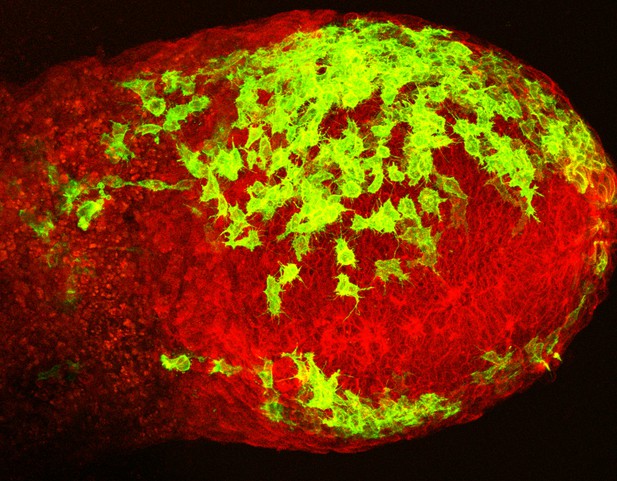
A mouse embryo after 7.5 days of development. The mesoderm cells are highlighted in green. Image credit: Saykali et al. (CC BY 4.0)
As an embryo develops, its cells divide and specialize to form different tissues and organs. Early in development the cells arrange into so-called germ layers, which each produce particular types of tissue. One of these layers, called the mesoderm, develops into the muscles, bones and circulatory system of the embryo. It also contributes to the support structures that feed and protect the embryo, such as the placenta, umbilical cord and yolk sac. If these ‘extra-embryonic’ structures do not develop correctly, the embryo may not grow properly.
Much of what we know about how the cells of the mesoderm move around to form different tissues comes from studies of species that lay eggs; for example, chicks, frogs and fish. The initial steps of embryo development in these animals are similar to how mammals develop, but bigger differences emerge as the extra-embryonic tissues start to form. Recent methodological advances are now making it possible to dynamically study this later stage of development in live mouse embryos.
Saykali et al. studied mouse embryos whose mesoderm cells contained a ‘reporter’ that allowed them to be identified when viewed using a microscopy technique known as two-photon live imaging. This approach allows cells to be tracked as they move through living tissue. Saykali et al. found that the mesoderm cells change shape depending on which region of the embryo they are in, and on which germ layer they are next to. The cells that become extra-embryonic are larger and longer, and develop small protrusions. Instead of moving directly to their destinations, they tend to zigzag.
Further experiments revealed that embryonic and extra-embryonic mesoderm cells produce different amounts of several proteins, including the distinct types of filaments that act as the cell’s internal skeleton. Mesoderm cells that are destined to become extra-embryonic depend less on signaling proteins called Rho GTPases to move around.
Knowing how mesoderm cells form extra-embryonic structures will help researchers to understand how problems with these structures can affect how embryos grow. The techniques used by Saykali et al. will also help to design new ways to cultivate mesoderm cells in the laboratory for future experiments. These could, for example, investigate whether human mesoderm cells develop in the same way as mice mesoderm cells.




