Dopamine drives Drosophila sechellia adaptation to its toxic host
Figures
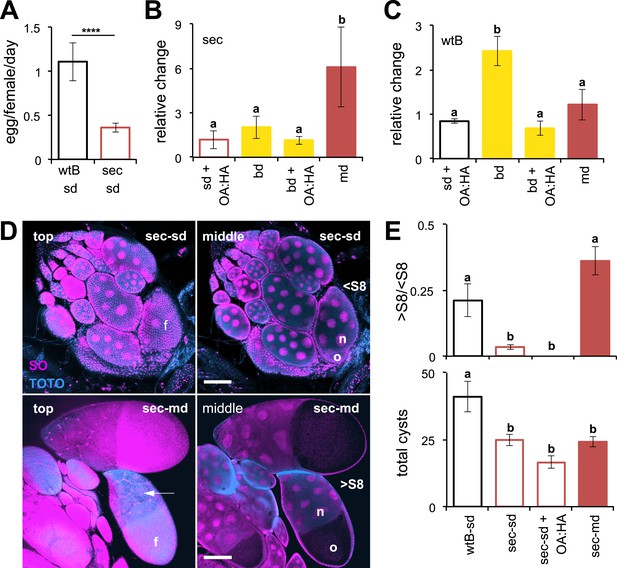
Morinda increases egg production in D. sechellia.
(A–C) Egg production (egg/female/day) (N > 20) (A) and its relative change (N > 5) (B and C) in D. sechellia (14021–0248.25, sec) and D. melanogaster wild-type Berlin (wtB) fed a standard diet (sd), or morinda diet (md), or banana diet (bd), or diets supplemented with morinda carboxylic acids (+OA:HA). (D) Confocal images showing the surface (top) or the interior (middle) of ovarioles stained with nucleic acid specific dyes (sytox orange (SO) and TOTO) of D. sechellia (14021–0248.25) fed a standard diet (sec-sd) or a morinda diet (sec-md). The follicle cells (f) surrounding the oocyte (o) or stretched over the nurse cells (n) (arrow) are indicated for early (<S8) or vitellogenic cysts (>S8). Scale bar 100 μm. (E) Rate of vitellogenesis (>S8/<S8, top graph) and number of cysts (total cysts, lower graph) (N > 8) in D. sechellia (14021–0248.25, sec) and D. melanogaster wild-type Berlin (wtB) fed a standard diet (sd), or a morinda diet (md), or a diet supplemented with morinda carboxylic acids (+OA:HA). Different letters denote significant differences (p < 0.01) using ANOVA followed by Tukey's test (B–E); ****p < 0.00001 using Student's t test to compare species (A). Error bars represent s.e.m.
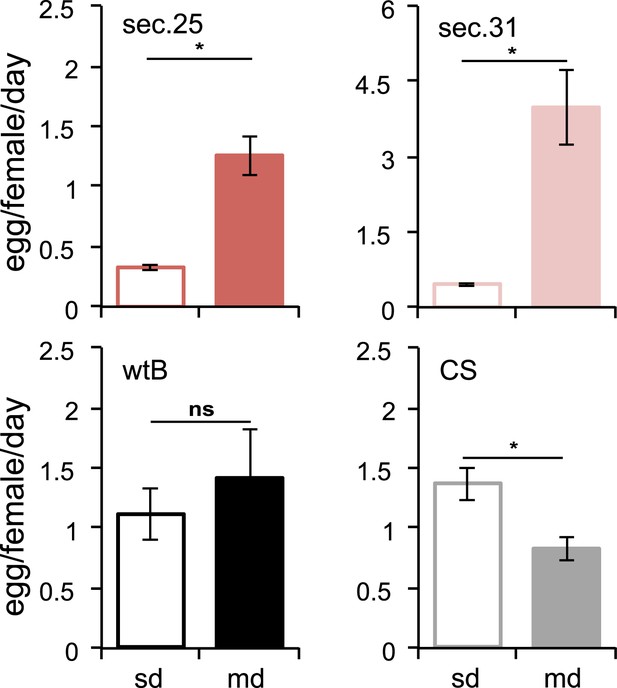
Morinda stimulates egg production in D. sechellia.
Egg production (egg/female/day) in D. sechellia 14021–0248.25 (sec.25, N = 3), D. sechellia 14021–0248.31 (sec.31, N = 3), D. melanogaster wild-type Berlin (wtB, N = 3) and D. melanogaster Canton-S (CS, N = 3), fed a standard diet (sd) or morinda diet (md). ns = non-significant, *p < 0.05 using Student's t test to compare diets in each group. Error bars represent s.e.m.
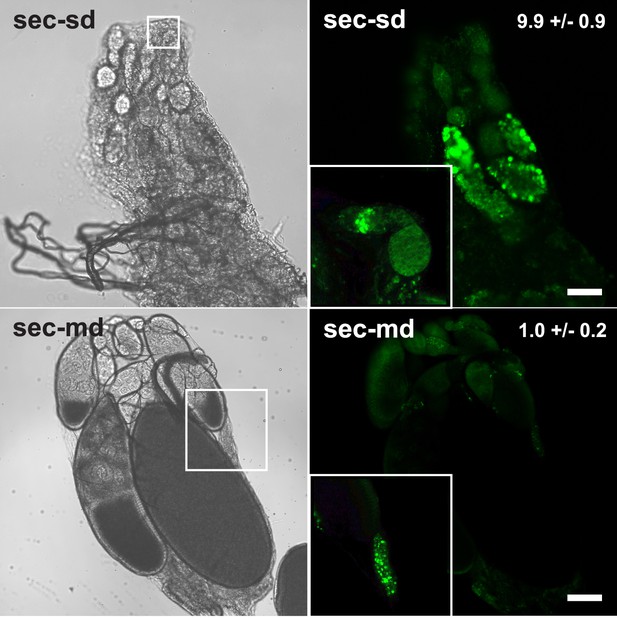
Apoptosis in D. sechellia ovaries.
Confocal fluorescent images (right) and light transmission images (left) of D. sechellia (14021–0248.25, sec) ovaries of flies fed a standard diet (sd) (top) or morinda diet (md) (bottom), stained with the vital die acridine orange to label apoptosis (Arama and Steller, 2006). The number of apoptotic cysts per ovary (average ± s.e.m.) is indicated for a standard diet (N = 11) and morinda diet (N = 10). Insets show in detail apoptosis occurring at the germanium (top) and an example of the occasional apoptotic cysts in ovaries of flies fed morinda (bottom). Scale bar 20 μm.
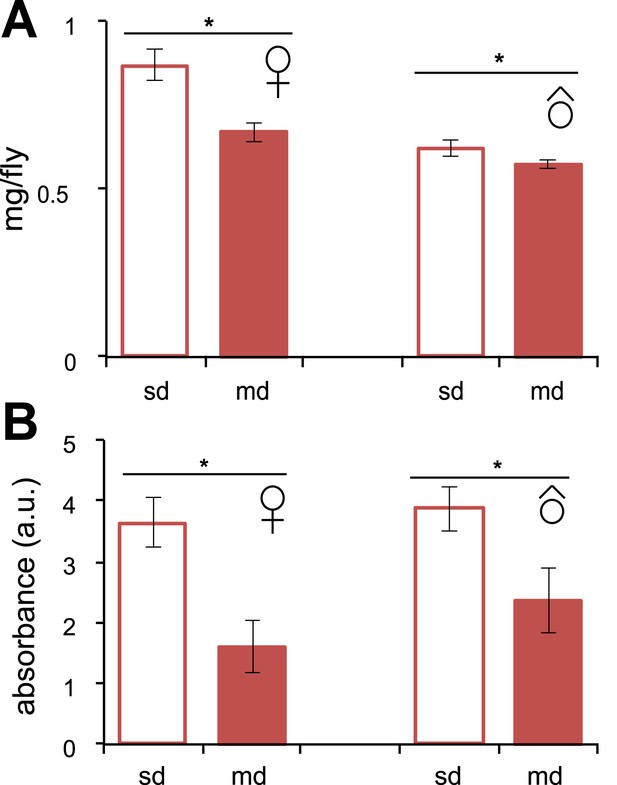
Feeding behavior in D. sechellia.
(A) Individual weight (mg/fly) (N > 20) of D. sechellia (14021–0248.25) mated females and males grown on standard diet (sd) or morinda diet (md). (B) Food intake (N = 3) measured as light absorbance (a. u., arbitrary units) of ingested sulforhodamine B added to a standard diet (sd) or morinda diet (md). *p < 0.01 using Student's t test to compare diets in each group. Error bars represent s.e.m.
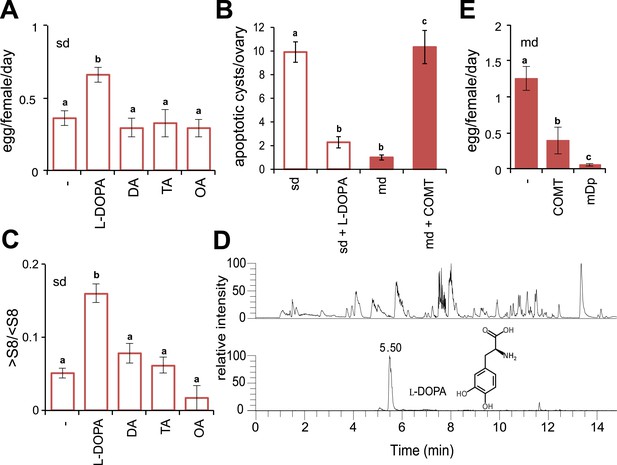
Morinda l-DOPA is required to stimulate egg production.
(A) Egg production (egg/female/day) (N > 6) (B) quantification of apoptosis (apoptotic cysts/ovary) (N > 6) and (C) rate of vitellogenesis (>S8/<S8) (N > 12) in D. sechellia (14021–0248.25) flies fed a non-supplemented (−) standard diet (sd) or a standard diet supplemented with L-3,4-dihydroxyphenylalanine (1 mg/ml, l-DOPA); dopamine (1 mg/ml, DA); tyramine (2 mg/ml, TA) or octopamine (2 mg/ml, OA); or a non- pretreated morinda diet (md) or a morinda diet pre-treated with catechol-O-methyltransferase (2.5 U per gram of fruit, md + COMT). Different letters denote significant differences (p < 0.01) using ANOVA followed by Tukey's test. Error bars represent s.e.m. (D) The total ion chromatogram (top trace) shows all compounds present in morinda extract, and the extracted ion chromatogram (lower trace) corresponds to the exact mass of sum formula of L-3,4-dihydroxyphenylalanine (l-DOPA) present in the fruit; as analysed by UHPLC-MS. (E) Egg production (eggs/female/day) (N > 3) in D. sechellia (14021–0248.25) flies fed a morinda diet (md) non-pre-treated (−), pre-treated with catechol-O-methyltransferase (2.5 U per gram of fruit, COMT) or α-methyl-DOPA (0.4 mM, mDp). Different letters denote significant differences (p < 0.01) using ANOVA followed by Tukey's test. Error bars represent s.e.m.
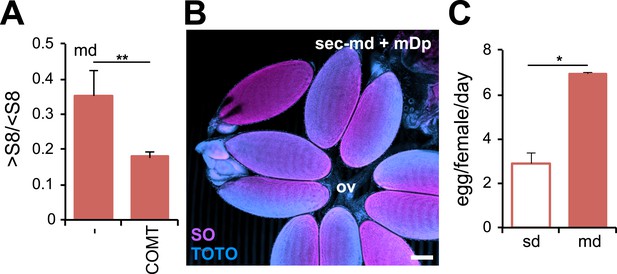
D. sechellia female fertility is modulated by morinda.
(A) Rate of vitellogenesis (>S8/<S8) (N > 6) in D. sechellia (14021–0248.25) flies fed a morinda diet (md) non-pre-treated (−) or pre-treated with catechol-O-methyltransferase (2.5 U per gram of fruit, COMT). **p < 0.002 using Student's t test. Error bars represent s.e.m. (B) Confocal image shows D. sechellia (14021–0248.25, sec) egg retention in the ovary of a fly fed a morinda diet (md) supplemented with α-methyl-DOPA (0.4 mM, mDp). Dissected ovaries were stained with nucleic-acid-specific dyes (sytox orange (SO) and TOTO). The oviduct (ov) is indicated. Scale bar, 100 μm. (C) Bar graph shows ovipositon (egg/female/day) (N = 3) stimulated in D. sechellia (14021–0248.25) flies offered fresh morinda (md) as oviposition substrate compared to flies offered a standard oviposition substrate (sd). *p < 0.02 using Student's t test. Error bars represent s.e.m.
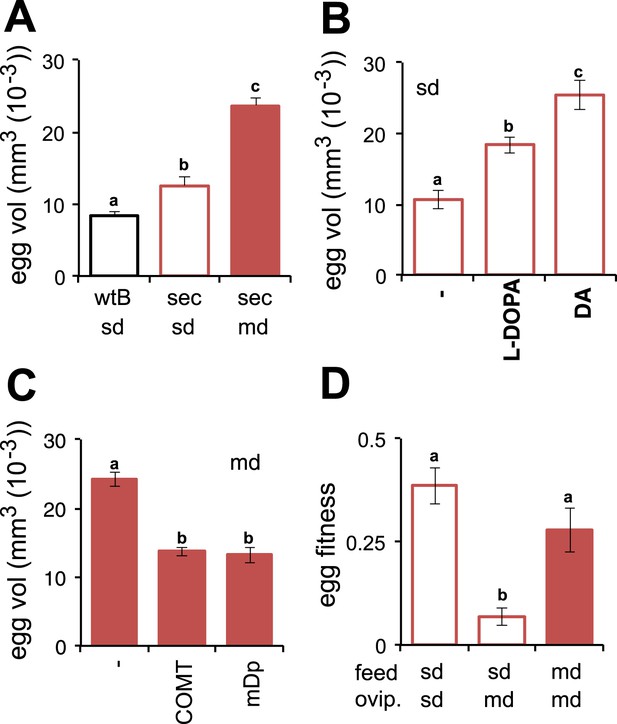
Morinda enhances early fitness.
(A–C) Volume (mm3(10−3)) of D. melanogaster wild-type Berlin (wtB) and D. sechellia (14021–0248.25, sec) eggs produced by flies fed a standard diet (sd) or morinda diet (md) (N > 15) (A); D. sechellia (14021–0248.25) flies fed a non-supplemented (−) standard diet (sd), or supplemented with L-3,4-dihydroxyphenylalanine (1 mg/ml, l-DOPA) or dopamine (1 mg/ml, DA) (N > 23) (B); D. sechellia (14021–0248.25) flies fed a non-pre-treated (−) morinda diet (md), or pre-treated with catechol-O-methyltransferase (2.5 U per gram of fruit, COMT) or α-methyl-DOPA (0.4 mM, mDp) (N > 10) (C). (D) Egg hatching rate (N > 5) in D. sechellia (14021–0248.25) fed (feed) a standard diet (sd) or morinda diet (md), ovipositing (ovip.) in either media. Different letters denote significant differences (p < 0.01) using ANOVA followed by Tukey's test. Error bars represent s.e.m.
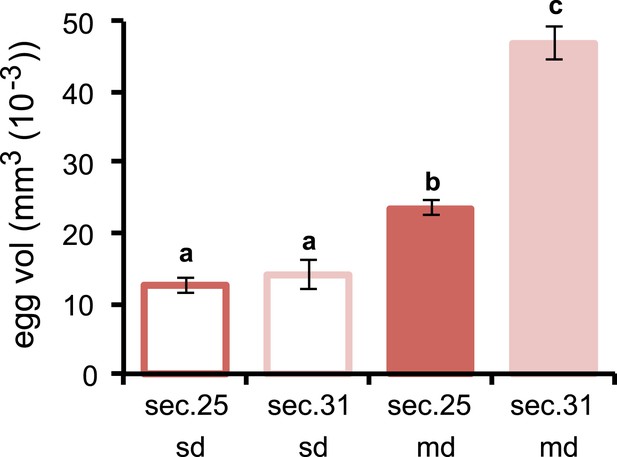
Volume of D. sechellia eggs is modulated by morinda diet.
Volume (mm3 (10−3)) of D. sechellia 14021–0248.25 (sec.25) and D. sechellia 14021–0248.31 (sec.31) eggs produced by flies fed a standard diet (sd) or morinda diet (md) (N > 7). Different letters denote significant differences (p < 0.01) using ANOVA followed by Tukey's test. Error bars represent s.e.m.
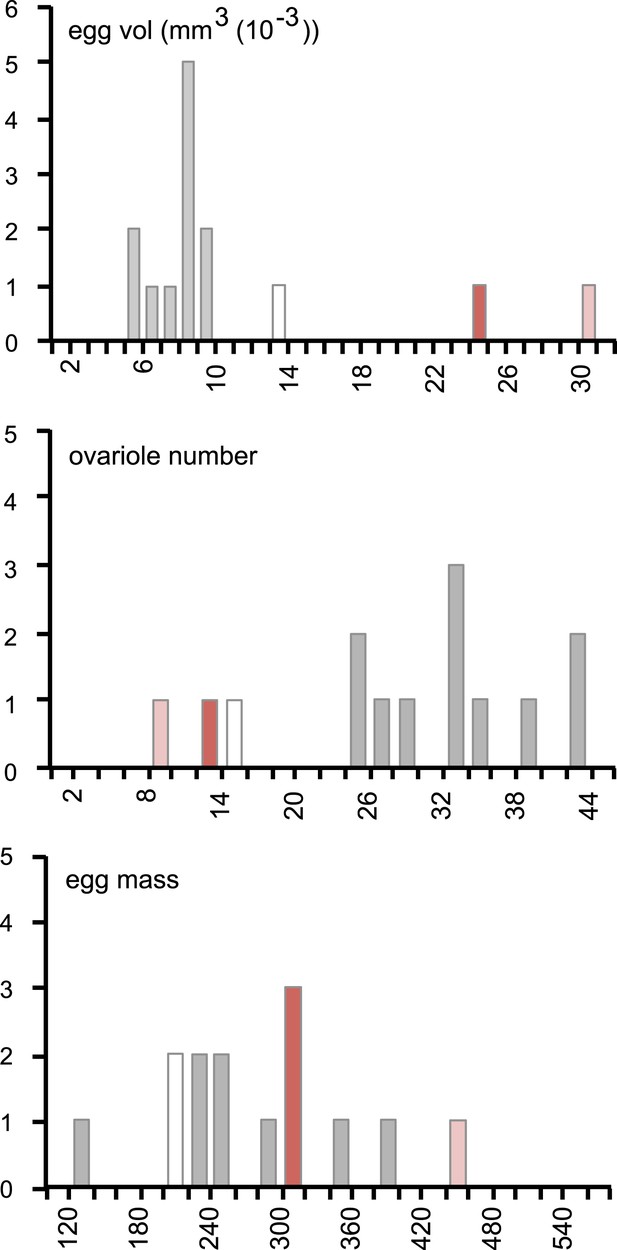
Female resource investment on fertility is conserved in D. sechellia.
Histograms of egg volume (mm3 (10−3)) (top), ovariole number (middle), and egg mass production (egg mass, calculated as egg volume × ovariole number) (bottom) in D. sechellia 14021–0248.25 (red bar) and D. sechellia 14021–0248.31 (light red bar) fed a morinda diet, compared to 12 Drosophila siblings (D. ananassae (14024–0371.13), D. erecta (14021–0224.01), D. melanogaster (wild type Berlin and 14021–0231.36), D. mojavensis (15081–1352.22), D. persimilis (14011–0111.49), D. pseudoobscura (14011–0121.94), D. sechellia (14021–0248.25, white bar), D. simulans (14021–0251.195), D. virilis (15010–1051.87), D. willistoni (14030–0811.24), D. yakuba (14021–0261.01), (data from Markow et al., 2009), (grey bar)) fed a standard diet.
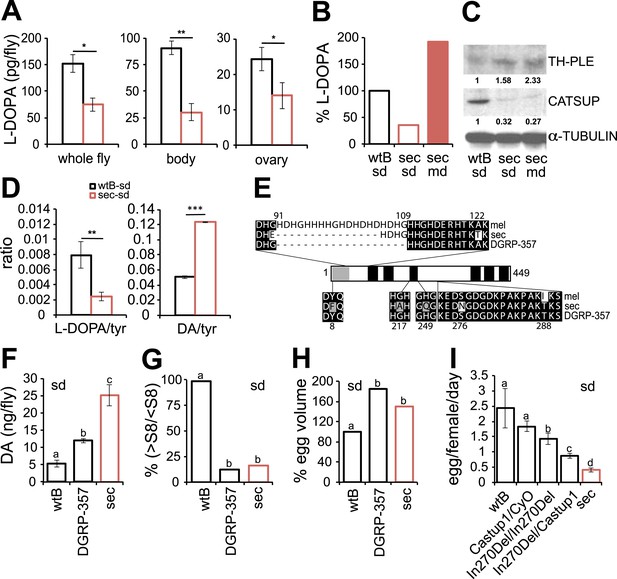
Dopamine metabolism is impaired in D. sechellia.
(A) L-3,4-dihydroxyphenylalanine quantification (l-DOPA pg/fly) (N = 3) in whole fly, bodies and ovaries of female D. melanogaster wild-type Berlin (wtB) and D. sechellia (14021–0248.25, sec) fed a standard diet (sd). *p < 0.05 and **p < 0.002 using Student's t test. (B) Relative L-3,4-dihydroxyphenylalanine (% pg l-DOPA per mg body, % l-DOPA) (N = 3) in female D. melanogaster wild-type Berlin (wtB) and D. sechellia (14021–0248.25, sec) fed a standard diet (sd) or morinda diet (md). p = 0.0062 and p = 0.018 using Student's t test D. melanogaster vs D. sechellia fed, respectively, a standard diet or morinda diet. (C) Western blots of total protein whole-fly extracts for TH-PLE, CATSUP, and α-TUBULIN as a loading control, in D. melanogaster wild-type Berlin (wtB) and D. sechellia (14021–0248.25, sec) fed a standard diet (sd) or morinda diet (md). The numbers under TH-PLE and CATSUP protein lanes indicate the relative protein levels (normalised to α-TUBULIN). (D) Ratios of L-3,4-dihydroxyphenylalanine (l-DOPA/tyr) (N = 3) and dopamine (DA/tyr) (N = 3) to tyrosine substrate in female D. melanogaster wild-type Berlin (wtB) and D. sechellia (14021–0248.25, sec) fed a standard diet (sd). **p < 0.007 and ***p < 0.000007 using Student's t test. (E) Drosophila CATSUP protein structure scheme showing a signal peptide (grey box) and six trans-membrane domains (black boxes). Deletions (dash) and exchanges (grey or white) of amino acids in D. sechellia (14021–0248.25, sec) compared to in D. melanogaster wild-type Berlin (wtB) and in D. melanogaster DGRP-357 (DGRP-357) CATSUP are indicated. (F–H) Dopamine (DA ng/fly) (N = 3) (F), relative rate of vitellogenesis (% >S8/<S8) (N > 10) (G), and egg-volume (% mm3(10−3)) (n > 10) (H) in D. sechellia (14021–0248.25, sec), D. melanogaster wild-type Berlin (wtB) and D. melanogaster DGRP-357 (DGRP-357) fed a standard diet (sd). (I) Egg production (egg/female/day) (N > 3) in D. melanogaster wild-type Berlin (wtB), heterozygote flies (Catsup1/CyO), D. melanogaster DGRP-357 (CatsupIn270Del/CatsupIn270Del), trans heterozygote flies (CatsupIn270Del/Catsup1) and D. sechellia (14021–0248.25, sec), fed a standard diet (sd). Different letters denote significant differences (p < 0.05) using ANOVA followed by Tukey's test (F–I). Error bars represent s.e.m.
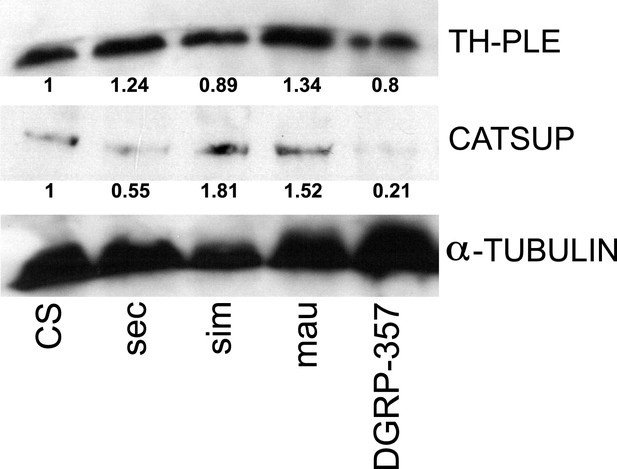
TH-PLE and CATSUP expression in Drosophila.
Western blots of total protein whole-fly extracts for TH-PLE, CATSUP, and α-TUBULIN as a loading control in D. melanogaster Canton-S (CS), D. sechellia (14021–0248.31, sec), D. simulans (14021–0251.004, sim), D. mauritiana (14021–0241.01, mau) and D. melanogaster DGRP-357 (DGRP-357) fed a standard diet. The numbers under TH-PLE and CATSUP protein lanes indicate the relative protein levels (normalised to α-TUBULIN).
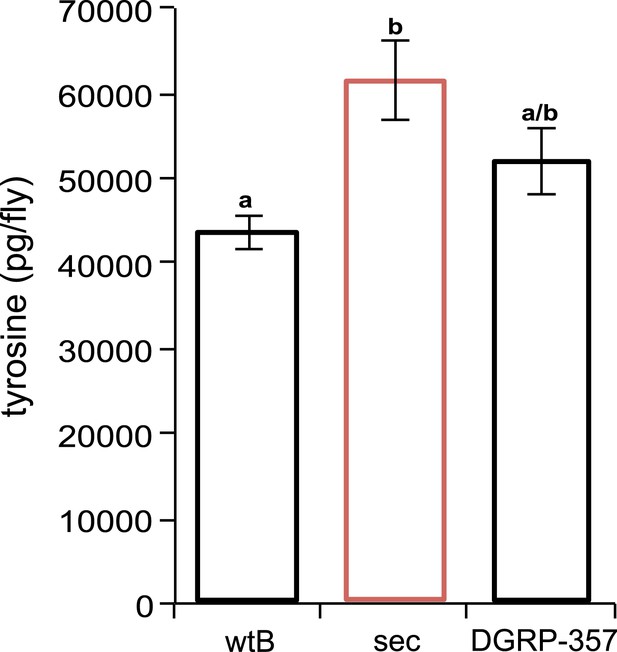
Tyrosine quantification in Drosophila.
D. sechellia (14021–0248.25) females (sec) show higher tyrosine content (expressed as picogram per fly [pg/fly]), compared to D. melanogaster wild-type Berlin females (wtB) and D. melanogaster DGRP-357 (DGRP-357) females, fed a standard diet. N = 3. Different letters denote significant differences (p < 0.05) using ANOVA followed by Tukey's test. Error bars represent s.e.m.
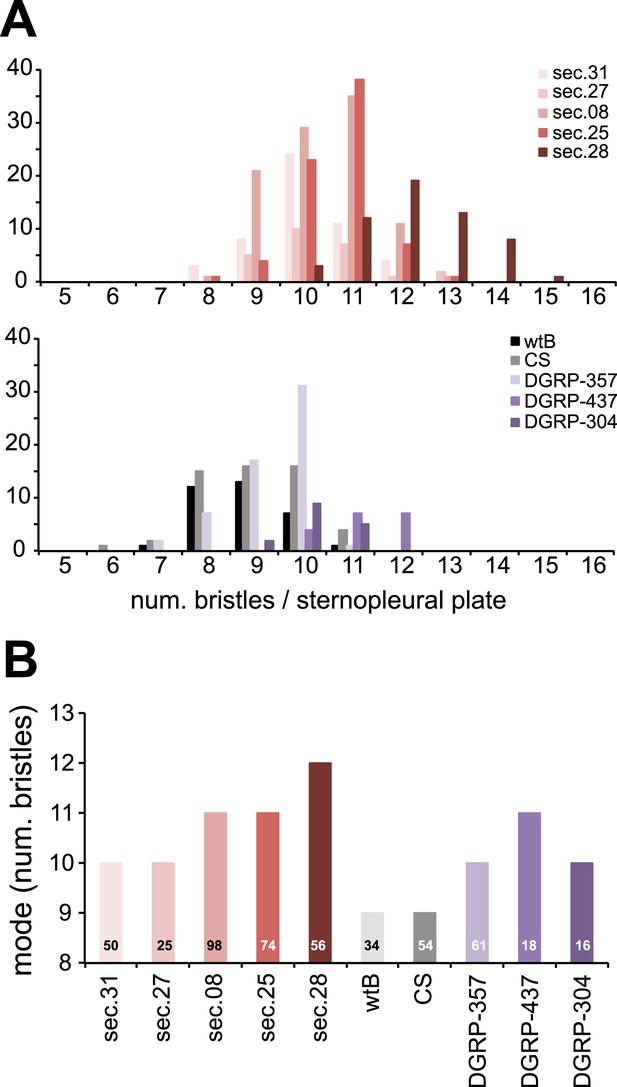
Sensory bristles.
(A) Histograms of total (macro and micro quetas) number of bristles per sternopleural plaque and (B) graph of mode (N is indicated for each species) of female D. melanogaster wild-type Berlin (wtB), D. melanogaster Canton-S (CS), D. melanogaster DGRP-357 (DGRP-357), D. melanogaster DGRP-437 (DGRP437), D. melanogaster DGRP-304 (DGRP-304), and D. sechellia (sec) original from Praslin (14021–0248.31 (sec.31) and 14021–0248.08 (sec.08), Seychelles 14021–0248.27 (sec.27)) and Cousin (14021–0248.25 (sec.25) and 14021–0248.28 (sec.28)) islands.
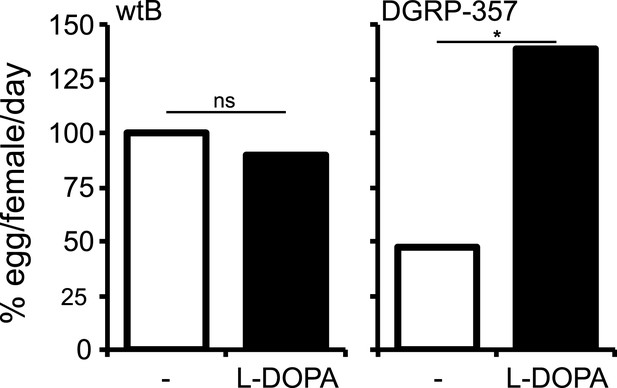
L-DOPA rescues diminished CatsupIn270Del/Catsup1 egg production.
Relative egg production (% egg/female/day) (N = 3) in D. melanogaster wild type Berlin (wtB) and D. melanogaster trans heterozygote flies (CatsupIn270Del/Catsup1) fed a non-supplemented (−) standard diet or a diet supplemented with L-3,4-dihydroxyphenylalanine (1 mg/ml, l-DOPA). ns = non-significant; *p < 0.015 using Student's t test to compare diets in each group.

Conserved Catsup sequence in D. sechellia.
Drosophila CATSUP amino acids sequence showing deletions (dash) and exchanges (grey or white) of amino acids in D. sechellia (14021–0248.03 (sec_03), 14021–0248.07 (sec_07), 14021–0248.08 (sec_08), 14021–0248.25 (sec_25), 14021–0248.28 (sec_28)) compared to in D. melanogaster wild-type Berlin (wtB).
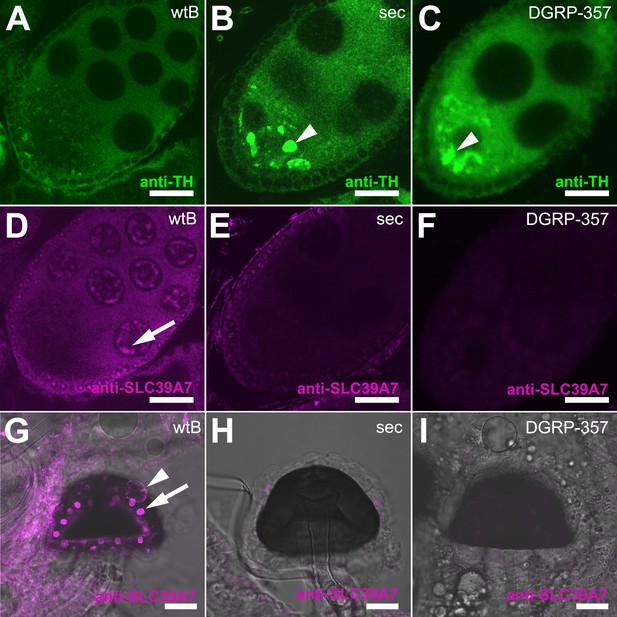
Expression of TH-PLE and CATSUP in Drosophila female reproductive system.
(A–C) Confocal images showing striking accumulation (arrowhead) of TH-PLE (green, anti-TH) in D. sechellia (14021–0248.25) (B) and D. melanogaster DGRP-357 (C), compared to in D. melanogaster wild-type Berlin (wtB) (A) oocytes. (D–F) Confocal images showing CATSUP (magenta, anti-SLC39A7) expressed in the nurse cells (arrow) of D. melanogaster wild-type Berlin (wtB) oocytes (D) and absent in the nurse cells of D. sechellia (14021–0248.25, sec) (E) and D. melanogaster DGRP-357 (DGRP-357) (F) oocytes. (G–I) Confocal image showing CATSUP expressed in the nuclei (arrow) and the membrane (arrowhead) of D. melanogaster wild-type Berlin (wtB) spermatheca secretory cells (G), and absent from D. sechellia (14021–0248.25) (H) and D. melanogaster DGRP-357 (DGRP-357) (I). Scale bar 20 μm.
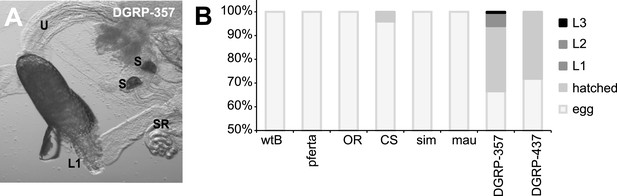
Egg hatching in morinda.
(A) D. melanogaster DGRP-357 first-instar larvae (L1) hatching inside the female reproductive system. U: uterus; S: spermatheca; SR: seminal receptacle. Scale bar 20 μm. (B) Proportion of eggs hatched or moulted to larva 1 (L1) larva 2 (L2) or larva 3 (L3), in D. melanogaster wild-type Berlin (wtB), D. melanogaster pferta (pferta), D. melanogaster Oregon-R (OR), D. melanogaster Canton-S (CS), D. melanogaster DGRP-357 (DGRE-357) D. melanogaster DGRP-437 (DGRP-437), D. simulans (14021–0251.004, sim) and D. mauritiana (14021–0241.01, mau).
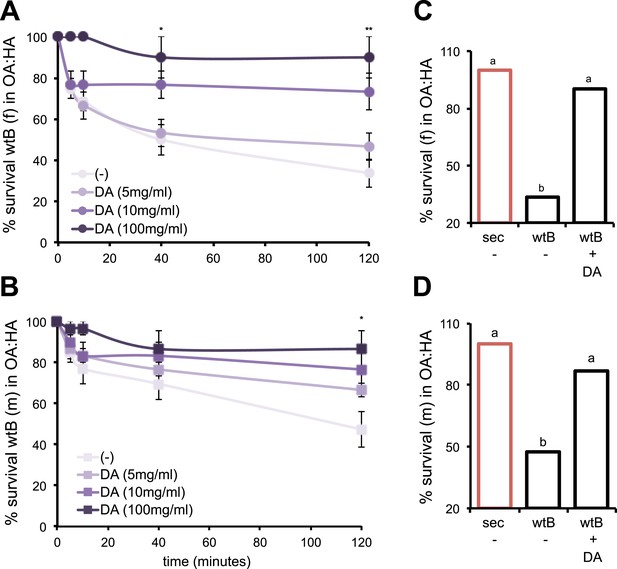
DA contributes to the behavioral resistance to morinda carboxylic acids.
(A–B) Survival kinetic curves for D. melanogaster wild-type Berlin (wtB) (A) females (f, N > 3) and (B) males (m, N > 3) exposed to morinda carboxylic acids (OA:HA) and fed a standard diet supplemented with either no (−), or increasing doses of DA (5 mg/ml, 10 mg/ml and 100 mg/ml). *p < 0.02 and **p < 0.002 using Student's t test. (C–D) Survival (%) (N > 3) of D. sechellia (14021–0248.25, sec) and D. melanogaster wild-type Berlin (wtB) (C) females (f) and (D) males (m), fed a non-supplemented (−) or DA (+DA, 100 mg/ml) supplemented standard diet upon 2 hr exposure to morinda carboxylic acids (OA:HA). Different letters denote significant differences (p < 0.01) using ANOVA followed by Tukey's test. Error bars represent s.e.m.





