Plant-virus Interactions: Caught in a TrAP
Viruses are parasites that depend on their host to be able to replicate. Animals have mobile immune cells that specialize in detecting and neutralizing viruses. However, plants do not have specialist immune cells so, instead, they rely on mechanisms that are found within all plant cells to block virus replication. Now, in eLife, Xiuren Zhang of Texas A&M University and co-workers – including Claudia Castillo-González as first author – report a new mechanism by which plants can defend themselves against viruses; Zhang and co-workers also report how these viruses manage to counter this defense mechanism (Castillo-González et al., 2015).
When a virus invades a cell and starts to replicate, the production of virus RNA molecules triggers a process known as post-transcriptional gene silencing in which host enzymes convert the RNA molecules into vsiRNAs (virus-derived small interfering RNA molecules). These small RNAs – which can also spread to other cells – are then incorporated into a complex of proteins that represses the expression of the viral genes throughout the plant (Llave, 2010).
The genome of a virus can be made of DNA or RNA and post-transcriptional gene silencing has evolved as a universal defense against both types of viruses. Plants can also defend against DNA viruses using a second process known as transcriptional gene silencing (Pumplin and Voinnet, 2013). This process – which is also used to regulate the expression of a plant’s own genes – can be used to halt virus replication by directly modifying the way DNA is packaged in the cell (Figure 1).
In plants and other eukaryotic organisms, DNA is wrapped around proteins called histones to form a structure called chromatin. Such packing is essential to fit all the genetic material inside the cell nucleus. However, a gene that is in a region of tightly wrapped DNA cannot be expressed. DNA and histones are often modified by the addition of chemical groups known as methyl groups. The pattern of “methylation” in a region of the chromatin influences how tightly it is condensed. Therefore, it rules how highly the genes in that region are expressed (Liu et al., 2010). To activate particular genes, the structure of the chromatin can be relaxed by altering the methylation pattern of its associated histones. However, unlike post-transcriptional gene silencing, researchers do not fully understand how plants use transcriptional gene silencing to defend themselves against viruses.
Geminiviridae is the largest known family of single-stranded DNA viruses in plants. These viruses use host plant histones to pack their DNA and form structures called minichromosomes. Plants control Geminivirus infections by depositing repressive methylation marks into these minichromosomes. It is known that both the Geminivirus DNA and the associated histones are methylated in infected cells (Raja et al., 2008). Remarkably, Castillo-González et al. show that an enzyme called KRYPTONITE binds to the minichromosomes in the plant Arabidopsis thaliana. This enzyme – which belongs to the SET domain family of methyltransferases – methylates the virus-associated histones and promotes DNA methylation: the end result is to condense the viral minichromosomes and stop virus replication.
Virtually all plant viruses produce suppressor proteins that block the plant defense mechanisms (Csorba et al., 2015). Geminiviruses produce a suppressor protein called TrAP that inhibits an enzyme that is required to produce the methyl groups needed for methylation. Thus, it was thought that Geminiviruses avoid transcriptional gene silencing by reducing the cell’s pool of methyl groups (Wang et al., 2005). Using cleverly designed in vivo and in vitro experiments Castillo-González et al. found that TrAP interacts with KRYPTONITE and blocks its enzymatic activity to relax the viral chromatin and allow the virus DNA to replicate (Figure 1).
The production of high levels of TrAP in plants also leads to the deregulation of many plant genes whose expression is usually controlled by transcriptional gene silencing. This deregulation could potentially explain the similarities in appearance between TrAP-producing plants and transcriptional gene silencing mutant plants. However, plants that lack a working KRYPTONITE enzyme do not present those physical features, which suggest that TrAP may also block other enzymes belonging to the SET domain family in A. thaliana.
In the future, it will be interesting to find out whether some plants are resistant to infection by Geminiviruses because they have methyltransferases that TrAP is unable to bind to. If that turns to be the case, the findings would be of great help to unravel the interactions between plants and viruses and how they have co-evolved. Some Geminiviruses, such as the Maize streak virus infect crops and can cause serious economical losses. The work from Castillo-González et al. might point biotechnologists into new ways to create resistant plants.
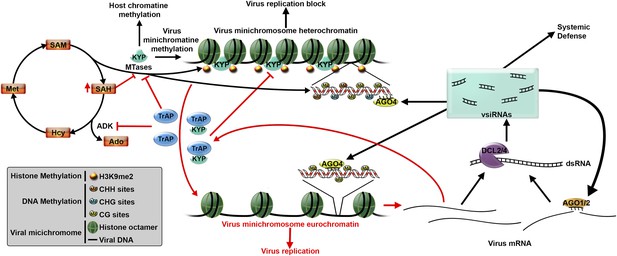
Plant defenses against virus infection can be overcome by a suppressor protein.
After infecting a plant cell, a Geminivirus starts to replicate (bottom right). This leads to the production of double stranded RNA molecules, which are processed by a DCL enzyme to produce virus-derived small interfering RNAs (vsiRNAs). These, in turn, trigger two defense mechanisms (black arrows) that aim to block virus replication. The vsiRNAs could be loaded into AGO1 and AGO2 enzymes to silence target viral mRNAs (known as post-transcriptional gene silencing), or could be loaded into AGO4 enzymes to direct DNA methylation (process called transcriptional gene silencing). KRYPTONITE (KYP), or another methyltranserase (MTase), methylates the histones in the viral minichromosome, which also promotes methylation of virus DNA. This results in the minichromosome becoming condensed, which blocks virus replication. However, many viruses produce suppressor proteins, such as TrAP, to counteract these defenses. TrAP blocks transcriptional gene silencing in two ways (red arrows): it inhibits the activity of the ADK enzyme leading to the accumulation of SAH (a molecule that blocks MTase activity) and a reduction in SAM (which is needed for methylation); TrAP can also directly bind to and inhibit the activity of KYP (and perhaps other MTases). Together these two process lead to the de-methylation of the minichromosome, which allows the virus to replicate. Abbreviations: DCL: Dicer-like ribonuclease; AGO: Argonaute; ADK: adenosine kinase; SAH: S-adenosylhomocysteine; SAM: S-adenosyl-methionine.
References
-
Histone methylation in higher plantsAnnual Review of Plant Biology 61:395–420.https://doi.org/10.1146/annurev.arplant.043008.091939
-
Virus-derived small interfering RNAs at the core of plant-virus interactionsTrends in Plant Science 15:701–707.https://doi.org/10.1016/j.tplants.2010.09.001
-
RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defenceNature Reviews. Microbiology 11:745–760.https://doi.org/10.1038/nrmicro3120
-
Viral genome methylation as an epigenetic defense against geminivirusesJournal of Virology 82:8997–9007.https://doi.org/10.1128/JVI.00719-08
Article and author information
Author details
Publication history
- Version of Record published: October 16, 2015 (version 1)
Copyright
© 2015, Ré and Manavella
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,469
- views
-
- 185
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
- Chromosomes and Gene Expression
Heat stress is a major threat to global crop production, and understanding its impact on plant fertility is crucial for developing climate-resilient crops. Despite the known negative effects of heat stress on plant reproduction, the underlying molecular mechanisms remain poorly understood. Here, we investigated the impact of elevated temperature on centromere structure and chromosome segregation during meiosis in Arabidopsis thaliana. Consistent with previous studies, heat stress leads to a decline in fertility and micronuclei formation in pollen mother cells. Our results reveal that elevated temperature causes a decrease in the amount of centromeric histone and the kinetochore protein BMF1 at meiotic centromeres with increasing temperature. Furthermore, we show that heat stress increases the duration of meiotic divisions and prolongs the activity of the spindle assembly checkpoint during meiosis I, indicating an impaired efficiency of the kinetochore attachments to spindle microtubules. Our analysis of mutants with reduced levels of centromeric histone suggests that weakened centromeres sensitize plants to elevated temperature, resulting in meiotic defects and reduced fertility even at moderate temperatures. These results indicate that the structure and functionality of meiotic centromeres in Arabidopsis are highly sensitive to heat stress, and suggest that centromeres and kinetochores may represent a critical bottleneck in plant adaptation to increasing temperatures.
-
- Chromosomes and Gene Expression
Splicing is the stepwise molecular process by which introns are removed from pre-mRNA and exons are joined together to form mature mRNA sequences. The ordering and spatial distribution of these steps remain controversial, with opposing models suggesting splicing occurs either during or after transcription. We used single-molecule RNA FISH, expansion microscopy, and live-cell imaging to reveal the spatiotemporal distribution of nascent transcripts in mammalian cells. At super-resolution levels, we found that pre-mRNA formed clouds around the transcription site. These clouds indicate the existence of a transcription-site-proximal zone through which RNA move more slowly than in the nucleoplasm. Full-length pre-mRNA undergo continuous splicing as they move through this zone following transcription, suggesting a model in which splicing can occur post-transcriptionally but still within the proximity of the transcription site, thus seeming co-transcriptional by most assays. These results may unify conflicting reports of co-transcriptional versus post-transcriptional splicing.





