Inhibitors of Rho kinases (ROCK) induce multiple mitotic defects and synthetic lethality in BRCA2-deficient cells
Figures
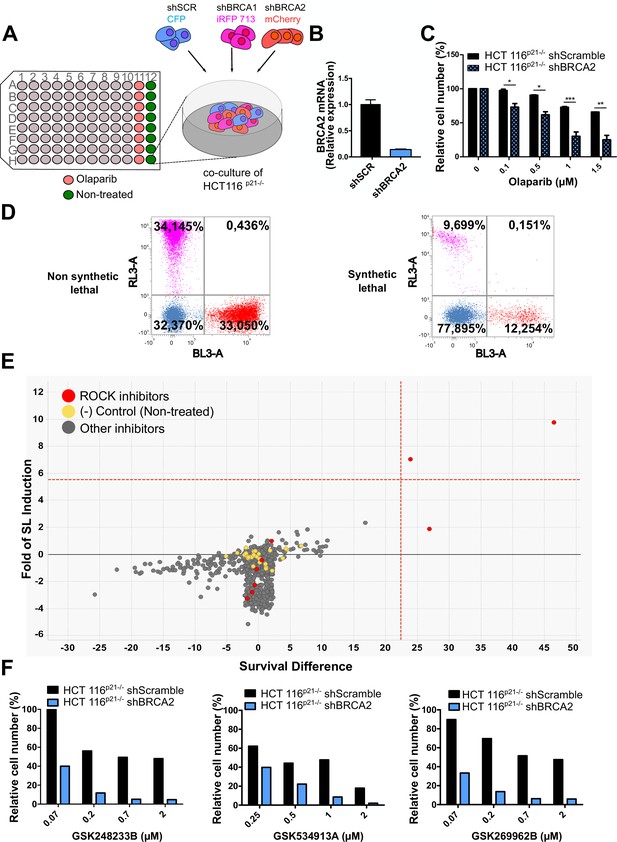
Phenotypic screening identifies ROCK kinases as potential targets for synthetic lethality in BRCA2 cells.
(A) The screening assay is based on the co-culture of isogeneic BRCA-proficient and BRCA-deficient cell lines in equal proportions on each well of 96-well plates. Such cell lines were generated as double stable cell lines tagged with different fluorescent proteins (CFP, iRFP, and mCherry) and expressing shRNAs for Scramble, BRCA1 or BRCA2 were generated as described in Carbajosa et al., 2019. (B) Quantitative real-time PCR of BRCA2 in shScramble and shBRCA2 HCT116p21-/- cells (N=2). Statistical analysis was performed with a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). (C) Relative cell number (%) of HCT116p21-/- cells expressing shScramble and shBRCA2 and treated with the indicated concentrations of olaparib (N=2). (D) Representative results expressed as RL-3-BL-3 dot plots (log scale, RL-3 780/60 nm filter, and BL-3 695/40 nm filter). A tested compound can be ‘non-synthetic lethal’ (the ratio between the populations' percentage remains unchanged when compared to the ratio used for seeding ~33% for each cell line); or ‘synthetic lethal’ (the ratio between cell types is altered when compared to the ratio used for seeding, with selective depletion of cells within the BRCA1- and/or BRCA2-deficient populations). (E) Screening results of PKIS2 library compounds (0.1 μM) in shBRCA2 HCT116p21-/- cells. Compounds were plotted based on their fold of SL (y axis) and their survival difference (x axis). A compound was considered a ‘hit’ if it exhibited a >5 standard deviations on these two variables. Fold of SL (y-axis): the ratios of the different populations in each individual well. Survival difference (x-axis): compares treated cells with the untreated control in the same plate. ROCK inhibitors and other inhibitors are plotted in red and gray, respectively. Please refer to Carbajosa et al., 2019 for statistical analysis of the screening. (F) Relative cell number (%) of shScramble and shBRCA2 HCT116p21-/- cells at different ROCK inhibitors. Data are shown as the average of independent experiments with the standard error of the mean.
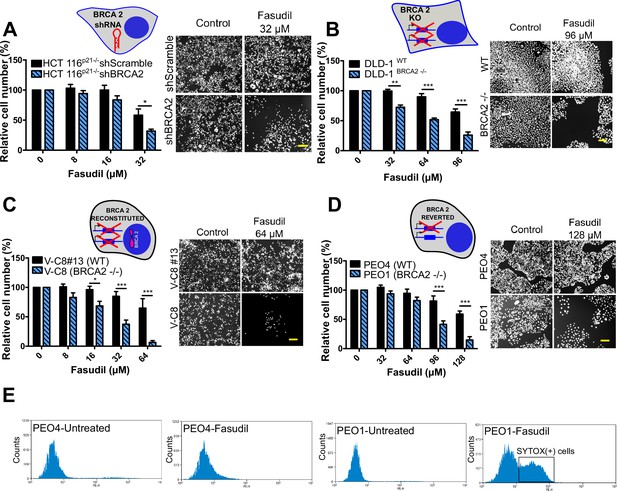
BRCA2-deficient cells are selectively killed by the ROCK kinase inhibitor fasudil.
(A) Relative cell number (%) of shScramble and shBRCA2 HCT116p21-/- cells after 6 days of treatment with fasudil (N=3). (B) Relative cell number (%) of DLD-1WTand DLD-1BRCA2-/- after 6 days of treatment with fasudil (N=2). (C) Relative cell number (%) of V-C8#13 and V-C8 cells after 6 days of treatment with fasudil (N=3). (D) Relative cell number (%) of PEO4 and PEO1 cells after 6 days of treatment with fasudil (N=4). Panels A-D: the cell cartoon shows the BRCA2 status caused by the modification introduced at last in each pair of cell lines (see Materials and methods for further details). Black borders indicate that the modification generated a BRCA2 proficient status and blue borders aBRCA2 deficiency. (E) FACS analysis of SYTOX green-stained PEO4 and PEO1 cells 6 days after fasudil treatment (128 μM, N=2). Statistical analysis was performed with a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data in A-D are shown as the average of independent experiments with the standard error of the mean.
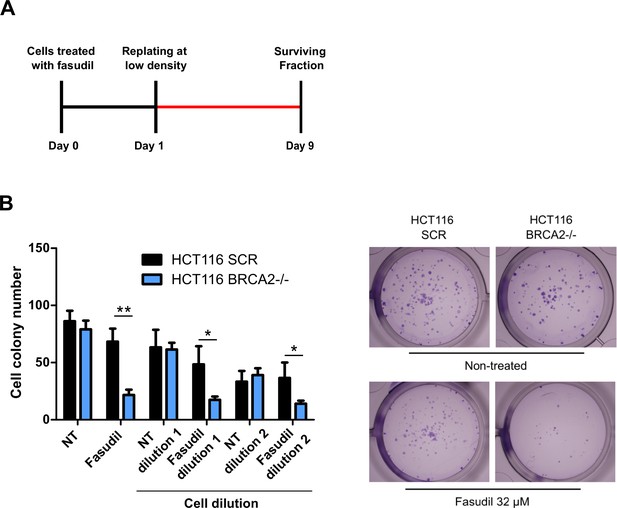
BRCA2-deficient cells are sensitive to the ROCK kinase inhibitor, fasudil.
(A) Scheme of the experimental design for the clonogenic assay. (B) Cell colony number of fasudil-treated shScramble and shBRCA2 HCT116 p21-/- cells (N=2). Representative images are shown on the right. Statistical analysis was performed with a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001).
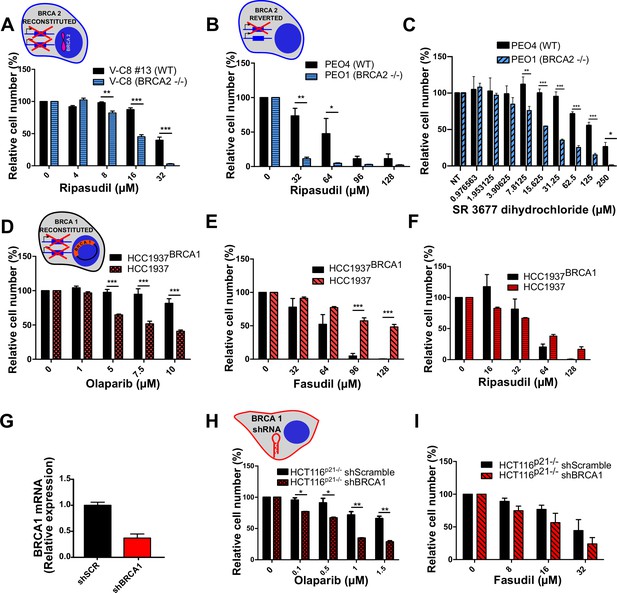
BRCA2-deficient cells are sensitive to the ROCK kinase inhibitor ripasudil.
(A) Relative cell number (%) of V-C8#13 and V-C8 cells after 6 days of treatment with ripasudil (N=1). (B) Relative cell number (%) of PEO4 and PEO1 cells after 6 days of treatment with ripasudil (N=2). (C) Relative cell number (%) of PEO4 and PEO1 cells after 6 days of treatment with SR 3677 dihydrochloride (N=2). (D) Relative cell number (%) of HCC1937BRCA1 and HCC1937 cells after 6 days of treatment with olaparib (N=3). (E) Relative cell number (%) of HCC1937BRCA1 and HCC1937 cells after 6 days of treatment with fasudil (N=3). (F) Relative cell number (%) of BRCA1-proficient HCC1937BRCA1 and BRCA1-deficient HCC1937 cells after 6 days of treatment with ripasudil (N=2). (G) Quantitative real-time PCR of BRCA1 in shScramble and shBRCA1 HCT116p21-/- cells. (H) Relative cell number (%) of shScramble and shBRCA1 HCT116p21-/- cells after 6 days of treatment with olaparib (N=2). (I) Relative cell number (%) of shScramble and shBRCA1 HCT116p21-/- cells after 6 days of treatment with fasudil (N=2). Statistical analysis was performed with a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data in A-F; H-I are shown as the average of independent experiments with the standard error of the mean. Panels A-F and H-I: the cartoon cells show the BRCA2 or BRCA1 status of the modification introduced at last in each pair of cell lines (see Materials and methods for further details). Black borders indicate that the modification generated a BRCA2 or BRCA1 proficient status, the blue borders indicate BRCA2 deficiency and the red borders represent BRCA1 deficiency.
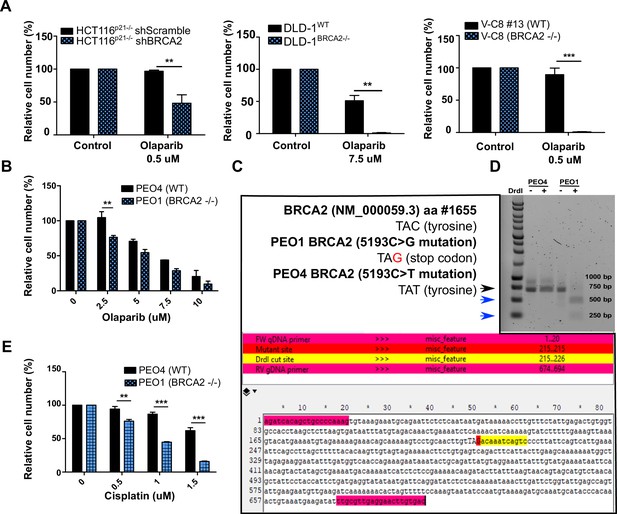
BRCA2-deficient cells are sensitive to olaparib.
(A) Relative cell number (%) of shScramble and shBRCA2 HCT116p21-/- (N=2), DLD-1WT and DLD-1BRCA2-/- (N=2), and V-C8#13 and V-C8 (N=3) cells after 6 days of treatment with olaparib. (B) Relative cell number (%) of PEO4 and PEO1 cells after 6 days of treatment with olaparib (N=2). (C) Nucleotide sequence (TAC, tyrosine) of aminoacid #1655 of the BRCA2 reference sequence NM_000059.3, and the nucleotide sequences found in PEO1 (TAG, stop codon) and PEO4 (TAT, tyrosine). Schematic of a fragment sequence of BRCA2 showing the primers (highlighted in magenta) used to amplify a 694 bp fragment around aminoacid #1655. The PEO1 mutation site TAG is highlighted in red. The DrdI enzyme digestion site is highlighted in yellow. (D) Agarose gel showing undigested and digested (DrdI enzyme) products of a 694 bp fragment of the BRCA2 gene from PEO4 and PEO1 cells (Black arrow head). The nonsense mutation in PEO1 (BRCA2-deficient cell lines) generates a restriction site for the DrdI enzyme giving rise to two digestion products of 480 bp and 214 bp (blue arrow head). (E) Relative cell number (%) of PEO4 and PEO1 cells treated with cisplatin (24 hr) followed by 5 days of growth in cisplatin-free media (N=2). Data are shown as the average of three technical replicates with the standard deviation. Panels A-E: Statistical analysis was performed using a t-test (*p<0.05, **p<0.01, ***p<0.001).
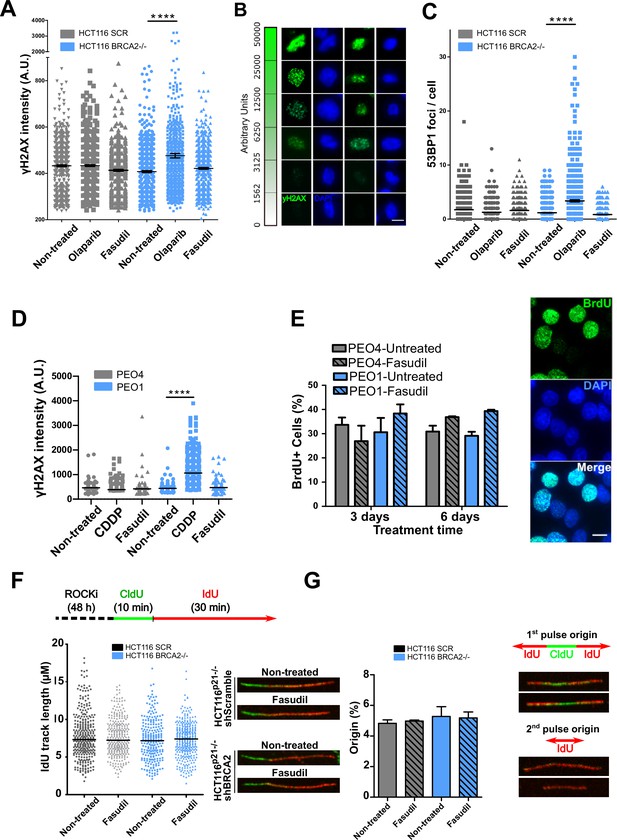
Fasudil does not induce acute replication stress in BRCA2-deficient cells.
(A) yH2AX intensity/cell of shScramble or shBRCA2 HCT116p21-/- cells (N=2). (B) Representative images of yH2AX intensity in single cells. (C) Number of 53BP1 foci/cell in shScramble and shBRCA2 HCT116p21-/- cells (N=2). (D) yH2AX intensity/cell in PEO1 or PEO4 cells (N=2). (E) Percentage of PEO4 and PEO1 cells stained with BrdU at 3 and 6 days after fasudil treatment (128 μM, N=2). A total of 500 cells were analyzed for each sample. Representative images of PEO1 cells after 3 days of fasudil treatment (BrdU shown in green, DAPI shown in blue). (F) Labelling scheme and IdU track lengths of shScramble and shBRCA2 HCT116p21-/- cells, treated with fasudil for 48 h (N=2). Representative images of individual DNA fibers are shown on the left side of the panel. (G) Origin firing frequency (percentage) of shScramble or shBRCA2 HCT116p21-/- cells in samples showed in E (N=2). Statistical analysis was performed using a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of independent experiments with the standard error of the mean.
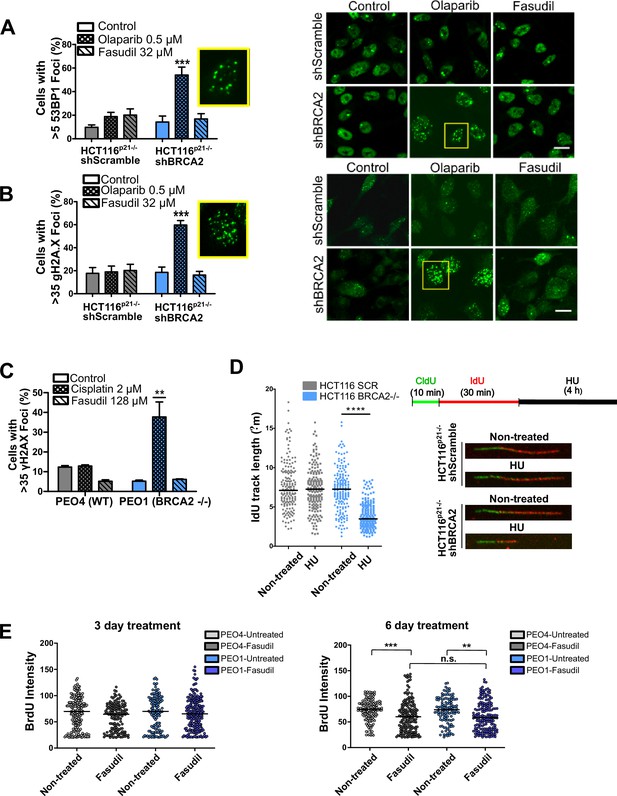
Fasudil does not alter S phase parameters in BRCA2-deficient cells.
(A) Percent of shScramble or shBRCA2 HCT116p21-/- cells with >5 53BP1 foci (N=3) and representative images of the treatments. A total of 300–400 cells were analyzed per independent experiment. Cells were treated for 48 hr. (B) Percent of shScramble or shBRCA2 HCT116p21-/- cells with >35 γH2AX foci (N=3) and representative images of the treatments. A total of 300–400 cells were analyzed per independent experiment. Cells were treated for 48 hr. (C) Percent of PEO4 and PEO1 cells with >35 γH2AX foci (N=2). A total of 300–400 cells were analysed per independent experiment. (D) Labelling scheme and IdU track lengths of shScramble and shBRCA2 HCT116p21-/- cells treated with HU for 4 h (N=2). On the right-hand side of the panel are representative images of individual DNA fibers. (E) BrdU intensity in experiments from Figure 3D. PEO4 (grey circles) and PEO1 (blue circles) cells after 3 or 6 days of fasudil treatment (128 μM, N=2). A total of 500 cells were analyzed for each sample. Individual intensity values per cell are displayed as a scatter plot and the average and standard deviation of each population are shown. Statistical analysis in A-D were performed with a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Statistical analysis in E was done with a Kruskal-Wallis non-parametric test followed by a Dunn’s multiple comparison test (*p<0.05, **p<0.01, ***p<0.001).
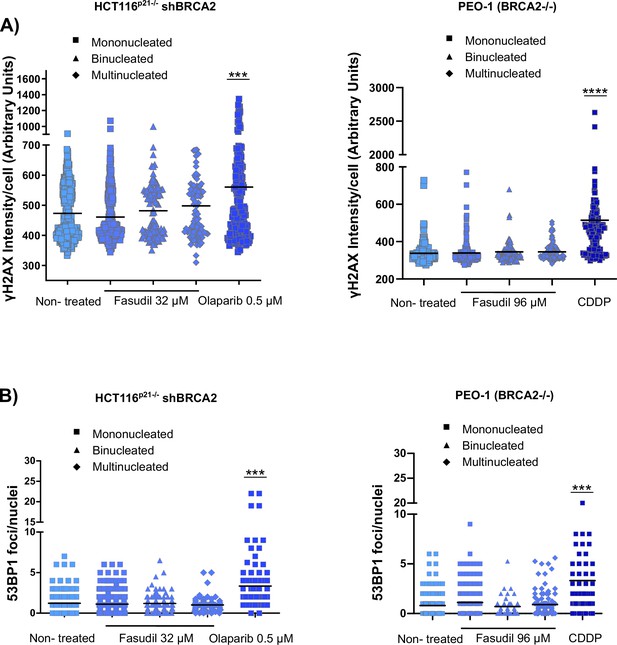
Fasudil does not induce acute replication stress after 6 days of treatment in BRCA2-deficient cells.
(A) γH2AX intensity/cell of shBRCA2 HCT116p21-/- and PEO1 (BRCA2-/-) cells after 6 days of fasudil treatment (N=2). Cells were divided according to their number of nuclei (mono, bi, and multinucleated cells). (B) Number of 53BP1 foci/nuclei in shBRCA2 HCT116p21-/- and PEO1 (BRCA2-/-) cells (N=2). Statistical analysis was performed with a one-way ANOVA test followed by a tuckey post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of independent experiments.
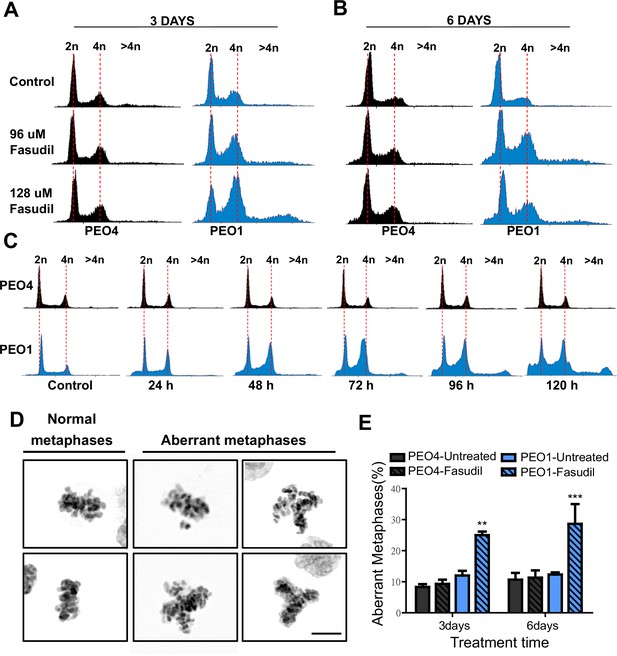
Fasudil treatment induces polyploidy and aberrant mitotic figures in BRCA2-deficient cells.
(A–B) Cell cycle analysis of PEO4 and PEO1 cells following 3 or 6 days of fasudil treatment (96 and 128 μM; N=3). Cells were stained with propidium iodide, and DNA content was analyzed via FACS (10,000 events per sample). (C) Cell cycle analysis of PEO4 and PEO1 cells following a time course with fasudil treatment (N=2; 1–5 days, 64 μM). Cells were stained with propidium iodide, and DNA content was analyzed via FACS (10,000 events per sample). (D) Representative images of DAPI-stained normal and aberrant metaphases. Aberrant metaphases include metaphases with DNA being pulled in multiple directions or metaphases with misaligned chromosomes. (E) Percent of aberrant metaphases in PEO4 and PEO1 cells 3 or 6 days after fasudil treatment (128 μM; N=3). A total of 100 metaphases were analyzed for each sample. Statistical analysis was performed using a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of independent experiments with the standard error of the mean.
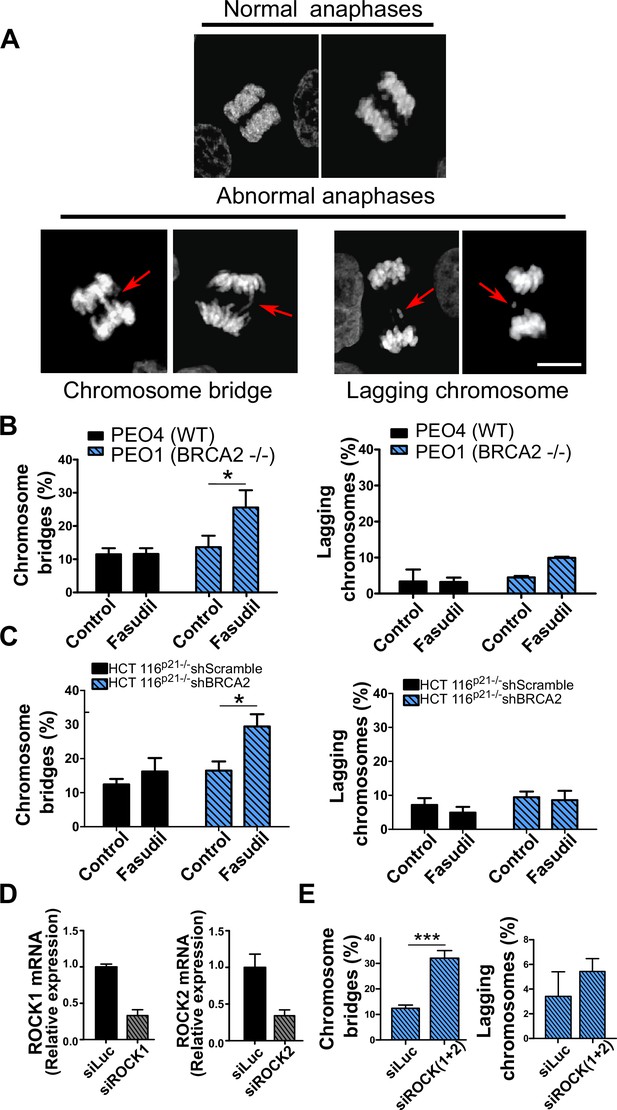
Mitotic DNA bridges accumulate in BRCA2-deficient cells following ROCK inhibition with fasudil.
(A) Representative images of normal and abnormal anaphases with bridges and lagging chromosomes. (B) Percentage of anaphases with chromosomes bridges and lagging chromosomes in PEO4 and PEO1 cells treated with fasudil (128 μM). Fifty to 70 anaphases per sample were analyzed in two independent experiments (N=2). (C) Percentage of anaphases with chromosomes bridges and lagging chromosomes in shScramble- or shBRCA2-transduced HCT116p21-/- cells treated with fasudil. 50–70 anaphases per sample were analyzed per independent experiment (N=3). (D) Quantitative real-time PCR of ROCK1 and ROCK2 in shBRCA2 HCT116p21-/- cells transfected with 150 μM of siROCK1 or siROCK2 (N=2). (E) Percentage of anaphases with chromosomes bridges and laggards in shBRCA2 HCT116p21-/- cells transfected with siROCK (1+2). A total of 50–70 anaphases per sample were analyzed in three independent experiments (N=2). The statistical analysis of the data was performed with a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of independent experiments with the standard error of the mean.
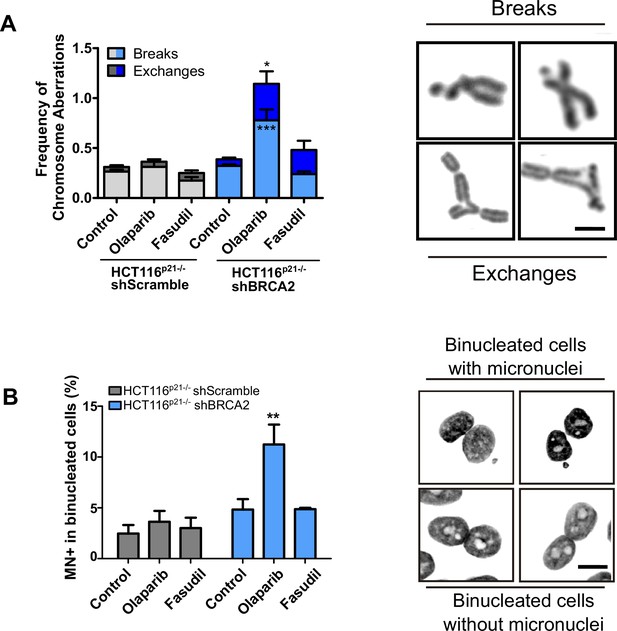
BRCA2-deficient cells treated with fasudil do not display replication stress-derived chromosome defects.
(A) Frequency of chromosome aberrations in shScramble or shBRCA2 HCT116p21-/- cells following treatment with olaparib (0.5 μM) or fasudil (32 μM) (N=3). A total of 50 metaphases were analyzed per condition. Chromosome aberrations include chromatid breaks and chromatid exchanges. Representative images of a chromatid break and a chromatid exchange are shown on the right. (B) Percent of shScramble or shBRCA2 HCT116p21-/- cells with micronuclei in binucleated cells (N=2). On the right are representative images of binucleated cells with and without micronuclei. Twenty-four h after seeding, cells were treated with the indicated inhibitors and 24 h later with cytochalasin B for 30 hr. A total of 300–400 cells were analyzed per independent experiment. Statistical analysis of all figures were performed with a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of independent experiments with the standard error of the mean.
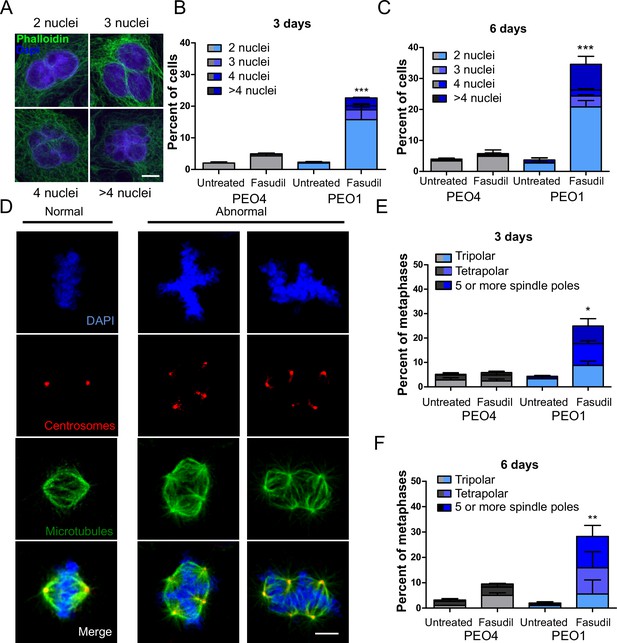
BRCA2-deficient cells exhibit cytokinesis failure, centrosome amplification and multipolar mitotic spindles following fasudil treatment.
(A) Representative pictures of PEO1 cells after fasudil treatment. Nuclei are stained with DAPI (shown in blue), and the cytoplasm of individual cells is stained with phalloidin which stains the actin cytoskeleton (shown in green). (B) Percent of binucleated and multinucleated PEO4 and PEO1 cells after 3 days of fasudil treatment (N=3, 128 μM). (C) Percent of binucleated and multinucleated number of PEO4 and PEO1 cells after 6 days of fasudil treatment (N=3, 128 μM). A total of 200 cells were analyzed per sample. (D) Representative pictures of PEO1 metaphases showing cells with normal and abnormal mitotic spindles. DNA, centrosomes, and microtubules are shown in blue, red, and green, respectively. (E) Percent of metaphases in PEO4 and PEO1 cells with multipolar spindles after 3 days of fasudil treatment (N=3, 128 μM). (F) Percent of metaphase in PEO4 and PEO1 cells with multipolar spindles after 6 days of fasudil treatment (N=2, 128 μM). Mitotic spindles were visualized by staining centrosomes (γ-tubulin) and microtubules (α-tubulin) and DNA was stained with DAPI. Cells were classified as having multipolar spindle (3, 4, or 5 or more spindles). A total of 100 metaphases were analyzed per sample. Statistical analysis was performed using a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of independent experiments with the standard error of the mean.
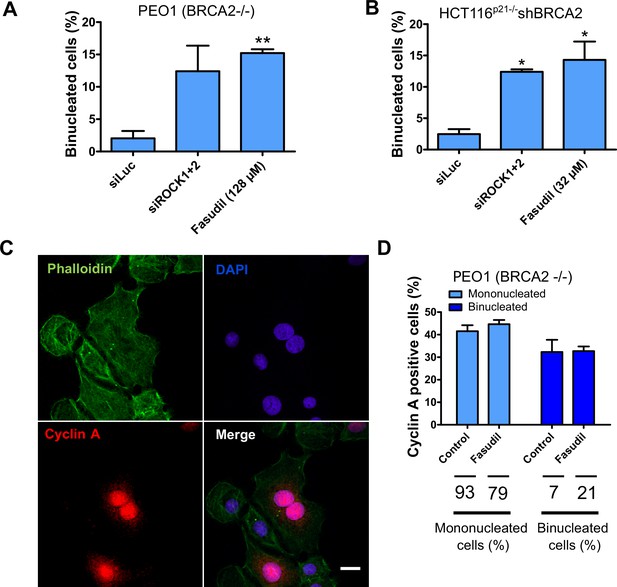
Multinucleated BRCA2-deficient cells resulting from fasudil treatment are able to transit through S phase.
(A) Percent of binucleated PEO1 cells transfected with siROCK (1+2) or treated with fasudil (N=2). (B) Percent of binucleated shBRCA2 HCT116p21-/- cells transfected with siROCK (1+2) or treated with fasudil (N=2). (C) Representative PEO1 cells stained with DAPI, Cyclin A (S phase marker, red) and Phalloidin (actin cytoskeleton, green) after fasudil treatment (3 days, 128 μM). (D) Quantification of cyclin A positive cells in each group: mononucleated; binucleated. The percentage of mononucleated or binucleated over total cells is shown in the lower part of the panel. Statistical analysis was performed using a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of two independent experiments with the standard error of the mean.
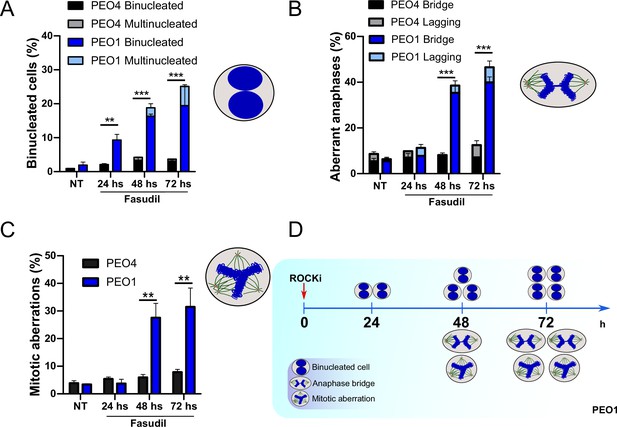
Binucleation precedes anaphase and mitotic aberrations in BRCA2-deficient cells.
(A) Percent of binucleated PEO1 and PEO4 cells treated with fasudil at the indicated time points after treatment (N=2). (B) Percent of aberrant anaphases in PEO1 and PEO4 cells treated with fasudil at the indicated time points after treatment (N=2). (C) Percent of mitotic aberrations in PEO1 and PEO4 cells treated with fasudil at the indicated time points after treatment (N=2). For panels A to C, statistical analysis was performed using a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of independent experiments with the standard error of the mean. (D) Representative scheme of the results obtained in A-C.
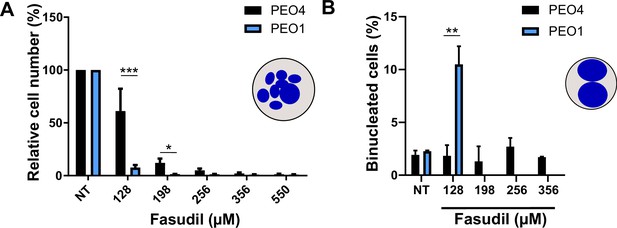
Binucleation-related cell death is not triggered in BRCA2-proficient cells.
(A) Relative cell number (%) of PEO4 and PEO1 cells at 6 days after treatment with different doses of fasudil (N=2). (B) Percent of binucleated PEO4 cells treated with different doses of fasudil (N=2). The percentage of binucleated in PEO1 cells was only quantified in fasudil 128 μM to serve as a positive control of binucleated cells. Statistical analysis was performed using a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of independent experiments with the standard error of the mean.
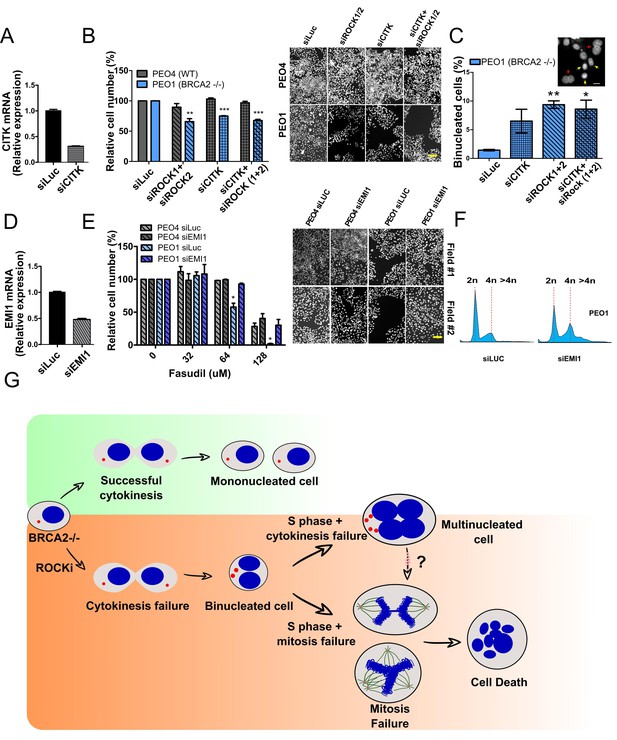
Mitosis as an alternative synthetic lethality strategy for BRCA2-deficient cells.
(A) Quantitative real-time PCR of CITK in shBRCA2 HCT116p21-/- cells transfected with 150 μM of siCITK (N=2). (B) Relative cell number (%) of PEO4 and PEO1 after 6 days of being transfected with siROCK (1+2), siCITK or siROCK (1+2)/siCITK and representative images of the transfected cells (N=2). (C) Percent of binucleated PEO1 cells transfected with siROCK (1+2), CITK or siROCK (1+2)/siCITK (N=2). (D) Quantitative real-time PCR of EMI1 in shBRCA2 HCT116p21-/- cells transfected with 150 μM of siEMI1 (N=2). (E) Relative cell number (%) of PEO4 and PEO1 after 6 days of being transfected with siEMI1 and treated with fasudil (N=2). Representative images of the transfected and treated cells. (F) Cell cycle analysis of PEO1 cells following transfection with siEMI1 for 48hs (N=2). Cells were stained with propidium iodide and DNA content was analyzed via FACS (10,000 events per sample). (G) Model depicting the events leading to BRCA2-deficient cell death after fasudil treatment. The inhibition or depletion of ROCK in BRCA2-deficient cells leads to cytokinesis failure. As a result, the daughter cells are binucleated (4N) and have extra centrosomes (two instead of one). We speculate that after a subsequent DNA duplication, these cells can attempt mitosis. Mitosis entry with increased DNA content and extra centrosomes may frequently give rise to abnormal and multipolar spindles, leading to misaligned chromosomes and mitotic failure due to multipolar spindle formation. Alternatively, cytokinesis may fail again, and cells may temporarily survive as multinucleated cells, possibly facing cell death during subsequent mitotic attempts.
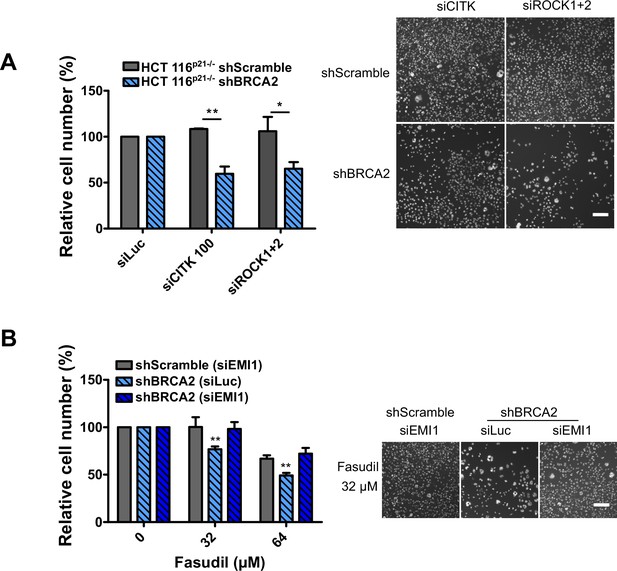
Mitosis as an alternative synthetic lethality strategy for BRCA2-deficient cells.
(A) Relative cell number (%) of shScramble and shBRCA2 HCT116p21-/- cells at 6 days after transfection with siCITK or siROCK (1+2) (N=2). Representative images are shown on the right. (B) Relative cell number (%) of shScramble and shBRCA2 HCT116p21-/- cells at 6 days after transfection with siEMI1. Samples were treated with fasudil when indicated (N=2). Representative images are shown on the right. Statistical analysis was performed using a two-way ANOVA test followed by a Bonferroni post-test (*p<0.05, **p<0.01, ***p<0.001). Data are shown as the average of independent experiments with the standard error of the mean.
Tables
Phenotypic screening identifies ROCK kinases as potential targets for synthetic lethality in BRCA2 cells.
(A) Table listing all ROCK inhibitors from the PKIS2 library and their corresponding survival difference at 0.1 and 1 μM.
| Survival difference | ||
|---|---|---|
| Inhibitor | 0.1 µM | 1 µM |
| GSK180736A | 0 | 8.15 |
| GSK248233B | 47.57 | 41.99 |
| GSK269962B | 25.58 | 28.49 |
| GSK270822A | 0 | 38.12 |
| GSK429286A | 0.29 | 18.11 |
| GSK466314A | 0 | 25.41 |
| GSK534911A | 25.5 | 33.72 |
| GSK534913A | 0 | 32.50 |
| SB-772077-B | 0 | 67.80 |





