant reduction in ATXN2 compared to SCA2 flies without dSesn expression (Figure 4C, F, quantified in Figure 7), similar to aforementioned reduction in disease protein after exercise. In contrast, ubiquitous expression of either ATXN3Q77 or β 1ACTQ70 led to developmental and early adult lethality (Tsou et al., 2016; Johnson et al., 2019), and dSesn expression did not improve outcomes (Figure 4—figure supplement 1).
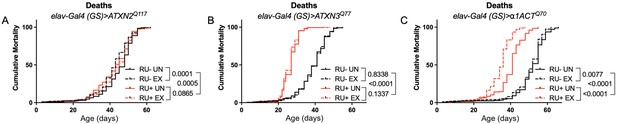
Expression of ATXN2 in adult neurons does not negatively impact lifespan.
Cumulative mortality in (A) elav-Gal4 (GS)> ATXN2Q117 flies is similar whether exercised or not (compare RU- UN to RU+ UN, and RU- EX to RU+ EX). (B) elav-Gal4 (GS)> ATXN3Q77 flies have reduced lifespan compared to uninduced controls, and exercise does not negatively affect either group. (C) elav-Gal4 (GS)>α1ACTQ70 flies have lower lifespan than uninduced controls, and exercise reduces lifespan further in this cohort. n ≥ 231, analyzed by log-rank tests.
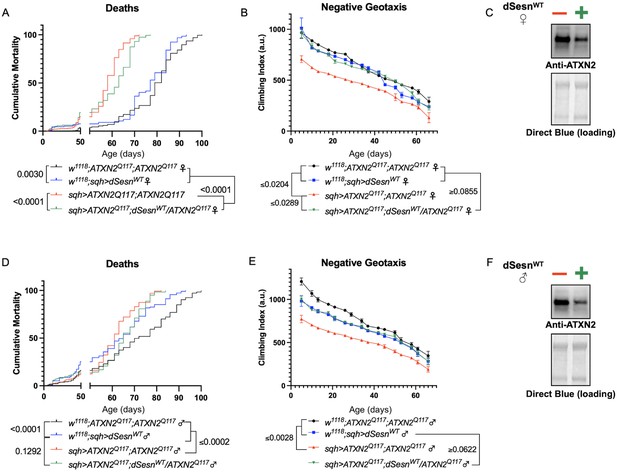
dSesn expression improves early death and low mobility in SCA2 flies, concurrent with reduction in disease protein.
Female (A, B) and male (D, E) flies ubiquitously expressing two copies of CAG-expanded ATXN2 (red lines) have early death (A, D) and lower climbing speed (B, E) than age-matched background control flies (blue and black lines). dSesn expression in flies also expressing polyQ-expanded ATXN2 (green lines) partially rescues early death (A, D) and fully rescues decreased mobility (B, E). ATXN2 protein levels are lower in both female (C) and male (F) flies ubiquitously expressing both dSesn and polyQ-expanded ATXN2. Survival and mobility experiments performed in triplicate. Individual survival experiments comprise ≥200 flies/genotype, scored every second day for death and analyzed by log-rank tests. Each individual climbing datum depicts average climbing speed for ≥5 vials of 20 flies, analyzed by 2-way ANOVA. Error bars indicate ± SD. Representative Western blots from five biological replicates (five flies per lysate), quantified in Figure 7.
-
Figure 4—source data 1
Uncropped, unedited blots from Figure 4C.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig4-data1-v4.zip
-
Figure 4—source data 2
Uncropped, unedited blots from Figure 4F.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig4-data2-v4.zip
Oxidative resistance and mTOR modulating functions of Sesn are necessary to improve SCA2 phenotypes
Sesn is a multi-functional protein with separate, previously identified oxidoreductase and mTOR interacting domains (Kim et al., 2015). The C86S (dSesnC86S) mutation abolishes oxidoreductase activity while the D424A (dSesnD424A) and D423A/D424A (dSesnD423A/D424A) substitutions disrupt TORC1 inhibiting and TORC2/Akt potentiating functions of dSesn (Kim et al., 2015). We previously showed that interaction with mTOR is required for the mobility-extending effects of dSesn in wild-type, sedentary flies (Kim et al., 2020). Overexpression of either wild-type or mutated dSesns did not increase longevity in a wild-type background (i.e. without polyQ protein expression; Figure 4—figure supplement 2). To explore the requirement of Sesn’s various functions for suppression of polyQ phenotypes, we performed mobility, survival, and protein expression experiments in which we ubiquitously overexpressed dSesnC86S, dSesnD424A, dSesnD423A/D424A, or wild-type dSesn (dSesnWT) in SCA2 flies.
First, we overexpressed dSesn without its antioxidant function(sqh>ATXN2Q; dSesnC86S;ATXN2Q117). Ubiquitous expression of dSesnC86S in SCA2 model flies did not provide any protection—in fact, it exacerbated premature death in both male and female flies (Figure 5A, D). However, we observed pronounced mobility improvements in female flies (Figure 5B) and mobility was fully rescued in males (Figure 5E). Whereas wild-type dSesn overexpression consistently reduced ATXN2 levels in both female (Figure 5C) and male (Figure 5F) SCA2 flies, the effect of dSesnC86S on ATXN2 levels was more variable (Figure 5C, F, quantified in Figure 7). These results suggest that while the oxidoreductase function of dSesn is 14 Figure 6: Interaction with mTOR is required for dSesn to improve survival and mobility inflies expressing polyQ-expanded ATXN2. Ubiquitous expression of dSesn harboring two separate mutations that abolish mTORC interaction dSesnD424A (A–D), dSesn D423A/D424A (E–H)(denoted by orange lines) exacerbates early death (A,E, females, C,G, males) and fails to rescuemobility (B,F, females, D,H, males) in SCA2 flies. Survival and mobility experiments performed intriplicate. Individual survival experiments are composed of ≥200 flies/genotype, scored every second day for death and analyzed by log-rank tests. Each individual climbing datum depictsaverage climbing speed for ≥5 vials of 20 flies, analyzed by 2-way ANOVA. Error bars indicate ± SD not required for protection against motility defects in SCA2 flies, it is essential for improved survival.
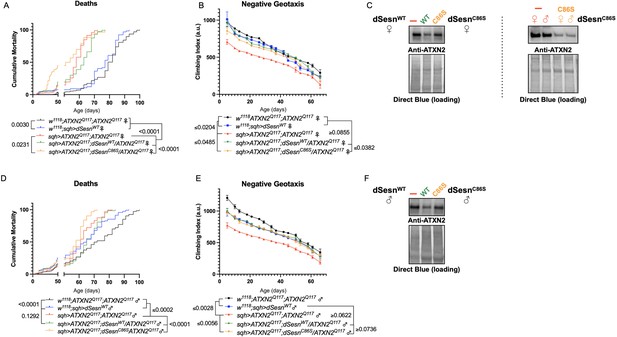
Oxidoreductase function is dispensable for mobility-extending effects of dSesn in flies expressing polyQ-expanded ATXN2.
Ubiquitous expression of dSesn harboring a point mutation that abolishes oxidoreductase activity (dSesnC86S, orange lines) exacerbates early death in SCA2 model flies (compare orange lines to red lines) in both females (A) and males (D). In contrast, climbing speed is partially rescued in the same female flies (expressing both dSesnC86S and polyQ-expanded ATXN2, in orange) (B) and fully rescued in males (E). (C, F) Both wild-type dSesn and dSesnC86S expression reduce ATXN2 levels, but the effect of dSesnC86S expression is variable (examples of variability shown in panel [C]). Survival and mobility experiments performed in triplicate. Individual survival experiments are composed of ≥200 flies/genotype, scored every second day for death and analyzed by log-rank tests. Each individual climbing datum depicts average climbing speed for 5 vials of 20 flies, analyzed by 2-way ANOVA. Error bars indicate ± SD. Representative Western blots from five biological replicates (five flies per lysate), quantified in Figure 7.
-
Figure 5—source data 1
Uncropped, unedited blots from Figure 5C (females) and Figure 5F (males).
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig5-data1-v4.zip
-
Figure 5—source data 2
Uncropped, unedited blots from Figure 5C depicting examples of variabliity.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig5-data2-v4.zip
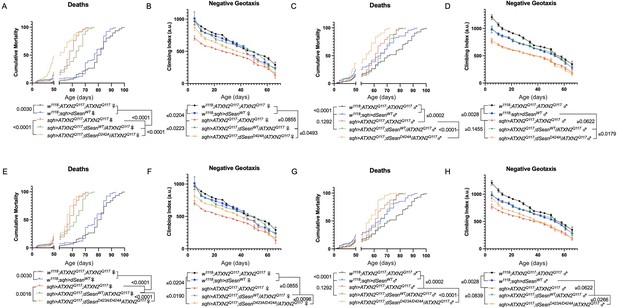
Interaction with mTOR is required for dSesn to improve survival and mobility in flies expressing polyQ-expanded ATXN2.
Ubiquitous expression of dSesn harboring two separate mutations that abolish mTORC interaction dSesnD424A (A–D), dSesn D423A/D424A (E–H) (denoted by orange lines) exacerbates early death (A,E, females, C,G, males) and fails to rescue mobility (B,F, females, D,H, males) in SCA2 flies. Survival and mobility experiments performed in triplicate. Individual survival experiments are composed of ≥200 flies/genotype, scored every second day for death and analyzed by log-rank tests. Each individual climbing datum depicts average climbing speed for ≥5 vials of 20 flies, analyzed by 2-way ANOVA. Error bars indicate ± SD.
We next ubiquitously expressed two separate amino-acid substitutions of dSesn that knock out its ability to modulate mTOR (Kim et al., 2015). Like dSesnC86S experiments, expression of either dSesnD424A (Figure 6A–D) or dSesnD423A/D424A (Figure 6E–H) increased early death in both female (Figure 6A, E) and male (Figure 6C, G) SCA2 flies. In female flies, both dSesnD424A and dSesn D423A/D424A conferred modest improvements to climbing speed compared to SCA2 model flies, but improvement did not equal that of wild-type dSesn expression and was absent at later timepoints (Figure 6B, F). Male flies ubiquitously expressing dSesnD424A did not display rescued mobility defects at any age (Figure 6D), while overexpression of dSesn D423A/D424A provided modest climbing speed improvements at early ages only (Figure 6H). Thus, interaction with mTOR appears critical for full rescue of climbing speed and protection against early death in SCA2 flies.
To examine the relative impact of dSesn expression on disease protein levels, we again performed Western blots on flies ubiquitously expressing polyQ-expanded ATXN2 with and without simultaneous expression of either wild-type dSesn, or dSesn harboring mutations that separately abolish its oxidative protection or mTOR modulating functions. ATXN2 protein in SCA2 model flies (sqh> ATXN2Q117;ATXN2Q117) was easily visible by log-phase of mortality (Figure 7A–C, F). All mutant dSesn-expressing cohorts had variable, but statistically significant reductions in ATXN2 protein levels.
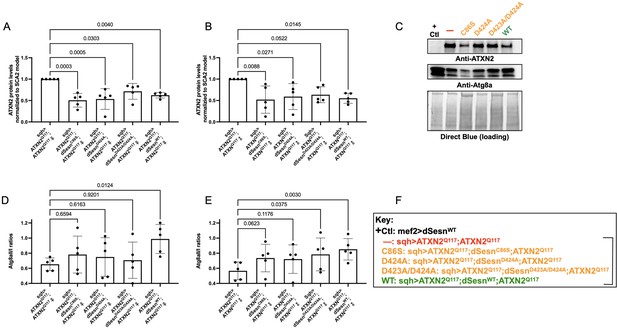
Wild-type dSesn expression reduces disease protein and increases autophagy in flies expressing polyQ-expanded ATXN2.
Ubiquitous expression of dSesn significantly reduces ATXN2 levels (A–C) and increases AtgIIa;AtgIa ratios (C–E). n = 5 biological replicates, 5 flies per lysate, analyzed by ANOVA with Tukey post-hoc comparison for significantly different groups. (F) Genotypes in representative Western blot and from independent biological replicates, quantified in (A, B, D, E). Bracket in (F) indicates quantifications in (A, B, D, E).
-
Figure 7—source data 1
Uncropped, unedited blots from Figure 7C.
- https://cdn.elifesciences.org/articles/75389/elife-75389-fig7-data1-v4.zip
Based on these results and on our prior observations in exercised flies and flies with muscle-specific dSesn overexpression (Kim et al., 2020; Sujkowski et al., 2020), we hypothesized that dSesn overexpression and reduced ATXN2 levels relate to changes in autophagy. We observed that AtgIIa/Ia ratios increased in both male and female SCA2 model flies that were overexpressing wild-type dSesn, while its mutated versions had higher variability, which largely trended towards increased autophagy, but without reaching significance (Figure 7C–F). These data may reflect different mechanisms of protection at play, discussed further below.
Finally, since dSesn overexpression improved survival in flies ubiquitously expressing two copies of the polyQ-expanded ATXN2 transgene (Figure 4A, D), we repeated our longevity experiments in exercised SCA2 flies expressing two copies of the ATXN2 transgene. Exercised female flies expressing two copies of CAG-expanded ATXN2 either ubiquitously (Figure 8A) or in adult neurons (Figure 8C) significantly improved survival compared to unexercised siblings. Male SCA2 flies trended toward improved survival in early life only, while they were still undergoing exercise training (Figure 8B, D). Altogether, these findings indicate protective effects from endurance exercise not only in terms of motility (Figures 1 and 2) but also in terms of longevity (Figure 8) in SCA2 flies.
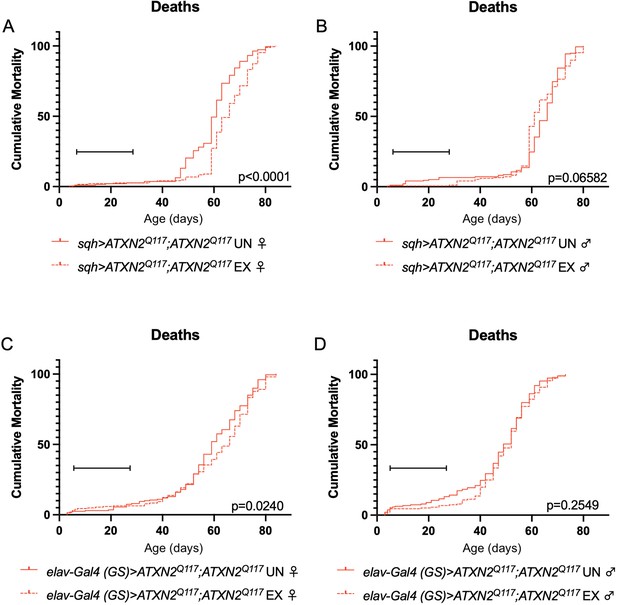
Exercise reduces early death in SCA2 model flies expressing two copies of ATXN2.
Exercised female flies expressing two copies of CAG-expanded ATXN2 ubiquitously (A) or in adult neurons (C) have increased survival compared to age-matched, unexercised siblings, while exercised male flies expressing two copies of CAG-expanded ATXN2 ubiquitously (B) or in adult neurons (D) trend toward increased survival only in the first 25 days, the period in which flies are still training. Brackets indicate exercise training period. p-Values indicate log-rank for entire survival curve, n ≥ 170, performed in duplicate.
In summary, both exercise and dSesn expression ameliorated mobility and survival defects in SCA2 model flies and these improvements correlated with reduced disease protein levels. Furthermore, these neuroprotective effects relied on known functions of dSesn related to mTOR and oxidoreductase activities (Figure 9).
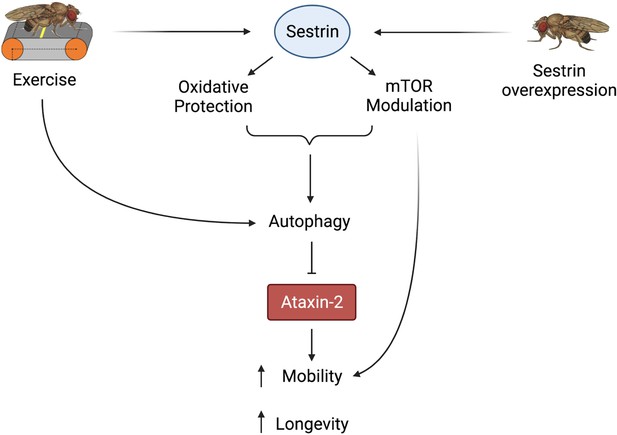
Proposed model of the effects of exercise and dSesn overexpression on SCA2 flies.
Sesn’s known functions and activation by exercise have been established previously (Kim et al., 2020; Kim et al., 2015). Wild-type Sesn overexpression or exercise can activate autophagy and reduce disease protein levels, improving function. Source data-all figures. Tab delimited excel file containing raw data for all figures and figure supplements. Uncropped, unedited Western blot data are included as separate images.
Discussion
Endurance exercise enhances quality of life and provides remedy for a number of conditions and diseases, but, until now, has not been systematically examined across several polyQ disorders. Lack of systematic studies in this respect also means that exercise-dependent molecular mechanisms that can be utilized to fight polyQ diseases remain largely unknown. Here, we explored in a highly controlled and rigorously quantified way the possibility that daily training is of benefit against some polyQ diseases. In addition, we identified a key factor of exercise’s protective effects that can be harvested towards therapeutic options.
Endurance exercise significantly rescued progressive motility defects in SCA2 flies, allowing their climbing speed and endurance to remain in wild-type range. Exercise also partially rescued SCA6 flies, with no beneficial effect on SCA3 model flies. Thus, exercise can have potent rescue effects for particular polyQ diseases. To obtain early insight into how exercise could lead to such dramatic improvement in SCA2 flies (albeit not for SCA3) we examined protein levels of ATXN2 and ATXN3, their respective disease proteins. Exercise led to reduction in protein levels of ATXN2, but did not noticeably affect ATXN3. In the context of endurance exercise, improved motility in SCA2 flies may be due to reduced disease protein levels.
In addition, we examined the effect of the powerful exercise-mimetic protein, Sesn, on lifespan, motility, and disease protein levels in SCA2 model flies. Sesns are induced by oxidative and genotoxic stress in flies and mammals and promote autophagy (Lee et al., 2010; Budanov and Karin, 2008). Sesns protect against oxidative stress-induced fly neuronal death (Singh and Chowdhuri, 2018) and reduce accumulation of protein aggregates in mammalian cells (Reddy et al., 2016). In previous work with wild-type flies, we found that expression of fly Sesn is sufficient to preserve speed and endurance and has no additive effect with exercise, consistent with a role as a key effector of exercise (Kim et al., 2020). Here, we found that Sesn was protective in SCA2 model flies, but did not confer benefit to either SCA3 or SCA6. Furthermore, Sesn’s oxidoreductase function was not necessary to rescue mobility defects in SCA2, while both its oxidative resistance and mTOR modulating activities were required for Sesn to improve early death. In fact, overexpression of Sesn with mutations eliminating either its oxidoreductase activity or mTOR modulating functions exacerbated premature death in SCA2 flies.
We have previously shown that the oxidoreductase activity of Sesn is dispensable for motility improvements in wild-type flies (Kim et al., 2020), similar to our observations with SCA2 flies in this study. Sesn overexpression is also not sufficient to extend lifespan in a wild-type background, shown here and elsewhere (Kim et al., 2020; Lee et al., 2010). Neurons are particularly susceptible to oxidative stress and reactive oxygen species can induce cell death during neurodegeneration (Fatokun et al., 2008). Perhaps, in the context of neurodegenerative disease, Sesn protects mobility until oxidative burden in neurons becomes insurmountable.
Wild-type Sesn overexpression in SCA2 model flies correlated with significantly reduced disease protein and increased autophagic flux. Overexpression of mutated versions of Sesn also led to reduced disease protein levels. This is not entirely surprising: mutated Sesns still had some positive impact on SCA2 fly mobility, but did not have the same, fully protective effect of wild-type Sesn. Collectively, these findings suggest the possibility of separate mechanisms at play: some protective mechanisms may directly depend on reduced toxic protein levels (as observed before in a model of HD and exercise Mangiarini et al., 1996; van Dellen et al., 2008); others may protect independently of changes in degenerative protein levels, or perhaps without directly relying on it. Future investigations are required to parse out such mechanisms and whether they function in a cell autonomous or non-cell autonomous manner.
To conclude, we propose endurance exercise as a promising therapeutic intervention for polyQ neurodegeneration, particularly in SCA2. We also demonstrate that Sesn can substitute for exercise benefits in flies that do not exercise. Identification of molecular targets capable of inducing exercise-like enhancements in the presence of neurodegeneration is particularly important, as many patients will eventually lose the ability to exercise. This work has begun to uncover exercise-induced mechanisms that can be utilized for neuroprotection against specific polyQ diseases and can be leveraged toward therapeutics in the future.
Materials and methods
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (H. sapiens) | ATXN2 | GenBank | FLYB: FBgn0267931 | |
| Gene (H. sapiens) | ATXN3 | GenBank | FLYB: FBgn0024961 | |
| Gene (H. sapiens) | CACNA1A | GenBank | FLYB: FBgn0283733 | |
| Gene (D. melanogaster) | Sesn | GenBank | FLYB: FBgn0034897 | |
| Genetic reagent (D. melanogaster) | UAS- ATXN2Q117 | Bloomington Drosophila Stock Center | BDSC68394: FBst0068394 | Flybase Symbol:w*; P{UAS-ATXN2.117Q}8B |
| Genetic reagent (D. melanogaster) | UAS- ATXN2Q117 | Bloomington Drosophila Stock Center | BDSC68395: FBst0068395 | Flybase Symbol: w*; P{UAS-ATXN2.117Q}9 A |
| Genetic reagent (D. melanogaster) | UAS-ATXN3Q77 | Tsou et al., 2016 | ||
| Genetic reagent (D. melanogaster) | UAS-α1ACTQ70 | Sutton et al., 2017 | ||
| Genetic reagent (D. melanogaster) | w1118;UAS-ATXN2Q117/CyO;UAS-ATXN2Q117 | This paper | See methods lines 305–306 | |
| Genetic reagent (D. melanogaster) | w1118;sqh/CyO;UAS- dSesnWT/TM3-Sb | This paper | See methods lines 311–314 | |
| Genetic reagent (D. melanogaster) | w1118;sqh/CyO;UAS- dSesnC86S/TM3-Sb | This paper | See methods lines 311–314 | |
| Genetic reagent (D. melanogaster) | w1118;sqh/CyO;UAS- dSesnD424A/TM3-Sb | This paper | See methods lines 311–314 | |
| Genetic reagent (D. melanogaster) | w1118;sqh/CyO;UAS- dSesnD423A/D424A/TM3-Sb | This paper | See methods lines 311–314 | |
| Antibody | anti-ataxin-2 (mouse monoclonal) | BD biosciences | 611,378 | (1:500) |
| Antibody | anti-ataxin-3 (mouse monoclonal) | Millipore | 1H9, MAB5360 | (1:1000) |
| Antibody | anti-GABARAP (rabbit polyclonal) | Abcam | Ab1398 | (1:1000) |
| Antibody | anti-dSesn (rabbit polyclonal) | Kim et al., 2020 | (1:500) |
Antibodies
Primary antibodies were obtained from the following sources: anti-ATXN2 (mouse monoclonal, 1:500; BD biosciences, Franklin Lakes, NJ) anti-ATXN3 (mouse monoclonal 1H9, MAB5360, 1:500–1000; Millipore, Burlington, MA), anti-dSesn (rabbit polyclonal, 1:500, Lee et al., 2010), anti-GABARAP (rabbit polyclonal, 1:1000, Abcam, Cambridge, United Kingdom). Peroxidase-conjugated secondary antibodies (goat anti-mouse, goat anti-rabbit, 1:5000; Jackson Immunoresearch, West Grove, PA).
Fly stocks and maintenance
Request a detailed protocolGifted stocks used in this study were sqh-Gal4 (Daniel Kiehart, Duke University, abbreviated “sqh” throughout), and w1118 (Russ Finley, Wayne State University). UAS-Q80, UAS-ATXN3Q77, UAS-α1ACTQ70 and elav-Gal4 (GS) were previously described in Tsou et al., 2016; Johnson et al., 2020; Johnson et al., 2019 UAS- ATXN2Q117 (BDSC#68394, BDSC#68395), on chromosomes II and III, respectively, were obtained from the Bloomington Drosophila Stock Center (Bloomington, IN). Both SCA2 lines were used for pilot work. A single line containing UAS-ATXN2Q117 on both chromosomes II and III was then generated using standard crosses (w1118;UAS-ATXN2Q117/CyO;UAS-ATXN2Q117).
Wild-type (dSesnWT) and C86S(dSesnC86S), D424A (dSesnD424A) or D423A/D424A (dSesnD423A/D424A) mutations are described in Kim et al., 2020; Kim et al., 2015. C86, D423 and D424 in dSesn correspond to C125, D406 and D407 in mammalian SESN2 (Kim et al., 2015). These four lines were separately recombined with sqh-Gal4 to create four independent lines with genotypes: w1118;sqh/CyO;UAS- dSesnXX/TM3-Sb. Simultaneous overexpression of both UAS-ATXN2Q117 and UAS-dSesnXX was achieved via a single cross between w1118; UAS-ATXN2Q117/CyO;UAS-ATXN2Q117 virgin females and w1118;sqh/CyO;UAS-dSesnXX/TM3-Sb males to generate w1118;UAS-ATXN2Q117;UAS-dSesnXX/ATXN2Q117. All insertions were validated by Western blotting (Tsou et al., 2015b; Tsou et al., 2016; Ristic et al., 2018; Johnson et al., 2020; Lee et al., 2010; Johnson et al., 2019; Blount et al., 2018).
Prior to all experiments, fly cultures were maintained at a constant density for at least two generations. About 20–25 virgin females (depending on genotype) and 5 males were mated in 300 mL bottles with 50 mL standard 10% sucrose 10% yeast spiked with 500 μL Penicillin-Streptomycin 10,000 u/mL, 10 mg/mL in 0.9% sterile NaCl (Sigma-Aldrich, St. Louis, MO). Adult progeny were synchronized by collecting within 6 hr of eclosion over a 24 hr time period. Groups of 20 age- and sex-matched flies were immediately transferred into narrow polypropylene vials containing 5 mL of standard 10% sucrose 10% yeast (no antibiotic) or RU486 food, as appropriate. Food vials were changed every day during exercise and every second day thereafter and scored for mobility and lifespan until no flies remained.
Flies were housed in a 25°C incubator on a 12:12 h light:dark cycle at 50% relative humidity. Control flies for all non-gene-switch Gal4 UAS experiments consisted of both the UAS and Gal4 lines into w1118. For gene-switch experiments, RU- flies of the same genotype served as the negative control. RU+ group received 100 μM RU486/mifepristone (Cayman Chemical, Ann Arbor, MI), which activates the gene switch (GS) driver, while RU- group received the same volume of vehicle solution (70% ethanol).
Exercise training
View detailed protocolTriplicate cohorts of at least 800 flies were collected under light CO2 anesthesia within 6 hr of eclosion and separated into vials of 20. Flies were then further separated into two large cohorts of at least 400 flies, which served as exercised and unexercised groups. Exercised groups received three weeks of ramped exercise as described previously (Piazza et al., 2009). The unexercised groups were placed on the exercise training device at the same time as the exercised groups, but were prevented from running by the placement of a foam stopper low in the vial. Both cohorts were housed in the same incubator with normal foam stopper placement at all times other than during an exercise bout.
Climbing speed
Request a detailed protocolNegative geotaxis was assessed in Rapid Negative Geotaxis (RING) assays in groups of at least 100 flies as described (Damschroder et al., 2018a). Briefly, vials of 20 flies were briskly tapped down, then measured for climbing distance after 2 s of inducing the negative geotaxis instinct. For each group of vials, an average of five consecutive trials was calculated and batch-processed using ImageJ (Bethesda, MD). Flies were longitudinally tested 2–3 times per week for 5–7 weeks. Between assessments, flies were returned to food vials and housed normally as described above. Negative geotaxis results were analyzed using two-way ANOVA analysis (age-effect and genotype effect) with post hoc Tukey’s multiple comparison tests in GraphPad Prism (San Diego, CA). All negative geotaxis experiments were performed in triplicate, with one complete trial shown in each graph.
Endurance
Request a detailed protocolAt least eight vials of 20 flies from each cohort were subjected to the endurance analysis on day 25 of adulthood, immediately after exercise was complete and after 2 days of recovery. For each session, the flies were placed on the Power Tower exercise machine (Sujkowski and Wessells, 2018) and the climbing instinct was induced until flies no longer responded to the negative geotaxis stimulus. Monitored at 15 min intervals, a vial of flies was visually determined to be fatigued when 10% or fewer flies could climb higher than 1 cm after three consecutive drops. Each vial was plotted as a single datum. Endurance experiments were performed in triplicate and at the same time as age-matched background controls and were scored blindly wherever possible. The time from the start of the assay to the time of fatigue was recorded for each vial, and the data analyzed using log-rank analysis in GraphPad Prism (San Diego, CA). Each graph represents an independent, representative repetition.
Longevity
Request a detailed protocolFor exercise experiments, appropriate food vials (RU486, vehicle, or standard 10% sucrose 10% yeast) were changed and deaths were recorded five times per week during exercise training and three times per week after completion of the training program. All other longevity experiments were scored three times per week. Dead flies were removed and counted until no flies remained. Differences in survival were plotted as cumulative mortality and assessed following censoring using log-rank analysis in GraphPad Prism (San Diego, CA). Longevity experiments were performed in triplicate and in parallel with background controls, with each individual graph depicting a representative biological repetition.
Western blots
Request a detailed protocolThree-to-five whole flies per biological replicate, depending on experiment, were homogenized in boiling lysis buffer (50 mM Tris pH 6.8, 2% SDS, 10% glycerol, 100 mM dithiothreitol), sonicated, boiled for 10 min, and centrifuged at 13,300xg at room temperature for 10 min. Western blots were developed ChemiDoc (Bio-Rad, Hercules, CA) and quantified with ImageLab (Bio-Rad, Hercules, CA). For direct blue staining, PVDF membranes were submerged for 10 min in 0.008% Direct Blue 71 (Sigma-Aldrich, St. Louis, MO) in 40% ethanol and 10% acetic acid, rinsed in 40% ethanol/10% acetic acid, air dried, and imaged. Western blots were performed using at least three biological replicates, and statistical analysis was performed in GraphPad Prism (San Diego, CA).
Statistical analysis
Request a detailed protocolSurvival and endurance analyses were analyzed by log-rank. Non-natural deaths were censored and did not exceed 10% of flies for any group. Climbing speed over time was analyzed by two-way ANOVA for age effect and genotype effect, with Tukey post-hoc comparison between significantly different groups. Western blots were analyzed using either student t-test or ANOVA depending on experiment. All statistics were performed using GraphPad Prism software version 9.2.0 (San Diego, CA, USA) for Macintosh.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting file; source data files have been provided for all figures and supplementary information.
References
-
Human neurodegenerative disease modeling using DrosophilaAnnual Review of Neuroscience 26:627–656.https://doi.org/10.1146/annurev.neuro.26.041002.131425
-
Lack of exercise is a major cause of chronic diseasesComprehensive Physiology 2:1143–1211.https://doi.org/10.1002/cphy.c110025
-
Ectopic expression in DrosophilaMethods in Cell Biology 44:635–654.https://doi.org/10.1016/s0091-679x(08)60936-x
-
Cycling regimen induces spinal circuitry plasticity and improves leg muscle coordination in individuals with spinocerebellar ataxiaArchives of Physical Medicine and Rehabilitation 96:1006–1013.https://doi.org/10.1016/j.apmr.2015.01.021
-
Emerging Roles of Sestrins in Neurodegenerative Diseases: Counteracting Oxidative Stress and BeyondJournal of Clinical Medicine 8:E1001.https://doi.org/10.3390/jcm8071001
-
Treadmill training increases the motor activity and neuron survival of the cerebellum in a mouse model of spinocerebellar ataxia type 1The Kaohsiung Journal of Medical Sciences 35:679–685.https://doi.org/10.1002/kjm2.12106
-
A genetic modifier suggests that endurance exercise exacerbates Huntington’s diseaseHuman Molecular Genetics 27:1723–1731.https://doi.org/10.1093/hmg/ddy077
-
Unbiased screen identifies aripiprazole as a modulator of abundance of the polyglutamine disease protein, ataxin-3Brain: A Journal of Neurology 139:2891–2908.https://doi.org/10.1093/brain/aww228
-
Drosophila tafazzin mutants have impaired exercise capacityPhysiological Reports 6:e13604.https://doi.org/10.14814/phy2.13604
-
Partial Body Weight-Supported Treadmill Training in Spinocerebellar AtaxiaRehabilitation Research and Practice 2018:7172686.https://doi.org/10.1155/2018/7172686
-
Caring for Machado-Joseph disease: current understanding and how to help patientsParkinsonism & Related Disorders 16:2–7.https://doi.org/10.1016/j.parkreldis.2009.08.012
-
Oxidative stress in neurodegeneration and available means of protectionFrontiers in Bioscience 13:3288–3311.https://doi.org/10.2741/2926
-
Exercise and genetic rescue of SCA1 via the transcriptional repressor CapicuaScience (New York, N.Y.) 334:690–693.https://doi.org/10.1126/science.1212673
-
Deactivation of TBP contributes to SCA17 pathogenesisHuman Molecular Genetics 23:6878–6893.https://doi.org/10.1093/hmg/ddu410
-
Treadmill exercise enhances spatial learning ability through suppressing hippocampal apoptosis in Huntington’s disease ratsJournal of Exercise Rehabilitation 11:133–139.https://doi.org/10.12965/jer.150212
-
Sestrins are evolutionarily conserved mediators of exercise benefitsNature Communications 11:190.https://doi.org/10.1038/s41467-019-13442-5
-
A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivoThe Journal of Biological Chemistry 289:12005–12015.https://doi.org/10.1074/jbc.M113.530527
-
Sestrin as a feedback inhibitor of TOR that prevents age-related pathologiesScience (New York, N.Y.) 327:1223–1228.https://doi.org/10.1126/science.1182228
-
Polyglutamine Repeats in Neurodegenerative DiseasesAnnual Review of Pathology 14:1–27.https://doi.org/10.1146/annurev-pathmechdis-012418-012857
-
Possible Neuroprotective Mechanisms of Physical Exercise in NeurodegenerationInternational Journal of Molecular Sciences 21:E5895.https://doi.org/10.3390/ijms21165895
-
Huntington’s disease: the coming of ageJournal of Genetics 97:649–664.https://doi.org/10.1007/s12041-018-0957-1
-
Polyglutamine spinocerebellar ataxias - from genes to potential treatmentsNature Reviews. Neuroscience 18:613–626.https://doi.org/10.1038/nrn.2017.92
-
Fruit flies on the front line: the translational impact of DrosophilaDisease Models & Mechanisms 9:229–231.https://doi.org/10.1242/dmm.024810
-
The Tiny Drosophila melanogaster for the Biggest Answers in Huntington’s DiseaseInternational Journal of Molecular Sciences 19:E2398.https://doi.org/10.3390/ijms19082398
-
Spinocerebellar Ataxia Type 2Advances in Experimental Medicine and Biology 1049:175–195.https://doi.org/10.1007/978-3-319-71779-1_8
-
Physical activity in obesity and metabolic syndromeAnnals of the New York Academy of Sciences 1281:141–159.https://doi.org/10.1111/j.1749-6632.2012.06785.x
-
Using Drosophila to Understand Biochemical and Behavioral Responses to ExerciseExercise and Sport Sciences Reviews 46:112–120.https://doi.org/10.1249/JES.0000000000000139
-
The protective role of exercise against age-related neurodegenerationAgeing Research Reviews 74:101543.https://doi.org/10.1016/j.arr.2021.101543
-
Spinocerebellar ataxia: an updateJournal of Neurology 266:533–544.https://doi.org/10.1007/s00415-018-9076-4
-
Endurance training protocol and longitudinal performance assays for Drosophila melanogasterJournal of Visualized Experiments 2012:3786.https://doi.org/10.3791/3786
-
BookPolyglutamine Repeat Disorders, including Huntington’s DiseaseIn: Waxman SG, editors. In Molecular Neurology. London: Academic Press. pp. 257–276.https://doi.org/10.1016/B978-012369509-3.50019-6
-
Ubiquitination regulates the neuroprotective function of the deubiquitinase ataxin-3 in vivoThe Journal of Biological Chemistry 288:34460–34469.https://doi.org/10.1074/jbc.M113.513903
-
DnaJ-1 and karyopherin α3 suppress degeneration in a new Drosophila model of Spinocerebellar Ataxia Type 6Human Molecular Genetics 24:4385–4396.https://doi.org/10.1093/hmg/ddv174
-
Repeat Expansion Disease ModelsAdvances in Experimental Medicine and Biology 1076:63–78.https://doi.org/10.1007/978-981-13-0529-0_5
-
BookImpact of Chronic Exercise on Invertebrate Functional AgingIn: Ram J, editors. In Conn’s Handbook of Models for Human Aging. Elesiver. pp. 461–471.
-
Glutamine repeats and neurodegenerationAnnual Review of Neuroscience 23:217–247.https://doi.org/10.1146/annurev.neuro.23.1.217
-
Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type 1The Journal of Biological Chemistry 284:7425–7429.https://doi.org/10.1074/jbc.R800041200
Article and author information
Author details
Funding
Wayne State University (Thomas C. Rumble Graduate Fellowship)
- Alyson Sujkowski
NIH Office of the Director (R01 AG059683)
- Robert J Wessells
NIH Office of the Director (R21 NS121276)
- Robert J Wessells
- Sokol V Todi
NIH Office of the Director (R01 NS086778)
- Sokol V Todi
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Henry Paulson for the anti-MJD antibody, and Todi lab members Kozeta Libohova, Wei-Ling Tsou, Gorica Ristic, Joanna Sutton, Jessica Blount, and Sean Johnson for generation and validation of the SCA lines used in this study. We acknowledge our collaborators Myungjin Kim and Jun Hee Lee for the original dSesn Drosophila stocks and reagents. This research was funded by the Wayne State University Thomas C Rumble Graduate Fellowship (to ALS), NIH R01 AG059683 (RJW), NIH R21 NS121276 (RJW and SVT), and NIH R01 NS086778 (SVT).
Copyright
© 2022, Sujkowski et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,928
- views
-
- 238
- downloads
-
- 22
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 22
- citations for umbrella DOI https://doi.org/10.7554/eLife.75389






