Female resistance to pneumonia identifies lung macrophage nitric oxide synthase-3 as a therapeutic target
Figures
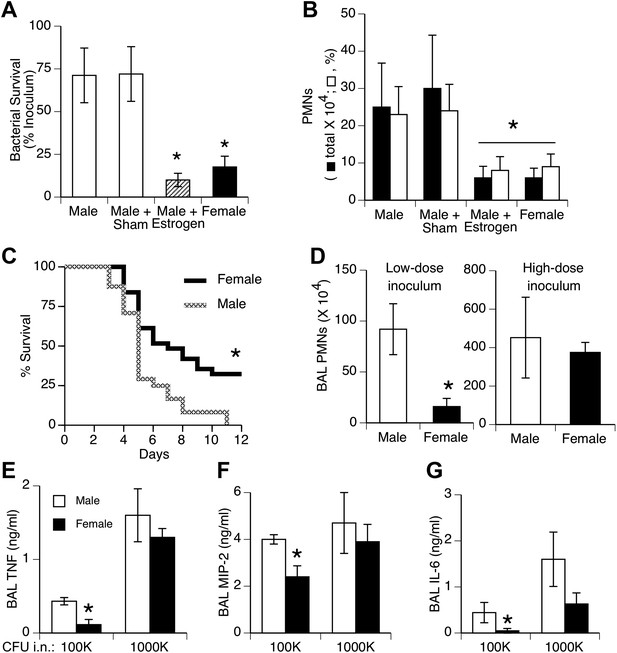
Females show greater resistance to pneumococcal pneumonia.
(A) Twenty-four hours after intranasal (i.n.) inoculation of S. pneumoniae (∼105 CFU), lung samples from female mice (and estrogen-treated male mice via subcutaneous slow-release 17-beta-estradiol pellets, ∼70 µg/day) contain fewer live bacteria than seen in male mice (n > 12, * = p < 0.01 vs control or sham-treated males) and (B) show less acute inflammation (BAL neutrophils, n > 12, * = p < 0.01). (C) After i.n. pneumococcus, female mice show significantly greater survival than male mice (2.5 × 105 CFU, n > 24, * = p < 0.01). Gender differences in pneumonic inflammation are seen with low (4 × 105 CFU), but not high (11 × 105), bacterial inocula, measured as BAL neutrophilia (D) or BAL cytokines TNF (E), MIP-2 (F), or IL-6 (G), (n > 3, * = p < 0.05).
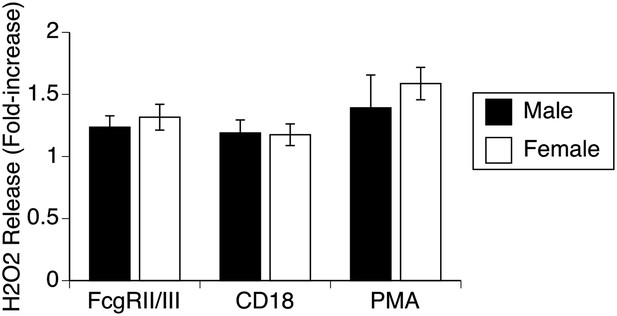
Respiratory burst by male and female alveolar macrophages.
Stimulation of normal AMs by antibodies to 2 different surface receptors (FcR, CD18) or with PMA leads to approximately equal increases in H2O2 release in both male and female AMs, indicating absence of gender differences in production of reactive oxygen species.
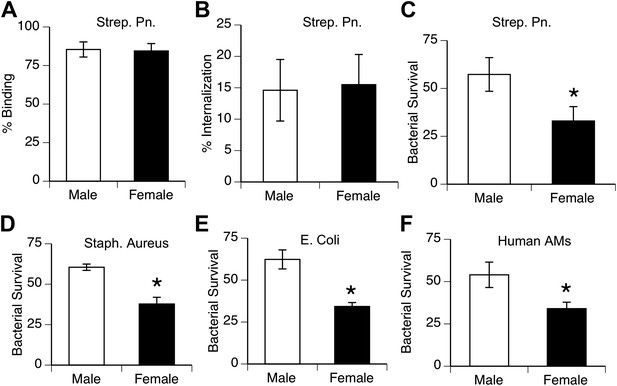
Female alveolar macrophages show better killing of ingested bacteria.
Binding (A) and internalization (B) of S. pneumoniae in normal male and female AMs is similar. Female AMs kill more internalized bacteria than male AMs in assays using pneumococci (C) (n > 11, * = p < 0.01), S. aureus (D) or E. coli (E), (n > 3, * = p < 0.01). (F). Normal human female AMs also show greater killing of internalized pneumococci, (n > 5, * = p < 0.01).
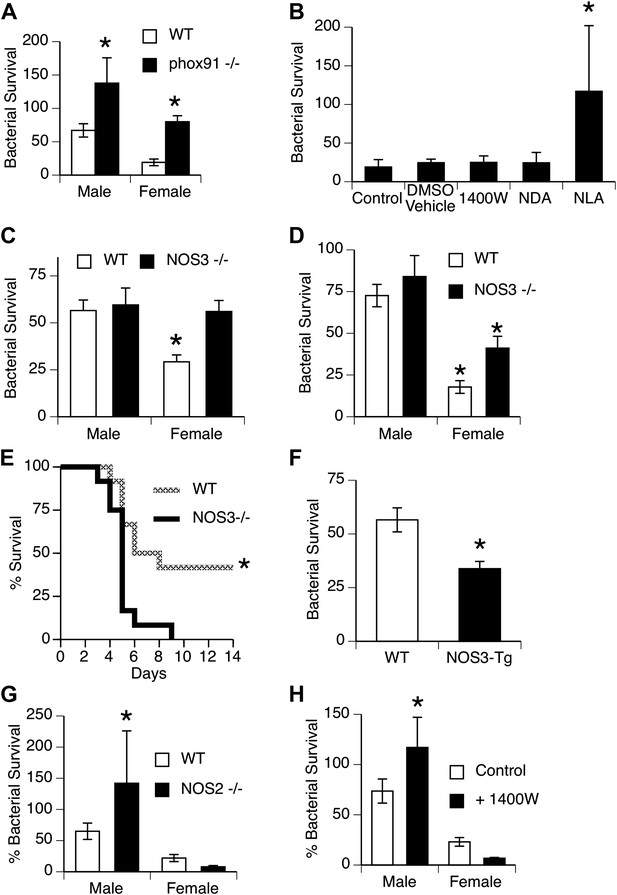
NOS3 and female resistance to pneumococcal pneumonia.
(A) NADPH oxidase deficient (phox91−/−) mice show comparable reduction in bacterial clearance in both male and female mice (n = 6, * = p < 0.01 vs wild-type). (B) In vitro killing of pneumococci by normal mouse female AMs is inhibited by the non-selective NOS inhibitor nitro-L-arginine (NLA), but not by its inactive stereo-isomer, nitro-D-arginine (NDA), nor by the type 2 NOS specific inhibitor 1400W (n = 3–4, * = p < 0.01). (C) Female AMs from Nos3−/− mice lose the in vitro killing advantage of wild-type female AMs and show the same killing rate as wild-type or NOS3 deficient male AMs (n = 3, * = p < 0.01 vs wild-type). (D) In vivo, absence of NOS3 reduces, but does not completely eliminate, the female advantage in bacterial clearance (n = 15, * = p<0.015 vs all 3 other groups) and results in increased mortality from pneumococcal pneumonia (E) (n = 12 female mice per group, * = p < 0.01). Conversely, transgenic male mice with increased expression of human NOS3 show enhanced killing of S. pneumoniae in vivo (F) (lower bacterial survival, n > 5, * = p < 0.01). In this low-dose inoculum model, NOS2 deletion (G) or inhibition (H) causes reduced bacterial clearance in male, but not female mice (n = 8, * = p < 0.05).
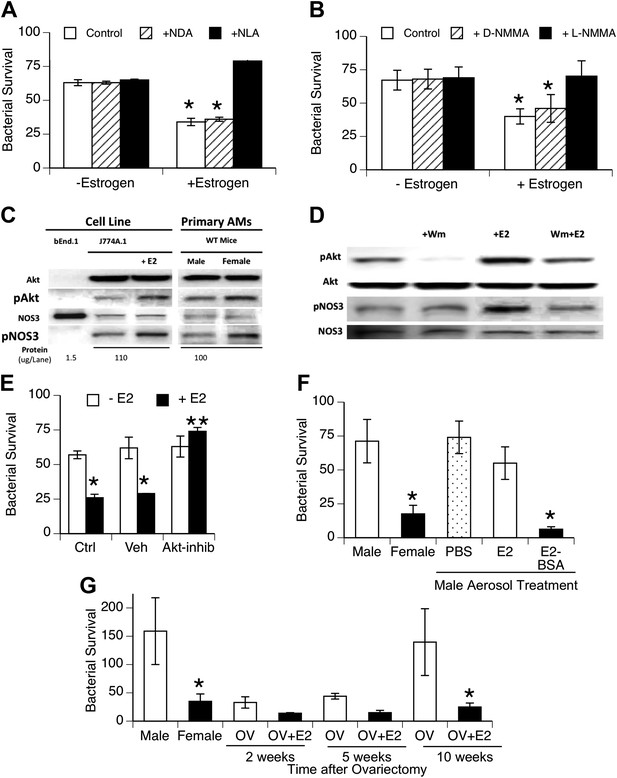
Estrogen-mediated activation of macrophage NOS3.
Estrogen treatment of J774A.1 mouse or human U937 macrophages (A and B) increases killing of ingested pneumococci; this increased killing is prevented by the NOS inhibitors NLA or l-NMMA, but not control stereoisomers (n = 3–4, * = p < 0.01). (C) Western blot analysis shows >100-fold NOS3 in macrophages compared to the endothelial cell line bEnd.1; after 30 min, estrogen-treated (E2, estradiol, 0.2 ng/ml) J774A.1 mouse macrophages show increased phosphorylation of Akt and NOS 3, while normal female AMs show basally increased pAkt and pNOS3 compared to male AMs; (D) basal- and estrogen-enhanced phosphorylation of Akt and NOS3 are inhibited by wortmannin (Wm, 50 nM). (E) Inhibition of Akt with 1L-6-hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate (10 µg/ml REF) prevents estrogen-mediated increased bacterial killing in J774A.1 cells (n = 3, * = p < 0.01). (F) Aerosol pre-treatment of male mice with albumin-conjugated estrogen 30 min before pneumococcal infection improves bacterial killing (n = 6, * = p < 0.01). (G) In ovariectomy-model of menopause, female mice lose their greater resistance to pneumococcal pneumonia after 10 weeks, an effect reversed by treatment with estrogen prior to infection, n > 8 for control, 10 week groups; n = 3 for 2 and 5 week groups; * = p < 0.01.
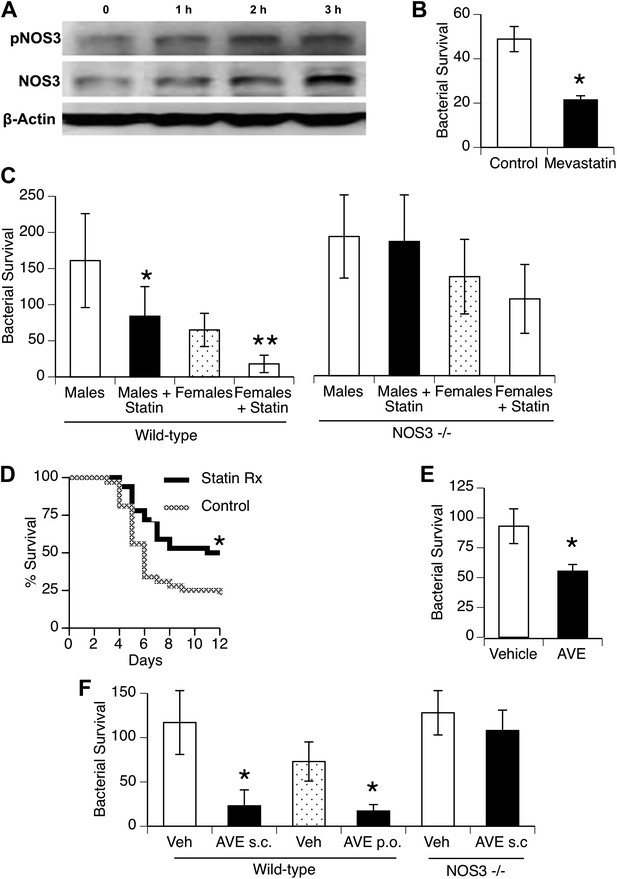
Statins enhance innate immune resistance to S. pneumoniae via NOS3.
(A) In vitro treatment of J774A.1 mouse macrophages with mevastatin (5 µM) increases levels of pNOS3 and NOS3 and (B) concomitantly increases killing of internalized bacteria (n = 4, * = p < 0.01). (C) In vivo, pre-treatment of mice with pravastatin (50 mg/kg) significantly improves bacterial clearance in wild-type mice (n = 8, * = p < 0.01 vs male controls; ** = p < 0.01 vs males, males + statin), but has no significant effect on either male or female NOS3−/− mice. (D) Statin-treated male mice with pneumococcal pneumonia show improved survival (n = 8, * = p < 0.01). (E) AVE3085, a small molecule activator of NOS3, increases bacterial killing by mouse macrophages in vitro (n = 3, * = p < 0.01) (F) Pre-treatment of male mice with AVE3085 by either subcutaneous or oral route improves in vivo bacterial clearance, an effect not seen in NOS3−/− male mice (n = 3–8, * = p < 0.01).
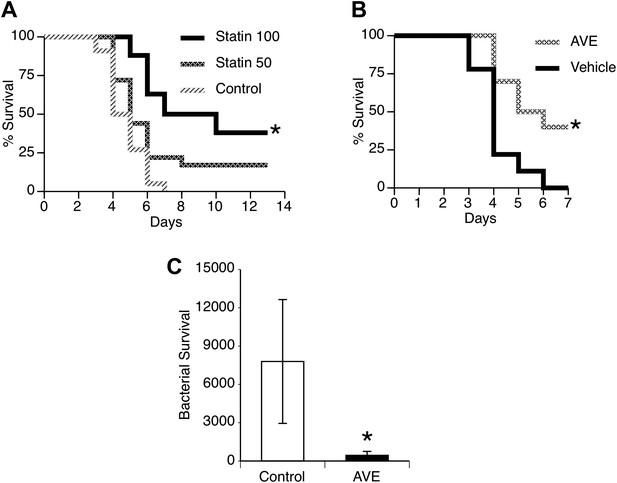
Statins and AVE3085 improve survival from post-influenza secondary pneumococcal pneumonia.
Male mice were allowed to recover 7 days from mild influenza (PR8 1 PFU i.n.) and then challenged with S. pneumoniae (500 CFU i.n.). Pre-treatment with (A) pravastatin (50 or 100 mg/kg) or (B) AVE3085 (0.75 mg, s.c.) caused a significant improvement in survival (n = 10, * = p < 0.01). (C) AVE3085 treatment also lead to improved bacterial clearance 24 hr after pneumococcal challenge in this post-influenza model (n = 6, * = p < 0.01).
Tables
Characteristics of 28,576 female subjects with first-time hospitalized pneumonia and 142,880 age-matched female population control subjects from Northern Denmark, 1997–2012
| Characteristic at time of pneumonia admission | Pneumonia cases | Population controls | Total |
|---|---|---|---|
| n | 28,576 | 142,880 | 171,456 |
| Age | |||
| 15–39 years | 2006 (7.0) | 10,062 (7.0) | 12,068 (7.0) |
| 40–64 years | 6383 (22.3) | 32,023 (22.4) | 38,406 (22.4) |
| 65–79 years | 9871 (34.5) | 49,267 (34.5) | 59,138 (34.5) |
| ≥80 years | 10,316 (36.1) | 51,528 (36.1) | 61,844 (36.1) |
| Charlson comorbidity index | |||
| Index low (0) | 12,031 (42.1) | 100,297 (70.2) | 112,328 (65.5) |
| Index medium (Mizgerd, 2006; Shrestha et al., 2013) | 11,324 (39.6) | 34,896 (24.4) | 46,220 (27.0) |
| Index high (≥3) | 5221 (18.3) | 7687 (5.4) | 12,908 (7.5) |
| Alcoholism-related conditions | 1398 (4.9) | 2225 (1.6) | 3623 (2.1) |
| Preadmission medication use | |||
| Antibiotic use (≤3 months) | 12,778 (44.7) | 20,002 (14.0) | 32,780 (19.1) |
| Statins, current use (≤ 6 months) | 3506 (12.3) | 16,111 (11.3) | 19,617 (11.4) |
| Estrogen, current use (≤ 6 months) | 2592 (9.1) | 13,225 (9.3) | 15,817 (9.2) |
| Statins and estrogen, current use (≤ 6 months) | 509 (1.8) | 2509 (1.8) | 3018 (1.8) |
| Statins and estrogen, no current use | 21,969 (76.9) | 111,035 (77.7) | 133,004 (77.6) |
| Marital status | |||
| Married | 10,109 (35.4) | 57,673 (40.4) | 67,782 (39.5) |
| Never married | 2825 (9.9) | 13,523 (9.5) | 16,348 (9.5) |
| Divorced or widowed | 15,642 (54.7) | 71,684 (50.2) | 87,326 (50.9) |
Odd ratios for first-time hospitalized pneumonia associated with current use of statins alone, estrogen alone, and estrogen + statins in combination
| Statin use | Pneumonia cases (n = 28,576) | Matched population controls (n = 142,880) | Crude OR (95% CI)* | Adjusted OR (95% CI)† |
|---|---|---|---|---|
| No current use of statins or estrogen | 21,969 (76.9) | 111,035 (77.7) | 1.0 (reference) | 1.0 (reference) |
| Current use of statins (≤ 180 days before admission) | 3506 (12.3) | 16,111 (11.3) | 1.12 (1.07–1.16) | 0.82 (0.78–0.85) |
| Current use of estrogen (≤ 180 days before admission) | 2592 (9.1) | 13,225 (9.3) | 0.99 (0.95–1.04) | 0.82 (0.78–0.86) |
| Current use of both statins and estrogen (≤ 180 days before admission) | 509 (1.8) | 2509 (1.8) | 1.04 (0.94–1.15) | 0.67 (0.60–0.75) |
-
*
Matched for age and hospitalization date.
-
†
Adjusted for level of Charlson's comorbidity index (19 different comorbidities), alcoholism-related conditions, antibiotics before admission, and marital status (see Table 1).





