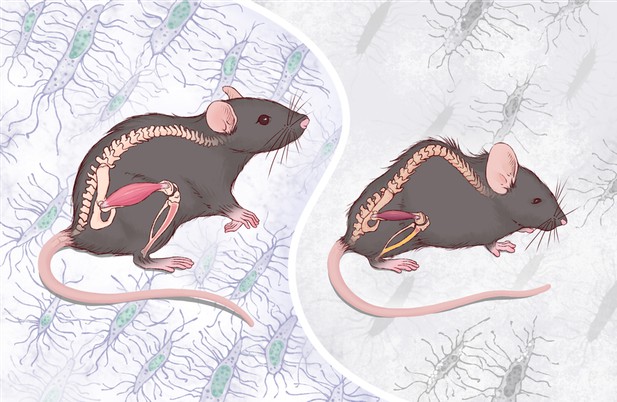
Mice with normal numbers of osteocytes (left) look healthy, while mice with fewer osteocytes (right) exhibit accelerated aging in their bones and muscles, as evidenced by the hunchback. Image credit: Ding, Gao, Gao et al. (CC BY 4.0)
A hallmark of aging is the weakening of our muscles and bones, which become more fragile as we get older. These gradual changes can result in a humpback and muscle shrinking among other conditions. At the same time little is known about what role osteocytes – the most abundant type of bone cell – play in the process of bone and muscle aging.
One way to investigate the role of osteocytes in aging is to remove them and observe what happens to nearby cells as they age. To achieve this Ding, Gao, Gao et al. genetically altered mice so that they would carry and activate a gene called DTA in their osteocytes. DTA is a gene derived from the bacterium that causes diphtheria, and when it is activated, it produces a toxin that accumulates in cells, eventually killing them. In the mice line developed by Ding, Gao, Gao et al. DTA slowly killed osteocytes, leading to adult mice lacking most of their osteocyte population that have a normal embryonic development. This is important because the fact that the mice develop normally before birth allowed the team to rule out embryonic defects when looking at their results.
Ding, Gao, Gao et al. found that, without enough osteocytes, the nearby bone and bone marrow cells aged faster than expected. Indeed, the skeleton and muscles of adult mice was severely affected by the loss of osteocytes, leading to fragile bones with lower mass and muscle shrinking. These mice looked old in their young age and died earlier.
At the cellular level, the removal of osteocytes impaired the formation of osteoblasts, the cells that are responsible for making bones. It also led to an increase in the numbers of osteoclasts – the cells that destroy bone tissue to repair it and maintain it – and fat tissue cells. Furthermore, cells in the bone marrow, which go on to make white blood cells, were also affected. The mechanisms through which osteocytes affect the growth of these other cells is yet to be fully understood. However, Ding, Gao, Gao et al. did observe that these cells acquired traits characteristic of aging cells, implying that osteocytes have a role in regulating cellular aging or senescence. Among these senescence traits is the increased production and secretion of molecules that interact with the immune system, a feature known as the ‘senescence-associated secretory phenotype’.
Overall, the results of Ding, Gao, Gao et al. suggest that reducing the number of osteocytes in mice leads to faster bone aging and affects the balance of the different cell types required for healthy bone and bone marrow growth. Future research could focus on finding drugs that allow osteocytes to keep performing their role during aging, and thus help maintain bone health. The findings of Ding, Gao, Gao et al. also suggest that osteocytes may be playing a previously underappreciated role in age-related diseases, which warrants further investigation.




