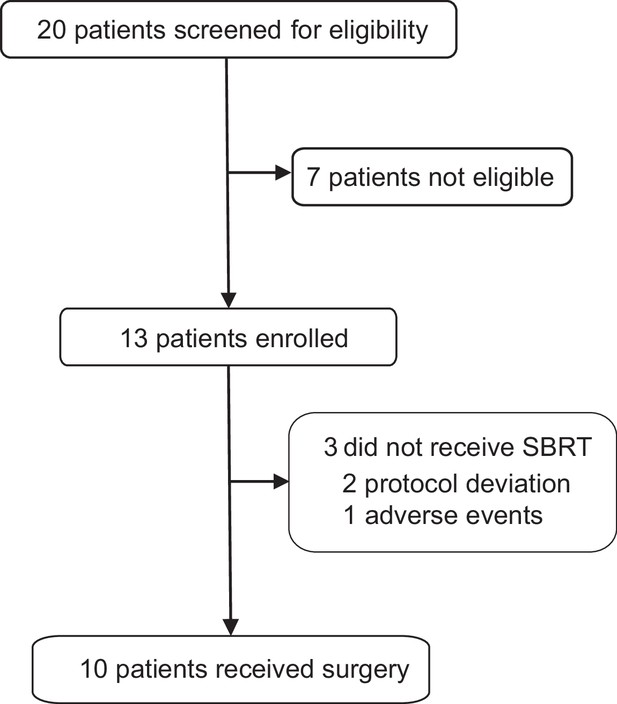
Effects of neoadjuvant stereotactic body radiotherapy plus adebrelimab and chemotherapy for triple-negative breast cancer: A pilot study
Figures
Figure 2
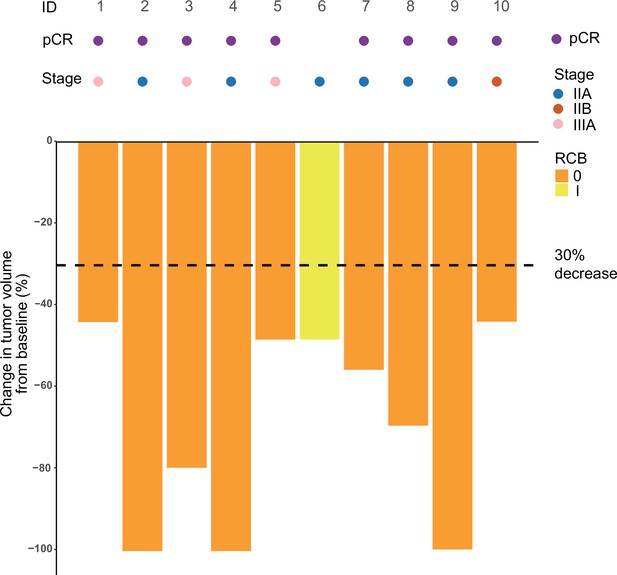
Swimming plot which demonstrates the pCR, RCB, and radiological response profile of 10 modified intention-to-treat population who received radiotherapy and undergone surgery.
Each round dot or column indicates a patient. Colors indicate different clinical stage. ID, identity; pCR, pathological complete response; RCB, residual cancer burden.
-
Figure 2—source code 1
R Code for Figure 2.
- https://cdn.elifesciences.org/articles/91737/elife-91737-fig2-code1-v1.zip
-
Figure 2—source data 1
Raw information for the pCR, RCB, and radiological response profile of 10 modified intention-to-treat population who received radiotherapy and underwent surgery.
- https://cdn.elifesciences.org/articles/91737/elife-91737-fig2-data1-v1.xlsx
Tables
Table 1
Baseline characteristics.
| Characteristic | Patients (n=13) |
|---|---|
| Age (median, range) | 51 (31–68) |
| Age group, years | |
| ≤50 | 6 (46.2%) |
| >50 | 7 (53.8%) |
| Menopausal status | |
| Premenopausal | 6 (46.2%) |
| Postmenopausal | 7 (53.8%) |
| Tumor size | |
| T2 | 10 (76.9%) |
| T3 | 3 (23.1%) |
| Lymph node status | |
| N0 | 6 (46.2%) |
| N1 | 2 (15.4%) |
| N2 | 5 (38.5%) |
| Clinical stage | |
| IIA | 6 (46.2%) |
| IIB | 1 (7.7%) |
| IIIA | 6 (46.2%) |
| Tumor grade | |
| II | 6 (46.2%) |
| III | 5 (38.5%) |
| Unknown | 2 (15.4%) |
| HER2 expression | |
| Negative | 8 (61.5%) |
| 1+ | 3 (23.1%) |
| 2+, FISH- | 2 (15.4%) |
Table 2
Pathological and clinical response.
| Variable | Patients (n=10) |
|---|---|
| Total pathological complete response | 9 (90%) |
| Residual cancer burden score | |
| 0 | 9 (90%) |
| I | 1 (10%) |
| II | 0 |
| III | 0 |
| Radiological response | |
| Complete response | 3 (30%) |
| Partial response | 7 (70%) |
| Stable disease | 0 |
| Objective response rate | 10 (100%) |
Table 3
Treatment-related adverse events.
| Patients (n=13) | |||
|---|---|---|---|
| Grade 1 or 2 | Grade 3 | Grade 4 | |
| Total | 5 (38.5%) | 6 (46.2%) | 1 (7.7%) |
| Anemia | 8 (61.5%) | 1 (7.7%) | 0 |
| Alopecia | 9 (69.2%) | 0 | 0 |
| Neutropenia | 4 (30.8%) | 3 (23.1%) | 1 (7.7%) |
| Hyponatremia | 8 (61.5%) | 0 | 0 |
| Nausea | 8 (61.5%) | 0 | 0 |
| Leukopenia | 6 (46.2%) | 1 (7.7%) | 0 |
| Lymphopenia | 7 (53.8%) | 0 | 0 |
| Thrombocytopenia | 5 (38.5%) | 1 (7.7%) | 0 |
| Elevated alanine aminotransferase level | 5 (38.5%) | 0 | 0 |
| Elevated aspartate aminotransferase level | 5 (38.5%) | 0 | 0 |
| Thyroid stimulating hormone decreased | 5 (38.5%) | 0 | 0 |
| Hyperuricemia | 5 (38.5%) | 0 | 0 |
| Vomiting | 5 (38.5%) | 0 | 0 |
| Fatigue | 4 (30.8%) | 0 | 0 |
| γ-Glutamyl transferase increased | 3 (23.1%) | 0 | 0 |
| Creatinine increased | 3 (23.1%) | 0 | 0 |
| Thyroid stimulating hormone increased | 3 (23.1%) | 0 | 0 |
| Creatine phosphokinase elevation | 1 (7.7%) | 1 (7.7%) | 0 |
| Hyperthyroidism | 2 (15.4%) | 0 | 0 |
| Free thyroid hormone decreased | 2 (15.4%) | 0 | 0 |
| Hyperglycemia | 2 (15.4%) | 0 | 0 |
| Hypokalemia | 2 (15.4%) | 0 | 0 |
| Rash | 2 (15.4%) | 0 | 0 |
| Peripheral neuropathy | 2 (15.4%) | 0 | 0 |
| Diarrhea | 0 | 1 (7.7%) | 0 |
| Immune mediated myositis | 1 (7.7%) | 0 | 0 |
| Ventricular extrasystole | 1 (7.7%) | 0 | 0 |
| Free thyroid hormone increased | 1 (7.7%) | 0 | 0 |
| Hypothyroidism | 1 (7.7%) | 0 | 0 |
| Troponin I elevated | 1 (7.7%) | 0 | 0 |
| Proteinuria | 1 (7.7%) | 0 | 0 |
| Constipation | 1 (7.7%) | 0 | 0 |
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/91737/elife-91737-mdarchecklist1-v1.docx
-
Reporting standard 1
The STROBE checklist.
- https://cdn.elifesciences.org/articles/91737/elife-91737-repstand1-v1.docx
Download links
A two-part list of links to download the article, or parts of the article, in various formats.
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Effects of neoadjuvant stereotactic body radiotherapy plus adebrelimab and chemotherapy for triple-negative breast cancer: A pilot study
eLife 12:e91737.
https://doi.org/10.7554/eLife.91737