Endogenous hydrogen peroxide positively regulates secretion of a gut-derived peptide in neuroendocrine potentiation of the oxidative stress response in Caenorhabditis elegans
eLife Assessment
This study presents convincing evidence of the role of an intestine-released neuropeptide, FLP-2, in the oxidative stress response of C. elegans, as well as for the neural circuit pathway that regulates its release in response to sensing reactive oxygen species (i.e., H2O2). These valuable results advance the understanding of gut-brain signaling and the neural circuit basis of behavioral responses to stress.
https://doi.org/10.7554/eLife.97503.3.sa0Valuable: Findings that have theoretical or practical implications for a subfield
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Convincing: Appropriate and validated methodology in line with current state-of-the-art
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
The gut-brain axis mediates bidirectional signaling between the intestine and the nervous system and is critical for organism-wide homeostasis. Here, we report the identification of a peptidergic endocrine circuit in which bidirectional signaling between neurons and the intestine potentiates the activation of the antioxidant response in Caenorhabditis elegans in the intestine. We identify an FMRF-amide-like peptide, FLP-2, whose release from the intestine is necessary and sufficient to activate the intestinal oxidative stress response by promoting the release of the antioxidant FLP-1 neuropeptide from neurons. FLP-2 secretion from the intestine is positively regulated by endogenous hydrogen peroxide (H2O2) produced in the mitochondrial matrix by sod-3/superoxide dismutase, and is negatively regulated by prdx-2/peroxiredoxin, which depletes H2O2 in both the mitochondria and cytosol. H2O2 promotes FLP-2 secretion through the DAG and calcium-dependent protein kinase C family member pkc-2 and by the SNAP25 family member aex-4 in the intestine. Together, our data demonstrate a role for intestinal H2O2 in promoting inter-tissue antioxidant signaling through regulated neuropeptide-like protein exocytosis in a gut-brain axis to activate the oxidative stress response.
Introduction
The gut-brain axis is critical for communication between the intestine and the nervous system to regulate behavior and maintain homeostasis, and altered gut-brain signaling is associated with neurodegeneration, obesity, and tumor proliferation (Carabotti et al., 2015; Grenham et al., 2011; Mayer et al., 2022; Mehrian-Shai et al., 2019; Vitali et al., 2022). Over the last decade the importance of peptides that function as signals in gut-brain signaling has gained recognition. Numerous gut peptides are distributed throughout the gastrointestinal (GI) tract with regional specificity (Haber et al., 2017), and gut-secreted peptides can modulate neurocircuits that regulate feeding behavior and glucose metabolism (Batterham and Bloom, 2003; Han et al., 2018; Song et al., 2019), inflammatory responses against pathogenic bacteria (Campos-Salinas et al., 2014; Yu et al., 2021), and satiety (Batterham et al., 2002; Chelikani et al., 2005; Gibbs et al., 1973; Lutz et al., 1995; Lutz et al., 1994; West et al., 1984). A gut-released peptide suppresses arousal through dopaminergic neurons during sleep in Drosophila (Titos et al., 2023). In Caenorhabditis elegans, gut-derived peptides regulate rhythmic behavior and behavioral responses to pathogenic bacteria (Lee and Mylonakis, 2017; Singh and Aballay, 2019; Wang et al., 2013). Conversely, peptides released from the nervous system regulate many aspects of intestinal function including gut mobility, inflammation, and immune defense (Browning and Travagli, 2014; Furness et al., 2014; Lai et al., 2017). In C. elegans, the secretion of peptides from various neurons regulates the mitochondrial unfolded protein response (UPRmt), the heat shock response, and the antioxidant response in the intestine (Jia and Sieburth, 2021; Maman et al., 2013; Prahlad et al., 2008; Shao et al., 2016). In spite of the many roles of peptides in the gut-brain axis, the mechanisms underlying the regulation of intestinal peptide secretion and signaling remain to be fully defined.
Hydrogen peroxide (H2O2) is emerging as an important signaling molecule that regulates intracellular signaling pathways by modifying specific reactive residues on target proteins. For example, H2O2-regulated phosphorylation of inhibitor of nuclear factor κB (NF-κB) kinase leads to the activation of NF-κB during development, inflammation, and immune responses (Kamata et al., 2002; Oliveira-Marques et al., 2009; Takada et al., 2003). In addition, H2O2-induced tyrosine and cysteine modifications contribute to redox regulation of c-Jun N-terminal kinase 2 (JNK2), Src family kinase, extracellular signal-regulated kinases 1 and 2 (ERK1/2), protein kinase C (PKC), and other protein kinases (Kemble and Sun, 2009; Konishi et al., 1997; Lee et al., 2003; Nelson et al., 2018). H2O2 signaling has been implicated in regulating neurotransmission and transmitter secretion. H2O2 at low concentration increases neurotransmission at neuromuscular junctions without influencing lipid oxidation (Giniatullin and Giniatullin, 2003; Giniatullin et al., 2019; Shakirzyanova et al., 2009), and enhanced endogenous H2O2 generation regulates dopamine release (Avshalumov et al., 2005; Avshalumov and Rice, 2003; Bao et al., 2005; Chen et al., 2001a; Chen et al., 2002). Acute H2O2 treatment increases exocytosis of ATP-containing vesicles in astrocytes (Li et al., 2019). Finally, mitochondrially derived H2O2 regulates neuropeptide release from neurons in C. elegans (Jia and Sieburth, 2021). Cellular H2O2 levels are tightly controlled through the regulation of its production from superoxide by superoxide dismutases (SODs) and cytoplasmic oxidases (Fridovich, 1995; Fridovich, 1997; Messner and Imlay, 2002; Zelko et al., 2002), and through its degradation by catalases, peroxidases, and peroxiredoxins (Chance et al., 1979; Marinho et al., 2014). In the intestine, endogenously produced H2O2 plays important roles as an antibacterial agent in the lumen, and in activating the ER unfolded protein response (UPRER) through protein sulfenylation (Botteaux et al., 2009; Corcionivoschi et al., 2012; Hourihan et al., 2016; Miller et al., 2020).
Here, we demonstrate a role for endogenous H2O2 signaling in the intestine in regulating the release of the intestinal FMRF-amide-like peptide, FLP-2, to modulate a neurocircuit that activates the antioxidant response in the intestine in C. elegans. Intestinal FLP-2 signaling functions by potentiating the release of the antioxidant neuropeptide-like protein FLP-1 from AIY interneurons, which in turn activates the antioxidant response in the intestine. FLP-2 secretion from the intestine is rapidly and positively regulated by H2O2, whose levels are positively regulated by superoxide dismutases in the mitochondrial matrix and cytosol, and negatively regulated by the peroxiredoxin-thioredoxin system in the cytosol. Intestinal FLP-2 release is mediated by aex-4/SNAP25-dependent exocytosis of dense core vesicles (DCVs) and H2O2-induced FLP-2 secretion is dependent upon the production of intestinal diacylglycerol (DAG) and on pkc-2/PKCα/β kinase activity.
Results
Neuronal FLP-1 secretion is regulated by neuropeptide signaling from the intestine
We previously showed that 10 min treatment with the mitochondrial toxin juglone leads to a rapid, reversible, and specific increase in FLP-1 secretion from AIY, as measured by a twofold increase in coelomocyte fluorescence in animals expressing FLP-1::Venus fusion proteins in AIY (Figure 1A and B; Jia and Sieburth, 2021). Coelomocytes take up secreted neuropeptides by bulk endocytosis (Fares and Greenwald, 2001) and the fluorescence intensity of Venus in their endocytic vacuoles is used as a measure of regulated neuropeptide secretion efficacy (Ailion et al., 2014; Ch’ng et al., 2008; Sieburth et al., 2007). To determine the role of the intestine in regulating FLP-1 secretion, we first examined aex-5 mutants. aex-5 encodes an intestinal subtilisin/kexin type 5 prohormone convertase that functions to proteolytically process peptide precursors into mature peptides in DCVs (Edwards et al., 2019; Thacker and Rose, 2000), and aex-5 mutants are defective in peptide signaling from the intestine (Mahoney et al., 2008). We found that aex-5 mutants expressing FLP-1::Venus in AIY exhibited no significant difference in coelomocyte fluorescence compared to wild-type controls in the absence of juglone (Figure 1B). However, coelomocyte fluorescence did not significantly increase in aex-5 mutants treated with juglone. Expression of aex-5 cDNA selectively in the intestine (under the ges-1 promoter) fully restored normal responses to juglone to aex-5 mutants, whereas aex-5 cDNA expression in the nervous system (under the rab-3 promoter) failed to rescue (Figure 1B). Thus, peptide processing in intestinal DCVs is necessary for juglone-induced FLP-1 secretion from AIY.
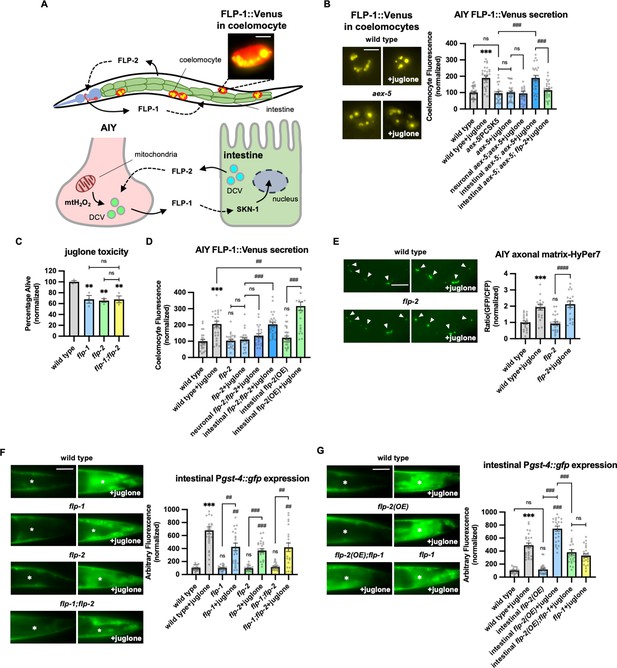
Peptidergic gut-to-neuron FLP-2 signaling potentiates the oxidative stress response.
(A) (Top) Schematic showing the positions of AIY, intestine, and coelomocytes of transgenic animals co-expressing FLP-1::Venus in the intestine and mCherry in coelomocytes. Representative image of the posterior coelomocyte that has taken up Venus into the endocytic compartment. Scale bar: 5 μM. (Bottom) Schematic showing FLP-1 and FLP-2 peptides as inter-tissue signals in gut-intestine regulation of the antioxidant response. (B) Representative images and quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-1::Venus fusion proteins in AIY following M9 or 300 μM juglone treatment for 10 min. Neuronal aex-5 denotes expression of aex-5 cDNA under the rab-3 promoter; intestinal aex-5 denotes expression of aex-5 cDNA under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’. n=30, 30, 24, 30, 26, 30, 30 independent animals. Scale bar: 5 μM. (C) Average percentage of surviving young adult animals of the indicated genotypes after 16 hr recovery following 4 hr juglone treatment. Unlined ** denotes statistical significance compared to ‘wild type’. n=213, 156, 189, 195 independent biological samples over three independent experiments. (D) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-1::Venus fusion proteins in AIY following M9 or 300 μM juglone treatment for 10 min. Neuronal flp-2 denotes expression of flp-2 gDNA under the rab-3 promoter; intestinal flp-2 denotes expression of flp-2 gDNA under the ges-1 promoter; intestinal flp-2(OE) denotes expression of flp-2 gDNA under the ges-1 promoter in wild-type animals. Unlined *** and ns denote statistical significance compared to ‘wild type’. n=20, 20, 25, 20, 20, 20, 25, 22 independent animals. (E) Representative images and quantification of fluorescence of mitochondrial matrix-targeted HyPer7 in the axon of AIY following M9 or 300 μM juglone treatment for 10 min. Arrowheads denote puncta marked by mito::HyPer7 fusion proteins (excitation: 500 and 400 nm; emission: 520 nm). Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation was used to measure H2O2 levels. Unlined *** and ns denote statistical significance compared to ‘wild type’. n=24, 22, 25, 24 independent animals. Scale bar: 10 μM. (F) Representative images and quantification of average fluorescence in the posterior intestine of transgenic animals expressing Pgst-4::gfp after 1h M9 or juglone exposure and 3 hr recovery. Asterisks mark the intestinal region used for quantification. Pgst-4::gfp expression in the body wall muscles, which appears as fluorescence on the edge animals in some images, was not quantified. Unlined *** and ns denote statistical significance compared to ‘wild type’; unlined ## and ### denote statistical significance compared to ‘wild type+juglone’. n=25, 26, 25, 25, 25, 25, 25, 25 independent animals. Scale bar: 10 μM. (G) Representative images and quantification of average fluorescence in the posterior region of transgenic animals expressing Pgst-4::gfp after 1 hr M9 or juglone exposure and 3 hr recovery. Asterisks mark the intestinal region for quantification. Pgst-4::gfp expression in the body wall muscles, which appears as fluorescence on the edge animals in some images, was not quantified. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical significance compared to ‘wild type+juglone’. n=23, 25, 25, 26, 24, 25 independent animals. Scale bar: 10 μM. (B–G) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, ** and ## p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 1—source data 1
Raw data used for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig1-data1-v1.xlsx
Next, we examined a number of mutants with impaired SNARE-mediated vesicle release in the intestine including aex-1/UNC13, aex-3/MADD, aex-4/SNAP25b, and aex-6/Rab27 (Figure 2C; Iwasaki et al., 1997; Mahoney et al., 2006; Thacker and Rose, 2000; Thomas, 1990; Wang et al., 2013), and they each exhibited no increases in FLP-1 secretion following juglone treatment above levels observed in untreated controls (Figure 1—figure supplement 1A). NLP-40 is a neuropeptide-like protein whose release from the intestine is presumed to be controlled by aex-1, aex-3, aex-4, and aex-6 (Lin-Moore et al., 2021; Mahoney et al., 2008; Shi et al., 2022; Wang et al., 2013). Null mutants in nlp-40 or its receptor, aex-2 (Wang et al., 2013), exhibited normal juglone-induced FLP-1 secretion (Figure 1—figure supplement 1B). These results establish a gut-to-neuron signaling pathway that regulates FLP-1 secretion from AIY that is likely to be controlled by peptidergic signaling distinct from NLP-40.
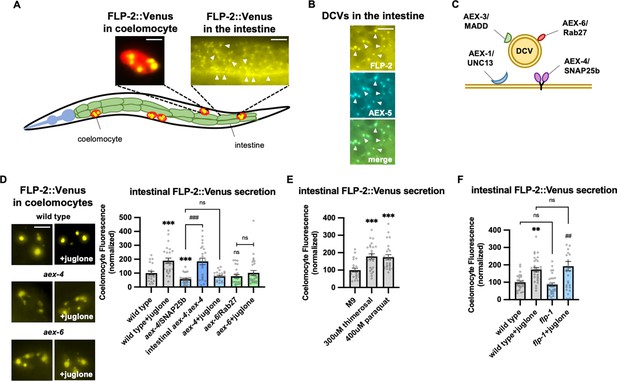
FLP-2 secretion from the intestine is stress regulated.
(A) Schematic showing the positions of intestine and coelomocytes of transgenic animals co-expressing FLP-2::Venus in the intestine and mCherry in coelomocytes. Representative images of the posterior coelomocyte that have taken up Venus into the endocytic compartment (scale bar: 5 μM) and the posterior intestinal region showing the distribution of FLP-2::Venus in puncta in the intestine are shown (scale bar: 15 μM). (B) Representative images of fluorescence distribution in the posterior intestinal region of transgenic animals co-expressing FLP-2::Venus and AEX-5::mTur2 fusion proteins. Arrowheads denote puncta marked by both fusion proteins. Scale bar: 5 μM. (C) Schematic showing the locations of AEX-1/UNC13, AEX-3/MADD, AEX-4/SNAP25, and AEX-6/Rab27 relative to a dense core vesicle (DCV). (D) Representative images and quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone for 10 min. Unlined *** and ns denote statistical significance compared to ‘wild type’. n=29, 25, 24, 30, 23, 30, 25, 25, 25 independent animals. Scale bar: 5 μM. (E) Quantification of average coelomocyte fluorescence of transgenic animals expressing FLP-2::Venus fusion proteins in the intestine following treatment with M9 buffer or the indicated stressors for 10 min. Unlined *** denotes statistical significane compared to ‘M9’. n=23, 25, 25 independent animals. (F) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Unlined ** denotes statistical significance compared to ‘wild type’; unlined ## denotes statistical significance compared to ‘flp-1’; a denotes statistical significance compared to ‘wild type+juglone’. n=30, 30, 30, 30 independent animals. (D–F) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, ** and ## p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 2—source data 1
Raw data used for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig2-data1-v1.xlsx
FLP-2 signaling from the intestine potentiates neuronal FLP-1 secretion and the oxidative stress response
flp-1 protects animals from the toxic effects of juglone (Jia and Sieburth, 2021). We reasoned that the intestinal signal that regulates FLP-1 secretion should also protect animals from juglone-induced toxicity. We identified the FMRF-amide neuropeptide-like protein, flp-2, in an RNA interference (RNAi) screen for neuropeptides that confer hypersensitivity to juglone toxicity upon knockdown (Jia and Sieburth, 2021). flp-2 signaling has been implicated in regulating lifespan, reproductive development, locomotion during lethargus, and the mitochondrial unfolded protein response (UPRmt) (Chai et al., 2022; Chen et al., 2016; Kageyama et al., 2022; Shao et al., 2016). Putative flp-2(ok3351) null mutants, which eliminate most of the flp-2 coding region, are superficially as healthy as wild-type animals, but they exhibited significantly reduced survival in the presence of juglone compared to wild-type controls (Figure 1C). The reduced survival rate of flp-2 mutants was similar to that of flp-1 mutants, and flp-1; flp-2 double mutants exhibited survival rates that were not more severe than those of single mutants (Figure 1C), suggesting that flp-1 and flp-2 may function in a common genetic pathway.
To determine whether flp-2 signaling regulates FLP-1 secretion from AIY, we examined FLP-1::Venus secretion. flp-2 mutants exhibited normal levels of FLP-1 secretion in the absence of stress, but FLP-1 secretion failed to significantly increase following juglone treatment of flp-2 mutants (Figure 1D). flp-2 is expressed in a subset of neurons as well as the intestine (Chai et al., 2022), and flp-2 functions from the nervous system for its roles in development and the UPRmt (Chai et al., 2022; Chen et al., 2016; Kageyama et al., 2022; Shao et al., 2016). Expressing a flp-2 genomic DNA, fragment (containing both the flp-2a and flp-2b isoforms that arise by alternative splicing), specifically in the nervous system failed to rescue the FLP-1::Venus defects of flp-2 mutants, whereas expressing flp-2 selectively in the intestine fully restored juglone-induced FLP-1::Venus secretion to flp-2 mutants (Figure 1D). These results indicate that flp-2 signaling is dispensable for FLP-1 secretion from AIY under normal conditions, but that flp-2 originating from the intestine is necessary to increase FLP-1 secretion during oxidative stress.
To address how flp-2 signaling regulates FLP-1 secretion from AIY, we examined H2O2 levels in AIY using a mitochondrially targeted pH-stable H2O2 sensor HyPer7 (mito-HyPer7, Pak et al., 2020). Mito-HyPer7 adopted a punctate pattern of fluorescence in AIY axons, and the average fluorescence intensity of axonal mito-HyPer7 puncta increased about twofold following 10 min juglone treatment (Figure 1E), in agreement with our previous studies using HyPer (Jia and Sieburth, 2021), confirming that juglone rapidly increases mitochondrial AIY H2O2 levels. flp-2 mutations had no significant effects on the localization or the average intensity of mito-HyPer7 puncta in AIY axons either in the absence of juglone or in the presence of juglone (Figure 1E), suggesting that flp-2 signaling promotes FLP-1 secretion by a mechanism that does not increase H2O2 levels in AIY. Consistent with this, intestinal overexpression of flp-2 had no effect on FLP-1::Venus secretion in the absence of juglone, but significantly enhanced the ability of juglone to increase FLP-1 secretion (Figure 1D). We conclude that both elevated mitochondrial H2O2 levels and intact flp-2 signaling from the intestine are necessary to increase FLP-1 secretion from AIY.
Previously we showed that FLP-1 signaling from AIY positively regulates the activation of the antioxidant transcription factor SKN-1/Nrf2 in the intestine. Specifically, flp-1 mutations impair the juglone-induced expression of the SKN-1 reporter transgene Pgst-4::gfp (Figure 1F; Jia and Sieburth, 2021). We found that mutations in flp-2 caused a similar reduction in juglone-induced Pgst-4::gfp expression as flp-1 mutants, and that flp-1; flp-2 double mutants exhibited similar impairments in juglone-induced Pgst-4::gfp expression as flp-1 or flp-2 single mutants (Figure 1F). Conversely, overexpression of flp-2 selectively in the intestine elevated juglone-induced Pgst-4::gfp expression, without altering baseline Pgst-4::gfp expression, and the elevated Pgst-4::gfp expression in juglone-treated animals overexpressing flp-2 was entirely dependent upon flp-1 (Figure 1G). It’s noteworthy that overexpressing flp-2 in the intestine did not enhance FLP-1::Venus release or Pgst-4::gfp expression in the absence of stress, indicating that the FLP-1 mediated antioxidant pathway by flp-2 is stress-activated. Together this data indicates that flp-2 signaling originating in the intestine positively regulates the stress-induced secretion of FLP-1 from AIY, as well as the subsequent activation of antioxidant response genes in the intestine. We propose that FLP-1 and FLP-2 define a bidirectional gut-neuron signaling axis, whereby during periods of oxidative stress, FLP-2 released from the intestine positively regulates FLP-1 secretion from AIY, and FLP-1, in turn, potentiates the antioxidant response in the intestine (Figure 1A).
FLP-2 secretion from the intestine is H2O2-regulated
To directly investigate the mechanisms underlying the regulation of FLP-2 secretion, we examined FLP-2::Venus fusion proteins expressed in the intestine under various conditions (Figure 2A). FLP-2::Venus fusion proteins adopted a punctate pattern of fluorescence throughout the cytoplasm of intestinal cells and at the plasma membrane (Figure 2A), and FLP-2::Venus puncta co-localized with the DCV cargo protein AEX-5/PCSK5 tagged to mTurquoise2 (AEX-5::mTur2, Figure 2B). FLP-2::Venus fluorescence was also observed in the coelomocytes (marked by mCherry) (Figure 2A and D), indicating that FLP-2 is released from the intestine. SNAP25 forms a component of the core SNARE complex, which drives vesicular membrane fusion and transmitter release (Chen and Scheller, 2001b; Goda, 1997; Jahn and Scheller, 2006). aex-4 encodes the C. elegans homolog of SNAP25, and mutations in aex-4 disrupt the secretion of neuropeptides from the intestine (Lin-Moore et al., 2021; Mahoney et al., 2008; Wang et al., 2013; Figure 2C). We found that aex-4 null mutations significantly reduced coelomocyte fluorescence in FLP-2::Venus-expressing animals, and expression of aex-4 cDNA selectively in the intestine fully restored FLP-2 secretion to aex-4 mutants (Figure 2D). Together these results suggest that intestinal FLP-2 can be packaged into DCVs that undergo release via SNARE-dependent exocytosis.
To test whether intestinal FLP-2 secretion is regulated by oxidative stress, we examined coelomocyte fluorescence in FLP-2::Venus-expressing animals that had been exposed to a number of different commonly used oxidative stressors. We found that 10 min exposure to juglone, thimerosal, or paraquat, which promote mitochondria-targeted toxicity (Castello et al., 2007; Elferink, 1999; Sharpe et al., 2012), each significantly increased Venus fluorescence intensity in the coelomocytes compared to untreated controls (Figure 2D and E). We conducted four controls for specificity: First, juglone treatment did not significantly alter fluorescence intensity of mCherry expressed in coelomocytes (Figure 2—figure supplement 1A). Second, impairing intestinal DCV secretion by either aex-4/SNAP25 or aex-6/Rab27 mutations (Lin-Moore et al., 2021; Mahoney et al., 2006; Mahoney et al., 2008; Thomas, 1990) blocked the juglone-induced increase in coelomocyte fluorescence in FLP-2::Venus-expressing animals (Figure 2C). Third, nlp-40 and nlp-27 encode neuropeptide-like proteins that are released from the intestine but are not implicated in stress responses (Liu et al., 2023; Taylor et al., 2021; Wang et al., 2013). Juglone treatment had no detectable effects on coelomocyte fluorescence in animals expressing intestinal NLP-40::Venus or NLP-27::Venus fusion proteins (Figure 2—figure supplement 1B and C), and NLP-40::mTur2 puncta did not overlap with FLP-2::Venus puncta in the intestine (Figure 2—figure supplement 1D). Finally, flp-1 mutants exhibited wild-type levels of FLP-2 secretion both in the absence and presence of juglone (Figure 2E). The distribution of FLP-2::Venus puncta in the intestine was not detectably altered by juglone treatment. Together, these results indicate that acute oxidative stress selectively increases the exocytosis of FLP-2-containing DCVs from the intestine, upstream of flp-1 signaling.
SOD-1 and SOD-3 superoxide dismutases regulate FLP-2 release
Juglone generates superoxide anion radicals (Ahmad and Suzuki, 2019; Paulsen and Ljungman, 2005) and juglone treatment of C. elegans increases ROS levels (de Castro et al., 2004) likely by promoting the global production of mitochondrial superoxide. Superoxide can then be rapidly converted into H2O2 by superoxide dismutase. To determine whether H2O2 impacts FLP-2 secretion, we first examined superoxide dismutase mutants. C. elegans encodes five superoxide dismutase genes (sod-1 through sod-5). sod-1 or sod-3 null mutations blocked juglone-induced FLP-2 secretion without altering baseline FLP-2 secretion, whereas sod-2, sod-4, or sod-5 mutations had no effect on FLP-2 secretion in the presence of juglone (Figure 3A and B, Figure 3—figure supplement 1A). sod-1; sod-3 double mutants exhibited juglone-induced FLP-2 secretion defects that was similar to single mutants, without significantly altering FLP-2 secretion in the absence of stress (Figure 3C). sod-1 encodes the ortholog of mammalian SOD1, which is a cytoplasmic SOD implicated in the development of amyotrophic lateral sclerosis and cancer (Giglio et al., 1994; Papa et al., 2014; Wang et al., 2021; Zhang et al., 2007). SOD-1::fusion proteins adopted a diffuse pattern of fluorescence in intestinal cells, consistent with a cytoplasmic localization (Figure 3D). Transgenes expressing the sod-1 cDNA selectively in the intestine fully rescued the juglone-induced FLP-2::Venus secretion defects of sod-1 mutants (Figure 3A). sod-3 encodes a homolog of mammalian SOD2, which is a mitochondrial matrix SOD implicated in protection against oxidative stress-induced neuronal cell death (Fukui and Zhu, 2010; Vincent et al., 2007). Intestinal SOD-3::GFP fusion proteins localized to round structures that were surrounded by the outer membrane mitochondrial marker TOMM-20::mCherry (Ahier et al., 2018), consistent with a mitochondrial matrix localization (Figure 3E). Expression of sod-3 cDNA in the intestine fully restored juglone-induced FLP-2 release to sod-3 mutants (Figure 3B). sod-3 variants lacking the mitochondrial localization sequence (sod-3(ΔMLS)) were no longer localized to mitochondria (Figure 3F) and failed to restore normal responsiveness to juglone to sod-3 mutants (Figure 3B). Thus, the generation of H2O2 by either SOD-1 in the cytoplasm or by SOD-3 in the mitochondrial matrix is necessary for juglone to increase FLP-2 secretion.
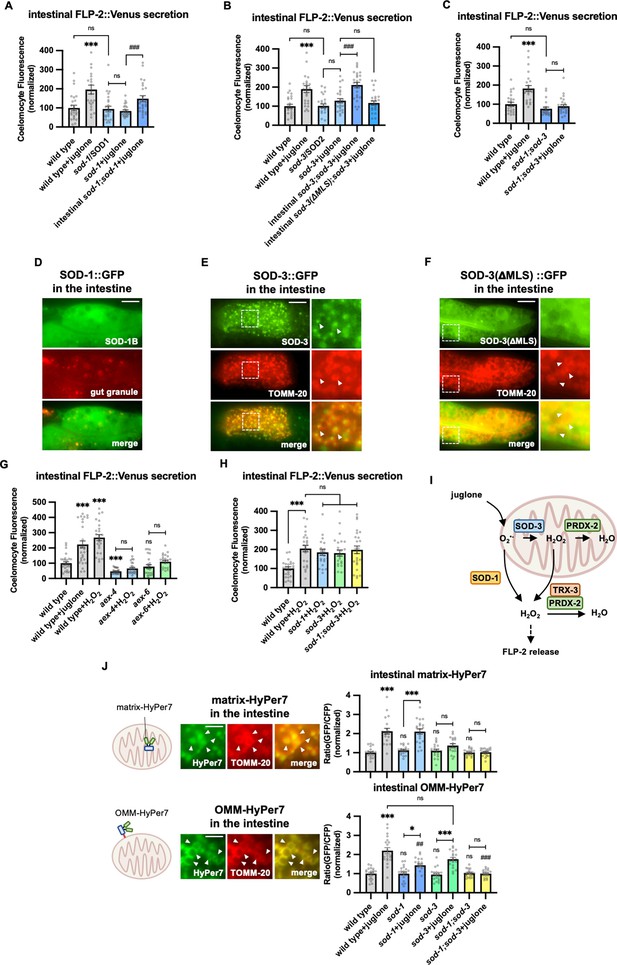
SOD-1/SOD-3 mediates endogenous H2O2 regulates FLP-2 release from the intestine.
(A) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal sod-1 denotes expression of sod-1b cDNA under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’. n=25, 22, 24, 24, 25 independent animals. (B) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal sod-3 and sod-3(ΔMLS) denote intestinal expression of sod-3 cDNA and sod-3(ΔMLS) variants, which lacks the mitochondrial localization sequence, under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’. n=25, 25, 25, 25, 25, 25 independent animals. (C) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Unlined *** denotes statistical significance compared to ‘wild type’. n=25, 25, 22, 25 independent animals. (D) Representative images of fluorescence distribution in the posterior intestinal region of transgenic animals expressing SOD-1b::GFP fusion proteins in contrast against autofluorescence of gut granules. Scale bar: 10 μM. (E) Representative images of fluorescence distribution in the posterior intestinal region of transgenic animals co-expressing SOD-3::GFP and TOMM-20::mCherry (to target mitochondria) fusion proteins. Scale bar: 15 μM. (F) Representative images of fluorescence distribution in the posterior intestinal region of transgenic animals co-expressing SOD-3(ΔMLS)::GFP and TOMM-20::mCherry fusion proteins. Scale bar: 15 μM. (G) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9, 300 μM juglone, or 1 mM H2O2 treatment for 10 min. Unlined *** and ns denote statistical significance compared to ‘wild type’. n=29, 30, 25, 25, 25, 24, 25 independent animals. (H) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 1 mM H2O2 treatment for 10 min. n=independent animals. (I) Schematic showing that SOD-1 and SOD-3 mediate juglone-induced H2O2 production in promoting FLP-2 release, and the PRDX-2/TRX-3 system detoxifies excessive H2O2. (J) Schematic, representative images and quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing mitochondrial matrix targeted HyPer7 (matrix-HyPer7) or mitochondrial outer membrane targeted HyPer7 (OMM-HyPer7) with TOMM-20::mCherry following M9 or 300 μM juglone treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. Unlined *** and ns denote statistical significance compared to ‘wild type’. Unlined ## and ### denote statistical significance compared to ‘wild type+juglone’. (Top) n=20, 20, 18, 20, 19, 19, 20, 20 independent animals. (Bottom) n=20, 20, 19, 20, 20, 20, 20, 20 independent animals. Scale bar: 5 μM. (A–C, G–H, and J) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, * p<0.05, ## p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 3—source data 1
Raw data used for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig3-data1-v1.xlsx
Next, to determine if H2O2 can regulate FLP-2 secretion, we treated animals acutely with exogenous H2O2. We found that 10 min H2O2 treatment increased FLP-2::Venus secretion to a similar extent as 10 min juglone treatment (Figue 3G). aex-4/SNAP25 or aex-6/Rab27 mutants exhibited no increase in FLP-2 secretion in response to H2O2 treatment compared to untreated controls (Figure 3G). In contrast, sod-1 or sod-3 mutants (or sod-1; sod-3 double mutants) exhibited an increase in FLP-2 secretion in response to H2O2 that was similar to that of wild-type controls (Figure 3H), suggesting that exogenous H2O2 can bypass the requirement of SODs but not SNAREs to promote FLP-2 secretion. Together these results suggest that H2O2 generated by SODs can positively regulate intestinal FLP-2 exocytosis from DCVs (Figure 3I).
SOD-1 and SOD-3 regulate intestinal mitochondrial H2O2 levels
To directly monitor H2O2 levels in the intestine, we generated transgenic animals in which HyPer7 was targeted to either the mitochondrial matrix (matrix-HyPer7) by generating fusion proteins with the cytochrome c MLS, or to the cytosolic face of the outer mitochondrial membrane (OMM-HyPer7) by generating fusion proteins with TOMM-20. When co-expressed in the intestine with the OMM marker TOMM-20::mCherry, matrix-HyPer7 formed round structures throughout the cytoplasm that were surrounded by the OMM, and OMM-HyPer7 formed ring-like structures throughout the cytoplasm that co-localized with the OMM marker (Figure 3J). Ten minute treatment with H2O2 significantly increased the fluorescence intensity by about twofold of both matrix-HyPer7 and OMM-HyPer7 without altering mitochondrial morphology or abundance (Figure 3J, Figure 3—figure supplement 1B and C), validating the utility of HyPer7 as a sensor for acute changes in H2O2 levels in and around intestinal mitochondria.
Juglone treatment for 10 min led to a similar twofold increase in matrix-HyPer7 fluorescence as H2O2 treatment (Figure 3J). sod-3 mutations did not alter baseline H2O2 levels in the matrix, but they completely blocked juglone-induced increased H2O2 levels, whereas sod-1 mutations had no effect on either baseline or juglone-induced increased H2O2 levels (Figure 3J). These results indicate that superoxide produced by juglone treatment is likely to be converted into H2O2 by SOD-3 in the mitochondrial matrix (Figure 3I).
Next, we examined H2O2 levels on the outer surface of mitochondria using OMM-HyPer7 and we found that juglone treatment led to a twofold increase in OMM-HyPer7 fluorescence, similar to H2O2 treatment (Figure 3J). sod-3 or sod-1 mutations did not alter baseline H2O2 levels on the OMM, but sod-1 single mutations attenuated juglone-induced increases in OMM H2O2 levels, while sod-3 mutations had no effect (Figure 3J). In sod-1; sod-3 double mutants, the juglone-induced increase in OMM H2O2 levels was completely blocked, whereas baseline H2O2 levels in the absence of stress were unchanged (Figure 3J). These results suggest that sod-3 and sod-1 are exclusively required for H2O2 production by juglone and that both mitochondrial SOD-3 and cytosolic SOD-1 contribute to H2O2 levels in the cytosol. One model that could explain these results is that juglone-generated superoxide is converted into H2O2 both by SOD-3 in the matrix and by SOD-1 in the cytosol, and that the H2O2 generated in the matrix can exit the mitochondria to contribute to cytosolic H2O2 levels needed to drive FLP-2 secretion (Figure 3I).
The peroxiredoxin-thioredoxin system regulates endogenous H2O2 levels and FLP-2 secretion
To determine whether endogenous H2O2 regulates FLP-2 secretion, we examined mutations in the peroxiredoxin-thioredoxin system. Peroxiredoxins and thioredoxins detoxify excessive H2O2 by converting it into water and they play a critical role in maintaining cellular redox homeostasis (Netto and Antunes, 2016; Figure 4A). C. elegans encodes two peroxiredoxin family members, prdx-2 and prdx-3, that are expressed at high levels in the intestine (Taylor et al., 2021). Null mutations in prdx-2 significantly increased FLP-2::Venus secretion compared to wild-type animals in the absence of stress (Figure 4B), whereas null mutations in prdx-3 had no effect on FLP-2 secretion (Figure 4—figure supplement 1A). We observed a corresponding increase in both matrix-HyPer7 and OMM-HyPer7 fluorescence intensity in prdx-2 mutants (Figure 4C and D), demonstrating that endogenous H2O2 is neutralized by peroxiredoxin and establishing a correlation between increased endogenous H2O2 levels and FLP-2 secretion. The increase in FLP-2 secretion in prdx-2 mutants was not further increased by juglone treatment (Figure 4B). These results suggest that elevation in the levels of endogenously produced H2O2 in the intestine can positively regulate FLP-2 secretion.
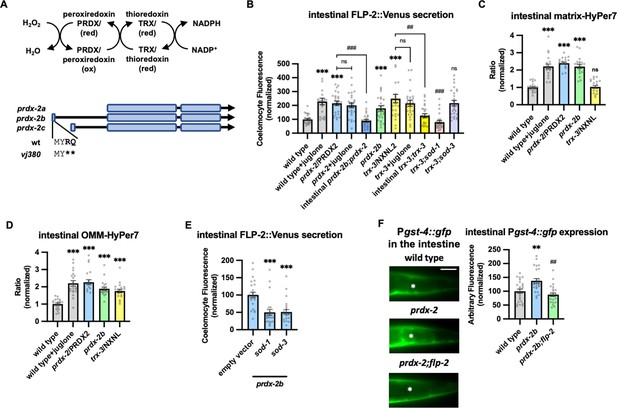
PRDX-2/PRDX and TRX-3/TRX regulate endogenous H2O2 and FLP-2 secretion.
(A) (Top) Schematic showing the PRDX/TRX system in H2O2 detoxification. (Bottom) Schematic showing the three isoforms of prdx-2 transcripts and vj380 allele of prdx-2b knockout. (B) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal prdx-2b denotes expression of prdx-2b cDNA under the ges-1 promoter. Intestinal trx-3 denotes expression of trx-3 cDNA under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ## and ### denote statistical significance compared to ‘trx-3’. n=25, 23, 25, 25, 25, 25, 25, 25, 25, 25, 25 independent animals. (C and D) Quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing matrix-HyPer7 (C) or OMM-HyPer7 (D) with TOMM-20::mCherry following M9 or 300 μM juglone treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. Unlined *** and ns denote statistical significance compared to ‘wild type’. (C) n=20, 20, 20, 20, 20 independent animals. (D) n=20, 20, 20, 20, 20 independent animals. (E) Quantification of average coelomocyte FLP-2::Venus fluorescence of transgenic animals fed with RNA interference (RNAi) bacteria targeting the indicated genes following M9 treatment for 10 min. Unlined *** denotes statistical significance compared to ‘empty vector’. n=25, 23, 24 independent animals. (F) Representative images and quantification of average fluorescence in the posterior region of transgenic animals expressing Pgst-4::gfp after 1 hr M9 or juglone exposure and 3 hr recovery. Asterisks mark the intestinal region for quantification. Pgst-4::gfp expression in the body wall muscles, which appears as fluorescence on the edge animals in some images, was not quantified. Unlined ** denotes statistical significance compared to ‘wild type’, unlined ## denotes statistical analysis compared to ‘prdx-2b’. n=25, 25, 25 independent animals. Scale bar: 10 μM. (B–F) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, ** and ## p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 4—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig4-data1-v1.xlsx
There are three isoforms of prdx-2 that arise by the use of alternative transcriptional start sites (Figure 4A). Expressing the prdx-2b isoform selectively in the intestine fully rescued the elevated FLP-2::Venus secretion defects of prdx-2 mutants, whereas expressing prdx-2a or prdx-2c isoforms failed to rescue (Figure 4B, Figure 4—figure supplement 1B and C). To independently verify the role of prdx-2b function in FLP-2 release, we generated a prdx-2b-specific knockout mutant by introducing an in-frame stop codon within the prdx-2b-specific exon 1 using CRISPR/Cas9 (prdx-2b(vj380); Figure 4A). prdx-2b(vj380) mutants exhibited increased H2O2 levels in the mitochondrial matrix and OMM (Figure 4C and D), as well as increased FLP-2::Venus secretion compared to wild-type controls that were indistinguishable from prdx-2 null mutants (Figure 4B). prdx-2b mutations could no longer increase FLP-2 secretion when either sod-1 or sod-3 activity was impaired (Figure 4E, Figure 4—figure supplement 1D). Thus, the prdx-2b isoform normally inhibits FLP-2 secretion likely by promoting the consumption of H2O2 in the mitochondrial matrix and/or cytosol.
Once oxidized, peroxiredoxins are reduced by thioredoxins (TRXs) for reuse (Netto and Antunes, 2016). trx-3 is an intestine-specific thioredoxin promoting protection against specific pathogen infections (Jiménez-Hidalgo et al., 2014; Miranda-Vizuete et al., 2000; Netto and Antunes, 2016). Mutations in trx-3 elevated FLP-2::Venus release in the absence of juglone and expressing trx-3 transgenes in the intestine restored wild-type FLP-2 release to trx-3 mutants (Figure 4B). Juglone treatment failed to further enhance FLP-2::Venus release in trx-3 mutants (Figure 4B). Mutations in cytoplasmic sod-1 but not in mitochondrial sod-3 reduced the elevated FLP-2::Venus release in trx-3 mutants to wild-type levels (Figure 4B). Mutations in trx-3 increased H2O2 levels in the OMM but had no effect on matrix H2O2 levels (Figure 4C and D). Thus, TRX-3 likely functions in the cytosol but not in the matrix to neutralize H2O2, and elevated H2O2 levels in the cytosol are sufficient to drive FLP-2 secretion without SOD-3-mediated H2O2 generation in the matrix.
Finally, to investigate the physiological significance of elevated endogenous H2O2 levels on the oxidative stress response, we examined the effects of prdx-2b mutations on expression of gst-4. prdx-2b mutants had significantly increased Pgst-4::gfp expression in the intestine compared to wild-type controls (Figure 4F). The increased Pgst-4::gfp expression in prdx-2b mutants was completely dependent upon flp-2 signaling, since gst-4 expression was reduced to wild-type levels in prdx-2b; flp-2 double mutants (Figure 4F). Together our data suggest that prdx-2b functions in the intestine to maintain redox homeostasis following SOD-1/SOD-3-mediated H2O2 production by regulating the secretion of FLP-2 (Figure 3I).
PKC-2/PKCα/β mediates H2O2-induced FLP-2 secretion from the intestine
H2O2 functions as a cellular signaling molecule by oxidizing reactive cysteines to sulfenic acid, and this modification on target proteins can regulate intracellular signaling pathways (García-Santamarina et al., 2014). One of the validated targets of H2O2 signaling is the PKC family of serine threonine kinases (Jia and Sieburth, 2021; Konishi et al., 1997; Konishi et al., 2001; Min et al., 1998). C. elegans encodes four PKC family members including pkc-1 and pkc-2, which are expressed at highest levels in the intestine (Islas-Trejo et al., 1997; Taylor et al., 2021). pkc-1 null mutants had no effect on baseline or juglone-induced FLP-2 secretion (Figure 5—figure supplement 1A). pkc-2 null mutations did not alter baseline intestinal FLP-2 secretion, but they eliminated juglone-induced FLP-2 secretion (Figure 5A). pkc-2 encodes a calcium and DAG stimulated PKCα/β PKC that regulates thermosensory behavior by promoting transmitter secretion (Edwards et al., 2012; Land and Rubin, 2017). Expressing pkc-2 cDNA selectively in the intestine fully restored juglone-induced FLP-2 secretion to pkc-2 mutants (Figure 5A), whereas expressing a catalytically inactive pkc-2(K375R) variant (Van et al., 2021) failed to rescue (Figure 5A). The intestinal site of action of pkc-2 is in line with prior studies showing that pkc-2 can function in the intestine to regulate thermosensory behavior (Land and Rubin, 2017). pkc-2 mutants exhibited wild-type H2O2 levels in the mitochondrial matrix and OMM of the intestine in both the presence and absence of juglone (Figure 5B and C). Increasing H2O2 levels by either acute H2O2 treatment or by prdx-2 mutation failed to increase FLP-2 secretion in pkc-2 mutants (Figure 5D and E). To determine whether pkc-2 can regulate the intestinal secretion of other peptides that are not associated with oxidative stress, we examined expulsion frequency, which is a measure of intestinal NLP-40 secretion (Mahoney et al., 2008; Wang et al., 2013). pkc-2 mutants showed wild-type expulsion frequency (Figure 5—figure supplement 1B), indicating that intestinal NLP-40 release is largely unaffected. Together, these results show that pkc-2 is not a general regulator of intestinal peptide secretion and instead functions downstream or in parallel to H2O2 to selectively promote FLP-2 secretion by a mechanism that involves phosphorylation of target proteins.
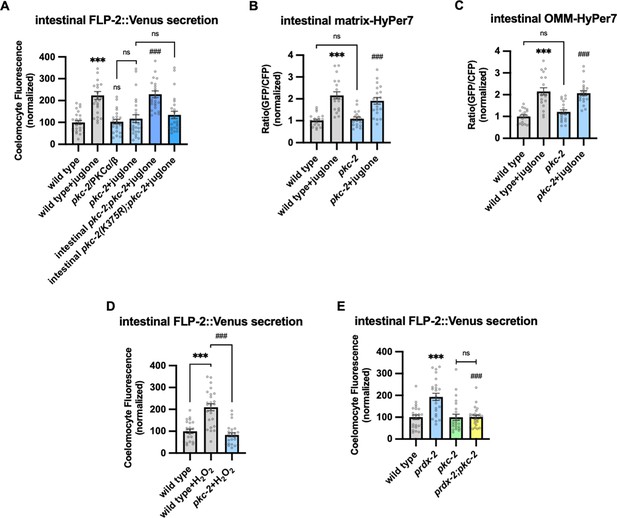
PKC-2/PKCα/β activation by H2O2 promotes FLP-2 secretion from the intestine.
(A) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal pkc-2 denotes expression of pkc-2b cDNA under the ges-1 promoter. Intestinal pkc-2b(K375R) denotes expression of pkc-2b(K375R) variants under the ges-1 promoter. Unlined *** and ns denote statistical significance compared to ‘wild type’; ### denotes statistical significance compared to ‘pkc-2+juglone’. n=24, 24, 25, 25, 25, 25 independent animals. (B and C) Quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing matrix-HyPer7 (B) or OMM-HyPer7 (C) with TOMM-20::mCherry following M9 or 300 μM juglone treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical analysis compared to ‘pkc-2’. (B) n=20, 20, 19, 20 independent animals, (C) n=20, 20, 20, 20 independent animals. (D) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 1 mM H2O2 treatment for 10 min. n=23, 25, 25 independent animals. (E) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 treatment for 10 min. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical significance compared to ‘prdx-2’. n=25, 25, 25, 25 independent animals. (A–E) Data are mean values ± s.e.m. normalized to wild-type controls. ns, not significant, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test.
-
Figure 5—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig5-data1-v1.xlsx
DAG positively regulates FLP-2 secretion
PKCα/β family members contain two N-terminal C1 domains (C1A and C1B) whose binding to DAG promotes PKC recruitment to the plasma membrane (Burns and Bell, 1991; Darby et al., 2017; Johnson et al., 2000; Kim et al., 2016; Ono et al., 1989; Yanase et al., 2011). To address the role of DAG in promoting FLP-2 secretion by PKC-2, we examined mutants that are predicted to have altered DAG levels. Phosphatidylinositol phospholipase C beta (PLCβ) converts phosphatidyl inositol phosphate (PIP2) to DAG and inositol triphosphate (IP3, Figure 6A), and impairing PLC activity leads to reduced cellular DAG levels (Nebigil, 1997). C. elegans encodes two PLC family members whose expression is enriched in the intestine, plc-2/ PLCβ and egl-8/ PLCβ (Taylor et al., 2021). plc-2 null mutants exhibited baseline and juglone-included FLP-2 secretion that were similar to wild-type controls (Figure 6—figure supplement 1). egl-8 loss-of-function mutants exhibited wild-type baseline FLP-2 secretion, but juglone-induced FLP-2 secretion was completely blocked (Figure 6B). H2O2 levels in egl-8 mutants were similar to wild-type controls, both in the presence and absence of juglone (Figure 6C and D). Thus, egl-8/PLCβ functions downstream of or in parallel to H2O2 production to promote FLP-2 secretion.
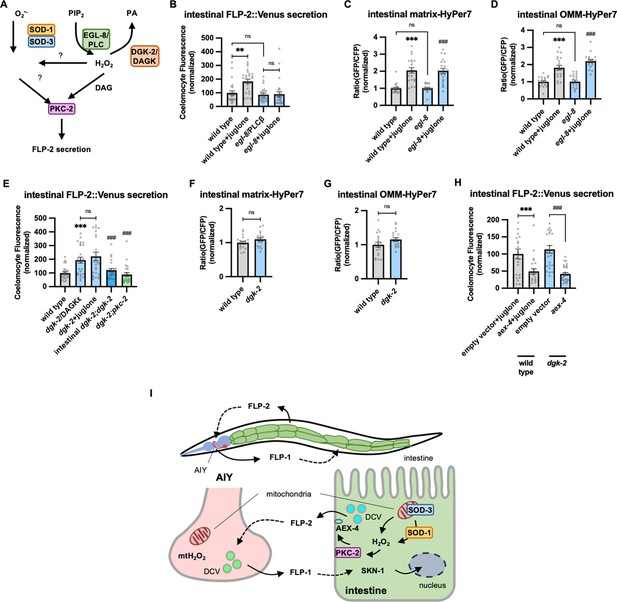
Diacylglycerol (DAG) promotes PKC-2-mediated FLP-2 secretion from the intestine.
(A) Schematic showing PLC and DGK mediates DAG metabolism and DAG functions in H2O2-mediated FLP-2 signaling. (B) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or juglone treatment for 10 min. n=25, 25, 25, 25 independent animals. (C and D) Quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing matrix-HyPer7 (C) or OMM-HyPer7 (D) with TOMM-20::mCherry following M9 or 300 μM juglone treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical significance compared to ‘egl-8’. (C) n=22, 20, 20, 21 independent animals, (D) n=20, 20, 20, 20 independent animals. (E) Quantification of average coelomocyte fluorescence of the indicated mutants expressing FLP-2::Venus fusion proteins in the intestine following M9 or 300 μM juglone treatment for 10 min. Intestinal dgk-2 denotes expression of dgk-2a cDNA under the ges-1 promoter. Unlined *** denotes statistical significance compared to ‘wild type’; unlined ### denotes statistical significance compared to ‘dgk-2/DGKε’. n=25, 25, 25, 25, 24 independent animals. (F and G) Quantification of fluorescence in the posterior region of the indicated transgenic animals co-expressing matrix-HyPer7 (F) or OMM-HyPer7 (G) with TOMM-20::mCherry following M9 treatment. Ratio of images taken with 500 nM (GFP) and 400 nM (CFP) for excitation and 520 nm for emission was used to measure H2O2 levels. (F) n=20, 20 independent animals, (G) n=20, 20 independent animals. (H) Quantification of average coelomocyte fluorescence of the indicated transgenic animals fed with RNA interference (RNAi) bacteria targeting the indicated genes in the intestine following M9 treatment for 10 min. n=25, 24, 25, 30 independent animals. (I) (Top) Schematic showing the position of intestine and AIY neurons in FLP-1-FLP-2-mediated axis. (Bottom) Schematic showing endogenous H2O2 promotes PKC-2/AEX-4-mediated FLP-2 release from the intestine in FLP-1-FLP-2-regulated inter-tissue axis. (B–H) Data are mean values ± s.e.m. normalized to wild-type controls. (B–E and H) ns, not significant, ** p<0.01, *** and ### p<0.001 by Brown-Forsythe and Welch ANOVA with Dunnett’s T3 multiple comparisons test. (F and G) ns, not significant by unpaired t test with Welch’s correction.
-
Figure 6—source data 1
Raw data for plotting the figures.
- https://cdn.elifesciences.org/articles/97503/elife-97503-fig6-data1-v1.xlsx
DAG kinase converts DAG into phosphatidic acid (PA), and is therefore a negative regulator or DAG levels (Figure 6A; van Blitterswijk and Houssa, 2000; Topham, 2006). In C. elegans, dgk-2/DGKε is the highest expressing DAG kinase in the intestine. Mutations in dgk-2 elevated FLP-2::Venus secretion (Figure 6E) without altering H2O2 levels in the intestinal mitochondrial matrix or OMM (Figure 6F and G). Expressing dgk-2 transgenes selectively in the intestine restored normal FLP-2::Venus secretion to dgk-2 mutants (Figure 6E). Finally, the increase in FLP-2 secretion in dgk-2 mutants was not further increased by juglone treatment, but it was completely blocked by pkc-2 mutations or aex-4/SNAP25 mutations (Figure 6E and H). These results show that FLP-2 secretion can be regulated bidirectionally by DAG, and they suggest that DAG and H2O2 function in a common genetic pathway upstream of pkc-2 to promote FLP-2 secretion (Figure 6A).
Discussion
By screening for intercellular regulators of FLP-1 signaling from the nervous system in promoting the antioxidant response, we have uncovered a function for peptidergic signaling in mediating gut-to-neuron regulation of the antioxidant response in C. elegans. We identified the neuropeptide-like protein FLP-2 as an inter-tissue signal originating in the intestine to potentiate stress-induced FLP-1 release from AIY neurons and the subsequent activation of SKN-1 in the intestine. We found that H2O2 generated endogenously in the intestine or exogenously by acute oxidant exposure increases FLP-2 secretion from intestinal DCVs. H2O2 promotes FLP-2 exocytosis through PKC-2 and AEX-4/SNAP25. The use of oxidant-regulated peptide secretion exemplifies a mechanism that can allow the gut and the nervous system to efficiently and rapidly communicate through endocrine signaling to promote organism-wide protection in the face of intestinal stress (Figure 6I).
A new function for flp-2 signaling in the antioxidant response
Previous studies have identified roles for flp-2 signaling in development and in stress responses. flp-2 promotes locomotion during molting (Chen et al., 2016), promotes entry into reproductive growth (Chai et al., 2022), regulates longevity (Kageyama et al., 2022), and activates the UPRmt cell non-autonomously during mitochondrial stress (Shao et al., 2016). The function we identified for flp-2 in the antioxidant response has some notable similarities with flp-2’s other functions. First, flp-2 mediates its effects at least in part by regulating signaling by other peptides. flp-2 signaling increases the secretion of the neuropeptide like protein PDF-1 during lethargus (Chen et al., 2016) and INS-35/insulin-like peptide for its roles in reproductive growth choice and longevity (Kageyama et al., 2022), in addition to regulating AIY FLP-1 secretion (Figure 5G). Second, the secretion of FLP-2 is dynamic. FLP-2 secretion decreases during lethargus (Chen et al., 2016) and increases under conditions that do not favor reproductive growth (Kageyama et al., 2022), as well increasing in response to oxidants (Figure 2C and D). However, in some instances, the regulation of FLP-2 secretion may occur at the level of flp-2 expression (Kageyama et al., 2022), rather than at the level of exocytosis (Figure 2C). Finally, genetic analysis of flp-2 has revealed that under normal conditions, flp-2 signaling may be relatively low, since flp-2 mutants show no defects in reproductive growth choice when animals are well fed (Chai et al., 2022), show only mild defects in locomotion during molting in non-sensitized genetic backgrounds (Chen et al., 2016), and do not have altered baseline FLP-1 secretion or antioxidant gene expression in the absence of exogenous oxidants (Figure 1D and E). It is notable that increased ROS levels are associated with molting (Back et al., 2012; Knoefler et al., 2012), aging (Back et al., 2012; Van Raamsdonk and Hekimi, 2010), starvation (Tao et al., 2017), and mitochondrial dysfunction (Dingley et al., 2010), raising the possibility that flp-2 may be used in specific contexts associated with high ROS levels to affect global changes in physiology, behavior, and development.
One major difference we found for flp-2 signaling in our study is that intestinal, but not neuronal flp-2 activates the oxidative stress response, whereas flp-2 originates from neurons for its reported roles in development and the UPRmt. The intestine is uniquely poised to relay information about diet to the rest of the animal, and secretion of a number of neuropeptide-like proteins from the intestine (e.g. INS-11, PDF-2, and INS-7) is proposed to regulate responses to different bacterial food sources (Lee and Mylonakis, 2017; Murphy et al., 2007; O’Donnell et al., 2018). Since bacterial diet can impact ROS levels in the intestine (Pang and Curran, 2014), secretion of FLP-2 from the intestine could function to relay information about bacterial diet to distal tissues to regulate redox homeostasis. In addition, the regulation of intestinal FLP-2 release by oxidants may meet a unique spatial, temporal, or concentration requirement for activating the antioxidant response that cannot be met by its release from the nervous system.
AIY as a target for flp-2 signaling
AIY interneurons receive sensory information from several neurons primarily as glutamatergic inputs to regulate behavior (Chalasani et al., 2007; Clark et al., 2006; Satoh et al., 2014). Our study reveals a previously undescribed mechanism by which AIY is activated through endocrine signaling originating from FLP-2 secretion from the intestine. FLP-2 could act directly on AIY, or it may function indirectly through upstream neurons that relay FLP-2 signals to AIY. frpr-18 encodes an orexin-like GPCR that can be activated by FLP-2-derived peptides in transfected mammalian cells (Larsen et al., 2013; Mertens et al., 2005), and frpr-18 functions downstream of flp-2 in the locomotion arousal circuit (Chen et al., 2016). frpr-18 is expressed broadly in the nervous system including in AIY (Chen et al., 2016), and loss-of-function frpr-18 mutations lead to hypersensitivity to certain oxidants (Ouaakki et al., 2023). FRPR-18 is coupled to the heterotrimeric G protein Gαq (Larsen et al., 2013; Mertens et al., 2005), raising the possibility that FLP-2 may promote FLP-1 secretion from AIY by directly activating FRPR-18 in AIY. However, flp-2 functions independently of frpr-18 in the reproductive growth circuit, and instead functions in a genetic pathway with the GPCR npr-30 (Chai et al., 2022). In addition, FLP-2-derived peptides (of which there are at least three) can bind to the GPCRs DMSR-1, or FRPR-8 in transfected cells (Beets et al., 2023). Identifying the relevant FLP-2 peptide(s), the FLP-2 receptor and its site of action will help to define the circuit used by intestinal flp-2 to promote FLP-1 release from AIY.
FLP-1 release from AIY is positively regulated by H2O2 generated from mitochondria (Jia and Sieburth, 2021). Here, we showed that H2O2-induced FLP-1 release requires intestinal flp-2 signaling. However, flp-2 does not appear to promote FLP-1 secretion by increasing H2O2 levels in AIY (Figure 1E), and flp-2 signaling is not sufficient to promote FLP-1 secretion in the absence of H2O2 (Figure 1D). These results point to a model whereby at least two conditions must be met in order for AIY to increase FLP-1 secretion: an increase in H2O2 levels in AIY itself, and an increase in flp-2 signaling from the intestine. Thus AIY integrates stress signals from both the nervous system and the intestine to activate the intestinal antioxidant response through FLP-1 secretion. The requirement of signals from multiple tissues for FLP-1 secretion may function to limit the activation of SKN-1, since unregulated SKN-1 activation can be detrimental to organismal health (Turner et al., 2024). AIY shows a sporadic Ca2+ response regardless of the presence of explicit stimulation (Shimizu et al., 2019; Chalasani et al., 2007; Clark et al., 2006), and FLP-1 secretion from AIY is calcium-dependent (Jia and Sieburth, 2021). How mitochondrial H2O2 levels are established in AIY by intrinsic or extracellular inputs, and how AIY integrates H2O2 and flp-2 signaling to control FLP-1 secretion remain to be defined.
A role for endogenous H2O2 in regulated neuropeptide secretion
Using HyPer7, we showed that acute juglone exposure results in a rapid elevation of endogenous H2O2 levels inside and outside intestinal mitochondria and a corresponding increase of FLP-2 release from the intestine that depends on the cytoplasmic superoxide dismutase sod-1, and mitochondrial sod-3. We favor a model whereby superoxide generated by juglone in the mitochondria is converted to H2O2 by SOD-3 in the matrix and by SOD-1 in the cytosol. In this case, both the superoxide generated by juglone and the H2O2 generated by SOD-3 would have to be able to exit the mitochondria and enter the cytosol. Superoxide and H2O2 can be transported across mitochondrial membranes through anion channels and aquaporin channels, respectively (Bienert and Chaumont, 2014; Ferri et al., 2003; Han et al., 2003; Kontos et al., 1985). The observation that both SOD-1 and SOD-3 activity are necessary to drive FLP-2 release suggests that H2O2 levels must reach a certain threshold in the cytoplasm to promote FLP-2 release, and this threshold requires the generation of H2O2 by both SOD-1 and SOD-3.
We identified a role for the antioxidant peroxiredoxin-thioredoxin system, encoded by prdx-2 and trx-3, in maintaining low endogenous H2O2 levels in the intestine and in negatively regulating FLP-2 secretion. We showed that the prdx-2b isoform functions to inhibit FLP-2 secretion and to lower H2O2 levels in both the mitochondrial matrix and on the cytosolic side of mitochondria. These observations are consistent with a subcellular site of action for PRDX-2B in either the matrix only or in both the matrix and cytosol. In contrast, trx-3 mutations do not alter mitochondrial H2O2 levels, suggesting that TRX-3 functions exclusively in the cytosol. Thus, the PRDX-2B-TRX-3 combination may function in the cytosol, and PRDX-2B may function with a different TRX family member in the matrix. There are several thioredoxin domain-containing proteins in addition to trx-3 in the C. elegans genome (including trx-5/NXNL2) that could be candidates for this role. Alternatively, prdx-2 may function alone or with other redox proteins. PRDX-2 may function without thioredoxins in its roles in light sensing and stress response in worms (Li et al., 2016; Oláhová et al., 2008; Oláhová and Veal, 2015). PRDX-2B contains a unique N-terminal domain that is distinct from the catalytic domain and is not found on the other PRDX-2 isoforms. This domain may be important for targeting PRDX-2B to specific subcellular location(s) where it can regulate FLP-2 secretion.
Regulation of FLP-2 exocytosis by PKC-2/PKCα/β and AEX-4/SNAP25
We demonstrated that pkc-2 mediates the effects of H2O2 on intestinal FLP-2 secretion, and H2O2- and DAG-mediated PKC-2 activation are likely to function in a common genetic pathway to promote FLP-2 secretion. Our observations that DAG is required for the effects of juglone (Figure 6B) are consistent with a two-step activation model for PKC-2, in which H2O2 could first modify PKC-2 in the cytosol, facilitating subsequent PKC-2 recruitment to the membrane by DAG. Alternatively, DAG could first recruit PKC-2 to membranes, where it is then modified by H2O2. We favor a model whereby H2O2 modification occurs in the cytosol, since H2O2 produced locally by mitochondria would have access to cytosolic pools of PKC-2 prior to its membrane translocation.
We defined a role for aex-4/SNAP25 in the fusion step of FLP-2 containing DCVs from the intestine under normal conditions as well as during oxidative stress. In neuroendocrine cells, phosphorylation of SNAP25 on Ser187 potentiates DCV recruitment into releasable pools (Nagy et al., 2002; Shu et al., 2008; Yang et al., 2007), and exocytosis stimulated by the DAG analog phorbol ester (Gao et al., 2016; Shu et al., 2008), without altering baseline SNAP25 function. Interestingly, the residue corresponding to Ser187 is conserved in AEX-4, raising the possibility that PKC-2 potentiates FLP-2 secretion by phosphorylating AEX-4. Since SNAP25 phosphorylation on Ser187 has been shown to increase its interaction with syntaxin and promote SNARE complex assembly in vitro (Gao et al., 2016; Yang et al., 2007), it is possible that elevated H2O2 levels could promote FLP-2 secretion by positively regulating SNARE-mediated DCV fusion at intestinal release sites on the basolateral membrane through AEX-4/SNAP25 phosphorylation by PKC-2. Prior studies have shown that PKC-2 phosphorylates the SNARE-associated protein UNC-18 in neurons to regulate thermosensory behavior (Edwards et al., 2012; Land and Rubin, 2017). Thus, PKC-2 may have multiple targets in vivo and target selection may be dictated by cell type and/or the redox status of the cell.
Similar molecular mechanisms regulating FLP-1 and FLP-2 release
The molecular mechanisms we identified that regulate FLP-2 secretion from the intestine are similar in several respects to those regulating FLP-1 secretion from AIY. First, the secretion of both peptides is positively regulated by H2O2 originating from mitochondria. Second, in both cases, H2O2 promotes exocytosis of neuropeptide-containing DCVs by a mechanism that depends upon the kinase activity of PKC. Finally, the secretion of both peptides is controlled through the regulation of H2O2 levels by superoxide dismutases and by the peroxiredoxin-thioredoxin system. H2O2-regulated FLP-1 and FLP-2 secretion differ in the identity of the family members of some of the genes involved. prdx-3-trx-2 and sod-2 family members regulate H2O2 levels in AIY, whereas prdx-2-trx-3 and sod-1/sod-3 family members regulate H2O2 levels in the intestine. In addition, pkc-1 promotes H2O2-induced FLP-1 secretion from AIY whereas pkc-2 promotes H2O2 -induced FLP-2 secretion from the intestine. Nonetheless, it is noteworthy that two different cell types utilize largely similar pathways for the H2O2-mediated regulation of neuropeptide release, raising the possibility that similar mechanisms may be utilized in other cell types and/or organisms to regulate DCV secretion in response to oxidative stress.
Materials and methods
A complete list of C. elegans strains used in this study.
Strains and transgenic lines
Request a detailed protocolC. elegans strains were maintained at 20°C in the dark on standard nematode growth medium (NGM) plates seeded with OP50 Escherichia coli as food source, unless otherwise indicated. All strains were synchronized by picking mid L4 stage animal either immediately before treatment (for coelomocyte imaging and intestine imaging) or 24 hr before treatment (for Pgst-4::gfp imaging). The wild-type strain was Bristol N2. Mutants used in this study were outcrossed at least four times.
Transgenic lines were generated by microinjecting plasmid mixes into the gonads of young adult animals following standard techniques (Mello et al., 1991). Microinjection mixes were prepared by mixing expression constructs with the co-injection markers pJQ70 (Pofm-1::rfp, 25 ng/μL), pMH163 (Podr-1::mCherry, 40 ng/μL), pMH164 (Podr-1::gfp, 40 ng/μL), or pDS806 (Pmyo-3::mCherry, 20 ng/μL) to a final concentration of 100 ng/μL. For tissue-specific expression, a 1.5 kb rab-3 promoter was used for pan-neuronal expression (Nonet et al., 1997), a 2.0 kb ges-1 or a 3.5 kb nlp-40 promoter was used for intestinal expression (Egan et al., 1995; Wang et al., 2013). At least three transgenic lines were examined for each transgene, and one representative line was used for quantification. Strains and transgenic lines used in this study are listed in Supplementary file 1.
Molecular biology
Request a detailed protocolAll gene expression vectors were constructed with the backbone of pPD49.26. Promoter fragments including Prab-3 and Pges-1 were amplified from genomic DNA; genes of interest, including cDNA fragments (aex-5, snt-5, sod-1b, sod-3, isp-1, prdx-2a, prdx-2b, prdx-2c, trx-3, pkc-2b, dgk-2a, aex-4) and genomic fragments (flp-2, flp-40, nlp-36, nlp-27), were amplified from cDNA library and genomic DNA respectively using standard molecular biology protocols. Expression plasmid of HyPer7 was designed based on reported mammalian expression plasmid for HyPer7 (Pak et al., 2020) and was synthesized by Thermo Fisher Scientific with codon optimization for gene expression in C. elegans. Plasmids and primers used in this study are listed in Supplementary file 1.
Toxicity assay
Request a detailed protocolA stock solution of 50 mM juglone in DMSO was freshly made on the same day of liquid toxicity assay. 120 μM working solution of juglone in M9 buffer was prepared using stock solution before treatment. Between 60–80 synchronized adult animals were transferred into a 1.5 mL Eppendorf tube with fresh M9 buffer and washed three times, and a final wash was done with either the working solution of juglone with or M9 DMSO at the concentrations present in juglone-treated animals does not contribute to toxicity since DMSO treatment alone caused no significant change in survival compared to M9-treated controls (Figure 1—figure supplement 1D). Animals were incubated in the dark for 4 hr on rotating mixer before being transferred onto fresh NGM plates seeded with OP50 to recover in the dark at 20°C for 16 hr. Percentage of survival was assayed by counting the number of alive and dead animals. Toxicity assays were performed in triplicates.
RNA interference
Request a detailed protocolPlates for feeding RNAi were prepared as described (Kamath and Ahringer, 2003). Around 20–25 gravid adult animals with indicated genotype were transferred onto the RNAi plates that were seeded with HT115(DE3) bacteria transformed with L4440 vectors with targeted gene inserts or empty L4440 vectors. Eggs were collected for 4 hr to obtain synchronized populations. L4 stage animals were collected for further assays. RNAi clones were from Ahringer or Vidal RNAi library, or made from genomic DNA. Details were listed in Supplementary file 1.
Behavioral assays
Request a detailed protocolThe defecation motor program was assayed as previously described (Liu and Thomas, 1994). Twenty to thirty L4 animals were transferred onto a fresh NGM plate seeded with OP50 E. coli and were stored in a 20°C incubator for 24 hr. After 24 hr, 10 consecutive defecation cycles were observed from three independent animals and the mean and the standard error was calculated for each genotype. The pBoc and aBoc steps were recorded using custom Etho software (James Thomas Lab website: http://depts.washington.edu/jtlab/software/otherSoftware.html).
Microscopy and fluorescence imaging
Request a detailed protocolApproximately 30–40 age matched animals were paralyzed with 30 mg/mL 2,3-butanedione monoxime (BDM) in M9 buffer and mounted on 2% agarose pads. Images were captured using the Nikon eclipse 90i microscope equipped with Nikon Plan Apo ×20, ×40, ×60, and ×100 oil objective (NA=1.40), and a Photometrics Coolsnap ES2 camera or a Hamamatsu Orca Flash LT+CMOS camera. Metamorph 7.0 software (Universal Imaging/Molecular Devices) was used to capture serial image stacks and to obtain the maximum intensity projection image for analysis.
For transcriptional reporter imaging, young adult animals were transferred into a 1.5 mL Eppendorf tube with M9 buffer, washed three times and incubated in 50 μM working solution of juglone or M9 buffer control with equivalent DMSO for 1 hr in the dark on rotating mixer before recovering on fresh NGM plates with OP50 for 3 hr in the dark at 20°C. The posterior end of the intestine was imaged with the ×60 objective and quantification for average fluorescence intensity of a 16-pixel diameter circle in the posterior intestine was calculated using Metamorph.
For coelomocyte imaging, L4 stage animals were transferred in fresh M9 buffer on a cover slide, washed six times with M9 before being exposed to 300 μM juglone in M9 buffer (diluted from freshly made 50 mM stock solution), 1 mM H2O2 in M9 buffer, or M9 buffer. DMSO at the concentrations present in juglone-treated animals does not alter neuropeptide secretion since DMSO treatment alone caused no significant change in FLP-1::Venus or FLP-2::Venus coelomocyte fluorescence compared to M9-treated controls (Figure 1—figure supplement 1D, Figure 2—figure supplement 1E). Animals were then paralyzed in BDM and images of coelomocytes next to the posterior end of intestine were taken using the ×100 oil objective. Average fluorescence intensity of Venus from the endocytic compartments in the posterior coelomocytes was measured in ImageJ.
For fusion protein fluorescence imaging, L4 stage animals were exposed to M9 buffer or indicated oxidants for 10 min before being paralyzed in BDM and images taken of the posterior end of the intestine using ×100 oil objective. For HyPer7 imaging, Z stacks were obtained using GFP (excitation/emission: 500 nm/520 nm) and CFP (excitation/emission: 400 nm/520 nm) filter sets sequentially, HyPer7 fluorescence signal was quantified as the ratio of GFP to CFP fluorescence intensity changes with respect to the baseline [(Ft − F0)/F0].
CRISPR/Cas9 editing
Request a detailed protocolprdx-2b(vj380) knockout mutants were generated using a co-CRISPR protocol (Arribere et al., 2014). An sgRNA and a repair single-stranded oligodeoxynucleotides (ssODN) targeting dpy-10 were co-injected with an sgRNA for genes of interest and an ssODN that induces homology-directed repair to introduce Cas9-mediated mutagenesis. Fifteen young adult animals were injected to produce around 30 singled F1 animals carrying Dpy or Rol phenotype. F2 animals were genotyped for mutations based on PCR and enzyme digest. Homozygous mutants were outcrossed with wild-type animals at least four times before being used for assays.
Statistics
Statistical analysis was performed on GraphPad Prism 9. Unpaired t test with two tails was used for two groups and one-way ANOVA with multiple comparison corrections was used for three or more groups to determine the statistical significance. Statistical details and n are specified in the Figure legends. All comparisons are conducted based on wild-type controls unless indicated by lines between genotypes. Bar graphs with plots were generated using GraphPad Prism 9.
Appendix 1
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (C. elegans) | flp-1(ok2811) IV | CGC | OJ6555 | Mutant |
| Genetic reagent (C. elegans) | flp-2(ok3351) X | CGC | OJ5490 | Mutant |
| Genetic reagent (C. elegans) | flp-1(ok2811);flp-2(ok3351) | This paper | OJ10228 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-4(sa22) X | CGC | OJ7466 | Mutant |
| Genetic reagent (C. elegans) | pkc-2(ok328) X | CGC | VC127 | Mutant |
| Genetic reagent (C. elegans) | vjIs150[pJQ60] | Jia and Sieburth, 2021 | OJ3614 | FLP-1::Venus |
| Genetic reagent (C. elegans) | aex-5(sa23);vjIs150[pJQ60] | this paper | OJ5616 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx1748[pJQ298]; aex-5(sa23);vjIs150[pJQ60] | this paper | OJ5780 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx1753[pJQ299]; aex-5(sa23);vjIs150[pJQ60] | this paper | OJ5785 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx1753[pJQ299];aex-5(sa23); flp-2(ok3351);vjIs150[pJQ60] | this paper | OJ6334 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-2(ok3351); vjIs150[pJQ60] | this paper | OJ5264 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2882[pJQ366];f lp-2(ok3351);vjIs150[pJQ60] | this paper | OJ8818 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2877[pJQ302]; flp-2(ok3511);vjIs150[pJQ60] | this paper | OJ8813 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2877[pJQ302];vjIs150 | this paper | OJ10229 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dvIs19[pAF15] | CGC | CL2166 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-1(ok2811);dvIs19 | this paper | OJ2547 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-2(ok3351);dvIs19 | this paper | OJ10230 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-1(ok2811);flp-2(ok3511);dvIs19 | this paper | OJ6544 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2877[pJQ302];dvIs19 | this paper | OJ10231 | Obtained in from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2877[pJQ302]; flp-1(ok2281);dvIs19 | this paper | OJ10232 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-1(sa9);vjIs150[pJQ60] | this paper | OJ5888 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-3(js815);vjIs150[pJQ60] | this paper | OJ5890 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-4(sa22);vIs150[pJQ60] | this paper | OJ5891 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-6(sa24);vjIs150[pJQ60] | this paper | OJ5892 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | nlp-40(tm4085);vjIs150[pJQ60] | this paper | OJ5615 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-2(sa3);vjIs150[pJQ60] | this paper | OJ5889 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2035[pJQ305] | this paper | OJ6405 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3069[pDY10]; vjEx2035[pJQ305] | this paper | OJ9469 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-4(sa22);vjEx2035[pJQ305] | this paper | OJ6409 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | aex-6(sa24);vjEx2035[pJQ305] | this paper | OJ8345 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-1(ok2811);vjEx2035[pJQ305] | this paper | OJ6641 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjIs40[pDS292] | this paper | OJ1002 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3263[pJQ370] | this paper | OJ10237 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3062[pDY14];vjEx2035[pJQ305] | this paper | OJ9567 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);vjEx2035[pJQ305] | this paper | OJ9797 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2814[pJQ419];sod-1 (tm783);vjEx2035[pJQ305] | this paper | OJ8588 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-3(tm760);vjEx0235[pJQ305] | this paper | OJ8341 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2910[pJQ389];sod-3 (tm760);vjEx2035[pJQ305] | this paper | OJ8933 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2973[pJQ408];sod-3 (tm760);vjEx2035[pJQ305] | this paper | OJ9106 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);sod-3(tm760); vjEx2035[pJQ305] | this paper | OJ10234 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3266[pJQ420] | this paper | OJ10243 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2993[pJQ407] | this paper | OJ9141 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2996[pJQ409(Pges-1::sod-3(∆MLS) cDNA::GFP)] | this paper | OJ9144 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3020[pJQ383] | this paper | OJ9230 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3014[pJQ411] | this paper | OJ9196 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);vjEx3020[pJQ383] | this paper | OJ9281 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-3(tm760);vjEx3020[pJQ383] | this paper | OJ9259 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);sod-3(tm760); vjEx3020[pJQ383] | this paper | OJ10244 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);vjEx3014[pJQ411] | this paper | OJ9795 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-3(tm760);vjEx3014[pJQ411] | this paper | OJ9280 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-1(tm783);sod-3(tm760); vjEx3014[pJQ411] | this paper | OJ10245 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-2(ok1030);vjEx2035[pJQ305] | this paper | OJ10238 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-4(gk101);vjEx2035[pJQ305] | this paper | OJ10239 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | sod-5(tm1146);vjEx2035[pJQ305] | this paper | OJ10240 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2(gk169);vjEx2035[pJQ305] | this paper | OJ8991 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380);vjEx2035[pJQ305] | this paper | OJ10251 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2926[pJQ381];prdx-2(gk169); vjEx2035[pJQ305] | this paper | OJ8996 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820);vjEx2035[pJQ305] | this paper | OJ9249 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3091[pJQ422];trx-3(tm2820); vjEx2035[pJQ305] | this paper | OJ9496 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820);sod-1(tm783); vjEx2035[pJQ305] | this paper | OJ10252 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820);sod-3(tm760); vjEx2035[pJQ305] | this paper | OJ10253 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2(gk169); vjEx3020[pJQ383] | this paper | OJ9237 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380); vjEx3020[pJQ383] | this paper | OJ10247 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820); vjEx3020[pJQ383] | this paper | OJ10249 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2(gk169); vjEx3014[pJQ411] | this paper | OJ10246 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380); vjEx3014[pJQ411] | this paper | OJ10248 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | trx-3(tm2820); vjEx3014[pJQ411] | this paper | OJ10250 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380);dvIs19 | this paper | OJ10254 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380);flp-2(tm3351);dvIs19 | this paper | OJ10255 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-3(gk529);vjEx2035[pJQ305] | this paper | OJ10256 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3268[pJQ380];prdx-2(gk169); vjEx2035[pJQ305] | this paper | OJ10258 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3270[pJQ399];prdx-2(gk169); vjEx2035[pJQ305] | this paper | OJ10260 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2b(vj380);sod-3(tm760); vjEx2035[pJQ305] | this paper | OJ9250 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | pkc-2(ok328);vjEx2035[pJQ305] | this paper | OJ9682 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2828[pJQ376];pkc-2(ok328); vjEx2035[pJQ305] | this paper | OJ8682 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx3131[pJQ446];pkc-2(ok328); vjEx2035[pJQ305] | this paper | OJ9657 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | pkc-2(ok328);vjEx3020[pJQ383] | this paper | OJ10279 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | pkc-2(ok328);vjEx3014[pJQ411] | this paper | OJ10280 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | prdx-2(gk169);pkc-2(ok328); vjEx2035[pJQ305] | this paper | OJ8939 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | pkc-1(nj3);vjEx2035[pJQ305] | this paper | OJ10278 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | egl-8(sa47);vjEx2035[pJQ305] | this paper | OJ9863 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | egl-8(sa47);vjEx3020[pJQ383] | this paper | OJ10281 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | egl-8(sa47);vjEx3014[pJQ411] | this paper | OJ10282 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dgk-2(gk124);vjEx2035[pJQ305] | this paper | OJ10263 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx327[pJQ460];dgk-2(gk124); vjEx2035[pJQ305] | this paper | OJ10264 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dgk-2(gk124);pkc-2(ok328); vjEx2035[pJQ305] | this paper | OJ10266 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dgk-2(gk124);vjEx3020[pJQ383] | this paper | OJ10283 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | dgk-2(gk124);vjEx3014[pJQ411] | this paper | OJ10284 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | plc-2(ok1761);vjEx2035[pJQ305] | this paper | OJ9809 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | vjEx2936[pJQ382] | this paper | OJ9028 | Obtained from Derek Sieburth lab |
| Genetic reagent (C. elegans) | flp-2(ok3351);vjEx2936[pJQ382] | this paper | OJ10595 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Prab-3::aex-5 cDNA (plasmid) | this paper | pJQ298 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::aex-5 cDNA | this paper | pJQ299 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Prab-3::flp-2 gDNA | this paper | pJQ366 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::flp-2 gDNA | this paper | pJQ302 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::aex-5::mTur2 | this paper | pDY10 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::flp-2::Venus | this paper | pJQ305 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::nlp-27 gDNA::Venus | this paper | pJQ370 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::nlp-40::mTur2 | this paper | pDY14 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-1a cDNA | this paper | pJQ419 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-3 cDNA | this paper | pJQ389 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-3(∆MLS) cDNA | this paper | pJQ408 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-1a cDNA::GFP | this paper | pJQ420 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-3 cDNA::GFP | this paper | pJQ407 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::sod-3(∆MLS) cDNA::GFP | this paper | pJQ409 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::MLS::HyPer7 | this paper | pJQ383 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::tomm-20::HyPer7 | this paper | pJQ411 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::prdx-2b cDNA | this paper | pJQ381 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::trx-3 cDNA | this paper | pJQ422 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::prdx-2a cDNA | this paper | pJQ380 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::prdx-2c cDNA | this paper | pJQ399 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::pkc-2b cDNA | this paper | pJQ376 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::pkc-2(K375R) cDNA | this paper | pJQ446 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pges-1::dgk-2a cDNA | this paper | pJQ460 | Obtained from Derek Sieburth lab |
| Recombinant DNA reagent | Pttx-3::MLS::HyPer7 | this paper | pJQ382 | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCGCTAGCAAAAATGAAATTAATTTTCCTGCTTTTGCTTTTTGG | this paper | aex-5_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCGGTACCTTATGACATTGTTCCCACCACT | this paper | aex-5_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGCAAGTTTCTGGAATCCTATCTGC | this paper | flp-2_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTATTGGA AGTCGTAATCTGGCAGC | this paper | flp-2_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCGGTACCTTATGACATTGTTCCCACCACT | this paper | aex-5_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCACCGGTTTGGAAGTCGTAATCTGGCAGCGG | this paper | flp-2_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGATTTCCACTTCTTCACTTCTTATCCTT | this paper | nlp-27_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCACCGGTCTTTCCCCATCCACCGTATCC | this paper | nlp-27_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGTT TATGAATCTTCTCACTCAGGTCTCC | this paper | sod-1a_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTCACTGGGGAGCAGCGAGAG | this paper | sod-1a_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGCTGCAATCTACTGCTCGC | this paper | sod-3_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTATTGTCGAGCATTGGCAAATCT | this paper | sod-3_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGAAGCACACTCTCCCAGA | this paper | sod-3(∆MLS)_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCCGGGCTGGGGAGCAGCGAGAGCAA | this paper | sod-1_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCCCCGGGTTGTCGAGCATTGGCAAATCTC | this paper | sod-3_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGTA TAGACAGATGTCGAAAGCATTC | this paper | prdx-2b_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTAGTGCT TCTTGAAGTACTCTTGG | this paper | prdx-2a/b/c_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGG CTAAGAACTTTTTCTCCGGA | this paper | trx-3_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTATGCACGGATTCTCTCGAGATT | this paper | trx-3_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGTCGAAAGCATTCATCGGAA | this paper | prdx-2a_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATG TCTCTCGCTCCAAAGATG | this paper | prdx-2c_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGTCGTTGAGCACGAACAGC | this paper | pkc-2b_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGATATCTCACGGTTCTACATCTTTGACATAAAAC | this paper | pkc-2b_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | ATTTCCTCACTGTTCTTGGAAGAGGATCGTTTG | this paper | pkc-2b(K375R)_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | ACACTTTTCCAAACGATCCTCTTCCAAGAACA | this paper | pkc-2b(K375R)_R | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGCTAGCAAAAATGGAAAT GGACGTGTATGATGAATTATTG | this paper | dgk-2a_F | Obtained from Derek Sieburth lab |
| Sequence-based reagent | CCCCGGTACCTTAGAAG AACATCCCACATCCGG | this paper | dgk-2a_R | Obtained from Derek Sieburth lab |
| Software, algorithm | Etho | James Thomas Lab | Defecation motor program analysis | http://depts.washington.edu/jtlab/software/otherSoftware.html |
| Software, algorithm | Metamorph 7.0 | Universal 709 Imaging/Molecular Devices | Image capture | |
| Software, algorithm | GraphPad Prism 9 | Prism | Statistical analysis |
Data availability
All data generated or analyzed during this study are included in the manuscript and source data files.
References
-
Exploring real-time in vivo redox biology of developing and aging Caenorhabditis elegansFree Radical Biology & Medicine 52:850–859.https://doi.org/10.1016/j.freeradbiomed.2011.11.037
-
The gut hormone peptide YY regulates appetiteAnnals of the New York Academy of Sciences 994:162–168.https://doi.org/10.1111/j.1749-6632.2003.tb03176.x
-
Aquaporin-facilitated transmembrane diffusion of hydrogen peroxideBiochimica et Biophysica Acta 1840:1596–1604.https://doi.org/10.1016/j.bbagen.2013.09.017
-
Potential role of Noxes in the protection of mucosae: H(2)O(2) as a bacterial repellentMicrobes and Infection 11:537–544.https://doi.org/10.1016/j.micinf.2009.02.009
-
Protein kinase C contains two phorbol ester binding domainsThe Journal of Biological Chemistry 266:18330–18338.https://doi.org/10.1016/S0021-9258(18)55274-1
-
Therapeutic efficacy of stable analogues of vasoactive intestinal peptide against pathogensThe Journal of Biological Chemistry 289:14583–14599.https://doi.org/10.1074/jbc.M114.560573
-
The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systemsAnnals of Gastroenterology: Quarterly Publication of the Hellenic Society of Gastroenterology 28:203–209.
-
Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brainThe Journal of Biological Chemistry 282:14186–14193.https://doi.org/10.1074/jbc.M700827200
-
Interneuron control of C. elegans developmental decision-makingCurrent Biology 32:2316–2324.https://doi.org/10.1016/j.cub.2022.03.077
-
Hydroperoxide metabolism in mammalian organsPhysiological Reviews 59:527–605.https://doi.org/10.1152/physrev.1979.59.3.527
-
H2O2 is a novel, endogenous modulator of synaptic dopamine releaseJournal of Neurophysiology 85:2468–2476.https://doi.org/10.1152/jn.2001.85.6.2468
-
SNARE-mediated membrane fusionNature Reviews Molecular Cell Biology 2:98–106.https://doi.org/10.1038/35052017
-
The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegansThe Journal of Neuroscience 26:7444–7451.https://doi.org/10.1523/JNEUROSCI.1137-06.2006
-
Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to jugloneFree Radical Biology & Medicine 37:139–145.https://doi.org/10.1016/j.freeradbiomed.2004.04.021
-
PKC-2 phosphorylation of UNC-18 Ser322 in AFD neurons regulates temperature dependency of locomotionThe Journal of Neuroscience 32:7042–7051.https://doi.org/10.1523/JNEUROSCI.4029-11.2012
-
Exploring neuropeptide signalling through proteomics and peptidomicsExpert Review of Proteomics 16:131–137.https://doi.org/10.1080/14789450.2019.1559733
-
Superoxide radical and superoxide dismutasesAnnual Review of Biochemistry 64:97–112.https://doi.org/10.1146/annurev.bi.64.070195.000525
-
Superoxide anion radical (o·̄2), superoxide dismutases, and related mattersJournal of Biological Chemistry 272:18515–18517.https://doi.org/10.1074/jbc.272.30.18515
-
The enteric nervous system and gastrointestinal innervation: integrated local and central controlAdvances in Experimental Medicine and Biology 817:39–71.https://doi.org/10.1007/978-1-4939-0897-4_3
-
The copper/zinc superoxide dismutase gene of Caenorhabditis elegansBiochemistry and Molecular Biology International 33:41–44.
-
Dual action of hydrogen peroxide on synaptic transmission at the frog neuromuscular junctionThe Journal of Physiology 552:283–293.https://doi.org/10.1113/jphysiol.2003.050690
-
Brain-gut-microbe communication in health and diseaseFrontiers in Physiology 2:94.https://doi.org/10.3389/fphys.2011.00094
-
Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosolThe Journal of Biological Chemistry 278:5557–5563.https://doi.org/10.1074/jbc.M210269200
-
SNAREs--engines for membrane fusionNature Reviews Molecular Cell Biology 7:631–643.https://doi.org/10.1038/nrm2002
-
Functional characterization of thioredoxin 3 (TRX-3), a Caenorhabditis elegans intestine-specific thioredoxinFree Radical Biology & Medicine 68:205–219.https://doi.org/10.1016/j.freeradbiomed.2013.11.023
-
The FMRFamide-like peptide FLP-2 is involved in the modulation of larval development and adult lifespan by regulating the secretion of the insulin-like peptide INS-35 in Caenorhabditis elegansBioscience, Biotechnology, and Biochemistry 86:1231–1239.https://doi.org/10.1093/bbb/zbac108
-
Coniferaldehyde inhibits LPS-induced apoptosis through the PKC α/β II/Nrf-2/HO-1 dependent pathway in RAW264.7 macrophage cellsEnvironmental Toxicology and Pharmacology 48:85–93.https://doi.org/10.1016/j.etap.2016.10.016
-
Sensory neuron regulation of gastrointestinal inflammation and bacterial host defenceJournal of Internal Medicine 282:5–23.https://doi.org/10.1111/joim.12591
-
Functional expression and characterization of the C. elegans G-protein-coupled FLP-2 Receptor (T19F4.1) in mammalian cells and yeastInternational Journal for Parasitology. Drugs and Drug Resistance 3:1–7.https://doi.org/10.1016/j.ijpddr.2012.10.002
-
Oxidative stress-induced apoptosis is mediated by ERK1/2 phosphorylationExperimental Cell Research 291:251–266.https://doi.org/10.1016/s0014-4827(03)00391-4
-
Regulation of a periodic motor program in C. elegansThe Journal of Neuroscience 14:1953–1962.https://doi.org/10.1523/JNEUROSCI.14-04-01953.1994
-
Reduction of food intake in rats by intraperitoneal injection of low doses of amylinPhysiology & Behavior 55:891–895.https://doi.org/10.1016/0031-9384(94)90076-0
-
Amylin decreases meal size in ratsPhysiology & Behavior 58:1197–1202.https://doi.org/10.1016/0031-9384(95)02067-5
-
Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegansMolecular Biology of the Cell 17:2617–2625.https://doi.org/10.1091/mbc.e05-12-1170
-
A neuronal GPCR is critical for the induction of the heat shock response in the nematode C. elegansThe Journal of Neuroscience 33:6102–6111.https://doi.org/10.1523/JNEUROSCI.4023-12.2013
-
The Gut-Brain AxisAnnual Review of Medicine 73:439–453.https://doi.org/10.1146/annurev-med-042320-014032
-
The gut-brain axis, paving the way to brain cancerTrends in Cancer 5:200–207.https://doi.org/10.1016/j.trecan.2019.02.008
-
Molecular characterization of two G protein-coupled receptor splice variants as FLP2 receptors in Caenorhabditis elegansBiochemical and Biophysical Research Communications 330:967–974.https://doi.org/10.1016/j.bbrc.2005.03.071
-
Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidaseThe Journal of Biological Chemistry 277:42563–42571.https://doi.org/10.1074/jbc.M204958200
-
Involvement of tyrosine phosphorylation and protein kinase C in the activation of phospholipase D by H2O2 in swiss 3T3 fibroblastsJournal of Biological Chemistry 273:29986–29994.https://doi.org/10.1074/jbc.273.45.29986
-
The mitochondrial thioredoxin systemAntioxidants & Redox Signaling 2:801–810.https://doi.org/10.1089/ars.2000.2.4-801
-
H2O2 oxidation of cysteine residues in C-Jun N-terminal kinase 2 (JNK2) contributes to redox regulation in human articular chondrocytesThe Journal of Biological Chemistry 293:16376–16389.https://doi.org/10.1074/jbc.RA118.004613
-
Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesiclesThe Journal of Neuroscience 17:8061–8073.https://doi.org/10.1523/JNEUROSCI.17-21-08061.1997
-
Role of hydrogen peroxide in NF-κB activation: from inducer to modulatorAntioxidants & Redox Signaling 11:2223–2243.https://doi.org/10.1089/ars.2009.2601
-
The natural toxin juglone causes degradation of p53 and induces rapid H2AX phosphorylation and cell death in human fibroblastsToxicology and Applied Pharmacology 209:1–9.https://doi.org/10.1016/j.taap.2005.03.005
-
Regulation of experience-dependent bidirectional chemotaxis by a neural circuit switch in Caenorhabditis elegansThe Journal of Neuroscience 34:15631–15637.https://doi.org/10.1523/JNEUROSCI.1757-14.2014
-
Food deprivation changes chemotaxis behavior in Caenorhabditis elegansBiophysics and Physicobiology 16:167–172.https://doi.org/10.2142/biophysico.16.0_167
-
PKC-1 regulates secretion of neuropeptidesNature Neuroscience 10:49–57.https://doi.org/10.1038/nn1810
-
Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinaseThe Journal of Biological Chemistry 278:24233–24241.https://doi.org/10.1074/jbc.M212389200
-
A look at the Caenorhabditis elegans Kex2/subtilisin-like proprotein convertase familyBioEssays: News and Reviews in Molecular, Cellular and Developmental Biology 22:545–553.https://doi.org/10.1002/(SICI)1521-1878(200006)22:63.0.CO;2-F
-
Signaling roles of diacylglycerol kinasesJournal of Cellular Biochemistry 97:474–484.https://doi.org/10.1002/jcb.20704
-
Protein kinase C fusion proteins are paradoxically loss of function in cancerThe Journal of Biological Chemistry 296:100445.https://doi.org/10.1016/j.jbc.2021.100445
-
Properties and functions of diacylglycerol kinasesCellular Signalling 12:595–605.https://doi.org/10.1016/s0898-6568(00)00113-3
-
Reactive oxygen species and aging in Caenorhabditis elegans: causal or casual relationship?Antioxidants & Redox Signaling 13:1911–1953.https://doi.org/10.1089/ars.2010.3215
-
Gut-brain axis: Insights from hippocampal neurogenesis and brain tumor development in a mouse model of experimental colitis induced by dextran sodium sulfateInternational Journal of Molecular Sciences 23:11495.https://doi.org/10.3390/ijms231911495
-
Cholecystokinin persistently suppresses meal size but not food intake in free-feeding ratsAmerican Journal of Physiology-Regulatory, Integrative and Comparative Physiology 246:R776–R787.https://doi.org/10.1152/ajpregu.1984.246.5.R776
-
A critical role of conventional protein kinase C in morphological changes of rodent mast cellsImmunology and Cell Biology 89:149–159.https://doi.org/10.1038/icb.2010.67
-
Phosphomimetic mutation of Ser-187 of SNAP-25 increases both syntaxin binding and highly Ca2+-sensitive exocytosisThe Journal of General Physiology 129:233–244.https://doi.org/10.1085/jgp.200609685
-
Interaction between familial amyotrophic lateral sclerosis (ALS)-linked SOD1 mutants and the dynein complexThe Journal of Biological Chemistry 282:16691–16699.https://doi.org/10.1074/jbc.M609743200
Article and author information
Author details
Funding
National Institute of Neurological Disorders and Stroke (R01NS071085)
- Derek Sieburth
National Institute of Neurological Disorders and Stroke (R01NS110730)
- Drew Young
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
C. elegans strains used in this study were provided by the Caenorhabditis Genetics Centre (CGC), which is funded by the NIH National Center for Research Resources (NCRR). We thank members of the Sieburth lab for critical reading and discussion of the manuscript. This work was supported by grants from National Institute of Health NINDS R01NS071085 and R01NS110730 to DS.
Version history
- Sent for peer review:
- Preprint posted:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.97503. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2024, Jia et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,386
- views
-
- 89
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.






