Cochlear progenitor number is controlled through mesenchymal FGF receptor signaling
Figures
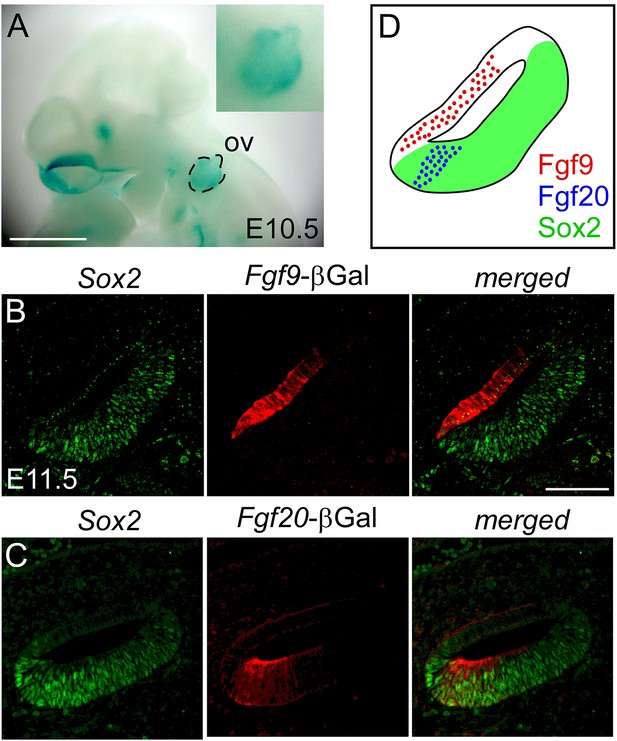
Fgf9 and Fgf20 are expressed in distinct regions of the otic vesicle.
(A) βGal activity in an Fgf9lacZ/+ embryo at E10.5 visualized with xGal staining. (B, C) βGal (red) and Sox2 (green) co-immunostaining showing that Fgf9 (B) is expressed in Sox2- non-sensory epithelium and Fgf20 (C) is expressed in Sox2+ sensory epithelium at E11.5. (D) Schematic diagram of FGF9, FGF20, and Sox2 immunostaining showing that FGF9 and FGF20 are expressed in distinct domains in the otic vesicle. ov, otic vesicle, scale bars, 100 μm.
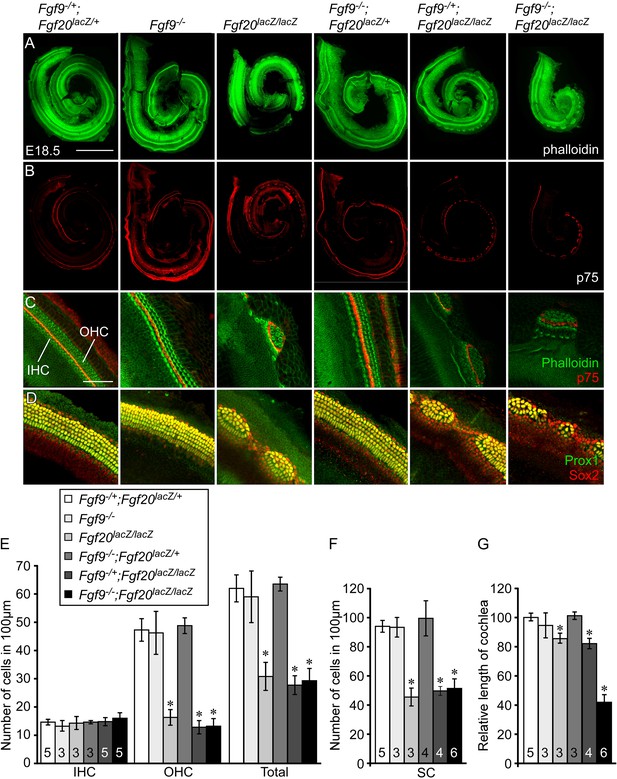
Fgf9 and Fgf20 regulate cochlear length.
(A, B) Phalloidin (A) and p75 immunostaining (B) of E18.5 whole cochlea showing hair cells (HCs) (phalloidin) and pillar cells (p75) in the cochlear duct of Fgf9−/+;Fgf20lacZ/+, Fgf9−/−, Fgf20lacZ/lacZ, Fgf9−/−;Fgf20lacZ/+, Fgf9−/+;Fgf20lacZ/lacZ and, Fgf9−/−;Fgf20lacZ/lacZ embryos. (C) Phalloidin (green) and p75 immunostaining (red) showing the orientation of HCs, pillar cells, and gaps in the sensory epithelium. (D) Prox1 (green) and Sox2 (red) co-immunostaining showing supporting cells (SCs) (yellow, Prox1 and Sox2) and undifferentiated sensory progenitors (red, Sox2). (E–G) Measurement of HC number (E), SC number (F), and length of cochleae (G) of E18.5 embryos. Scale bars, A, 500 μm; C, 100 μm. For statistical analysis, all samples were compared with Fgf9−/+;Fgf20lacZ/+ double heterozygous controls. *p < 0.001. Sample numbers (n) are indicated in data bars.
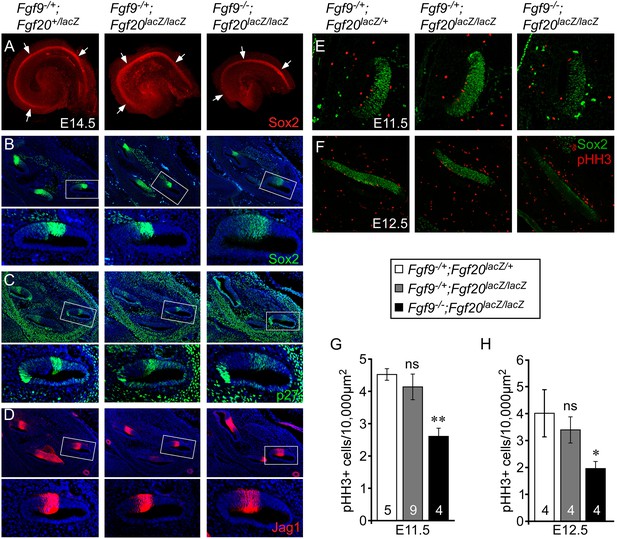
Fgf9 and Fgf20 are required for sensory progenitor proliferation.
(A) Sox2 immunostaining of whole E14.5 cochlea to identify the progenitor domain (arrows). (B–D) Sox2 (B), p27 (C), and Jag1 (D) immunostaining of E14.5 Fgf9−/+;Fgf20lacZ/+, Fgf9−/+;Fgf20lacZ/lacZ, and Fgf9−/−;Fgf20lacZ/lacZ embryo sections. Boxed regions of the cochlear duct are magnified below each image and were chosen in regions where the sections perpendicularly transect the cochlear duct. (E, F) Sox2 and phospho-Histone H3 (pHH3) co-immunostaining of E11.5 (E) and E12.5 (F) Fgf9−/+;Fgf20lacZ/+, Fgf9−/+;Fgf20lacZ/lacZ and, Fgf9−/−;Fgf20lacZ/lacZ embryo sections. (G, H) Measurement of Sox2+ sensory progenitor proliferation at E11.5 (G) and E12.5 (H). All samples were compared with Fgf9−/+;Fgf20lacZ/+ double heterozygous controls. *p < 0.01, **p < 0.001. Sample numbers (n) are indicated in data bars. See also Figure 3—figure supplements 1, 2.
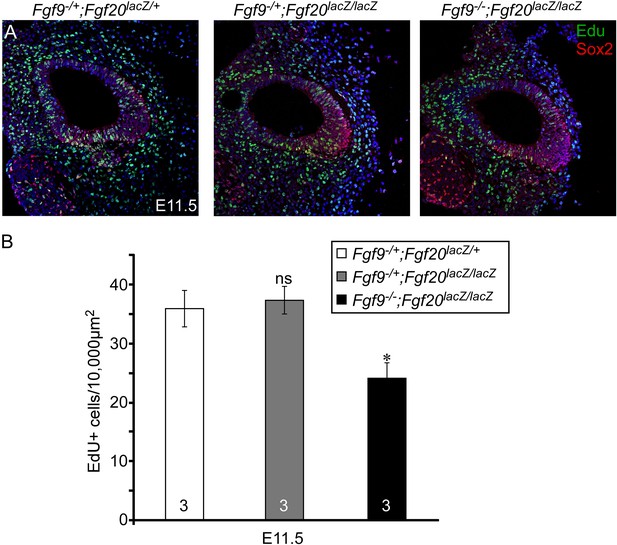
Proliferation of sensory progenitors.
(A) Sox2 and EdU staining of E11.5 Fgf9−/+;Fgf20lacZ/+, Fgf9−/+;Fgf20lacZ/lacZ, and Fgf9−/−;Fgf20lacZ/lacZ embryo sections. (B) Measurement of Sox2+ sensory progenitor proliferation at E11.5. All samples were compared with Fgf9−/+;Fgf20lacZ/+ double heterozygous controls. *p < 0.01. ns, not significant. Sample numbers (n) are indicated in data bars.
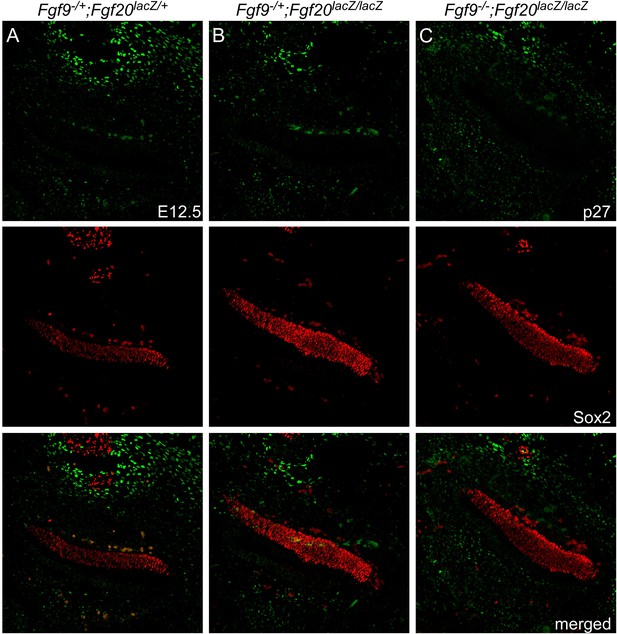
Fgf9 and Fgf20 loss do not cause premature cell cycle exit.
(A–C) p27 (green) and Sox2 (red) co-immunostaining of E12.5 Fgf9−/+;Fgf20lacZ/+ (A), Fgf9−/+;Fgf20lacZ/lacZ (B), and Fgf9−/−;Fgf20lacZ/lacZ (C) embryo sections.
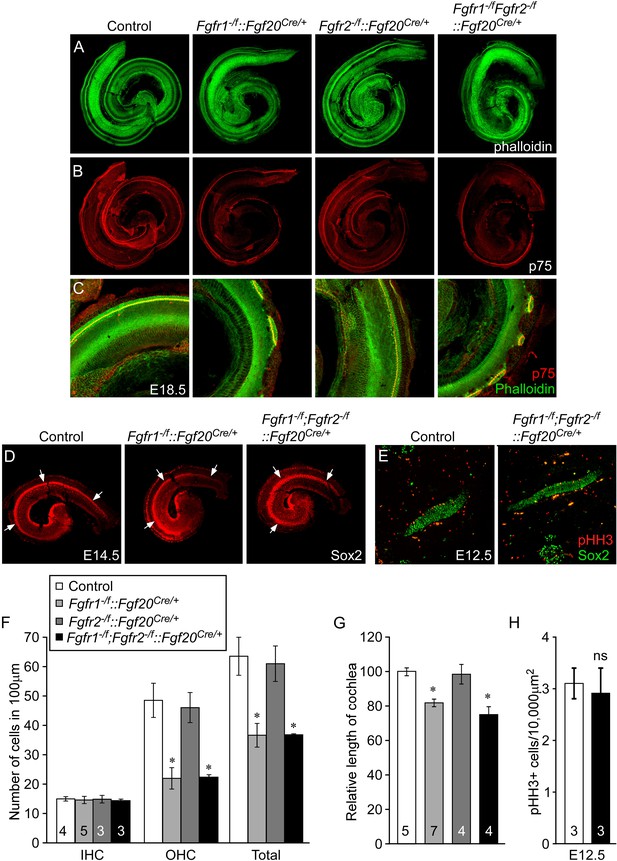
Cell-autonomous regulation of sensory progenitor differentiation requires epithelial Fgfr1 but not Fgfr2.
(A, B) Phalloidin (A) and p75 immunostaining (B) of E18.5 whole cochlea showing HCs (phalloidin) and pillar cells (p75) in the cochlear duct of control, Fgfr1−/f::Fgf20Cre/+ (Fgfr1−/f;Fgfr2+/f::Fgf20Cre/+), Fgfr2−/f::Fgf20Cre/+ (Fgfr1+/f;Fgfr2−/f::Fgf20Cre/+) and, Fgfr1−/f;Fgfr2−/f::Fgf20Cre/+ embryos. (C) Phalloidin (green) and p75 immunostaining (red) showing the patterning of HCs and pillar cells in the cochlear duct. (D) Sox2 immunostaining of E14.5 whole cochlea to identify progenitor domains (arrows). (E) Sox2 and pHH3 co-immunostaining of E12.5 embryo sections. (F, G) Measurement of HC number (F) and length of cochleae (G) of E18.5 control embryos. (H) Measurement of Sox2+ sensory progenitor proliferation in E12.5 embryos. All samples were compared with controls. *p < 0.001; ns, not significant. Sample numbers (n) are indicated in data bars. See also Figure 4—figure supplements 1, 2.
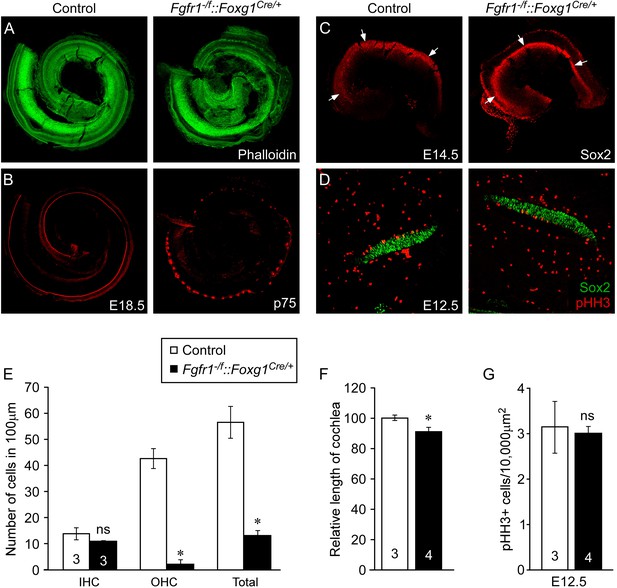
Epithelial Fgfr1 is required for lateral compartment differentiation and HC and SC patterning.
(A, B) Phalloidin (A) and p75 immunostaining (B) of E18.5 whole cochlea showing HCs (phalloidin) and pillar cells (p75) in the cochlear duct of control and Fgfr1−/f::Foxg1Cre/+ embryos. (C) Sox2 immunostaining of E14.5 whole cochlea to identify progenitor domains (arrows). (D) Sox2 and pHH3 co-immunostaining of E12.5 embryo sections. (E, F) Measurement of HC number (E) and length of cochleae (F) of E18.5 control and Fgfr1−/f::Foxg1Cre/+ embryos. (G) Measurement of Sox2+ sensory progenitor proliferation in E12.5 cochleae. *p < 0.001 in E and *p < 0.01 in F. Sample numbers (n) are indicated in data bars.
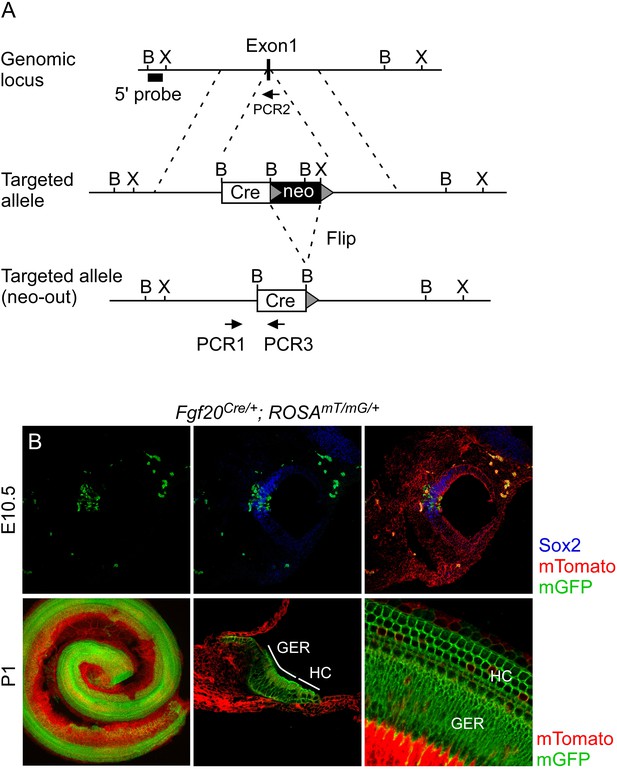
Generation of an Fgf20Cre knockin mouse line.
(A) Schematic diagram showing targeting of the Fgf20 genomic locus. Homologous recombination in mouse ES cells was used to insert a GFP:Cre (Cre) gene and neo selection cassette (flanked by Flip recombination target sequences, grey triangles) into exon 1 of Fgf20. F1 mice were bred to mice that express Flip recombinase in the germline to excise the neo selection cassette. Arrows indicate PCR primers used for genotyping. B, BamH1; X, Xho1. (B) Fgf20Cre/+; ROSA26mT/mG/+ (ROSAmT/mG/+) double transgenic mice showing the cumulative lineage of Fgf20Cre expressing cells (green) in the Sox2+ prosensory domain (blue) at E10.5 and in HCs, SCs, and the greater epithelial ridge (GER) at P1. Recombination at the ROSA26mT/mG locus silences membrane localized Tomato (mT) and activates membrane localized GFP (mG).
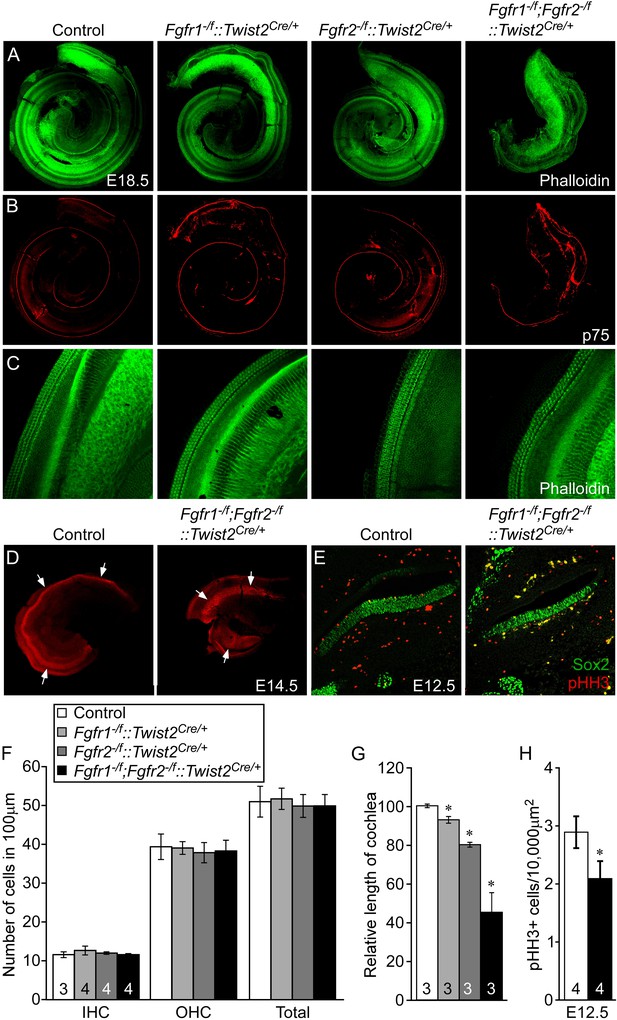
Mesenchymal Fgfr1 and Fgfr2 regulate the length of the cochlear duct and sensory progenitor proliferation.
(A, B) Phalloidin (A) and p75 immunostaining (B) of E18.5 whole cochlea showing HCs (phalloidin) and pillar cells (p75) in the cochlear duct of control, Fgfr1−/f::Twist2Cre/+ (Fgfr1−/f;Fgfr2+/f::Twist2Cre/+), Fgfr2−/f::Twist2Cre/+ (Fgfr1+/f;Fgfr2−/f::Twist2Cre/+), and Fgfr1−/f;Fgfr2−/f::Twist2Cre/+ embryos. (C) Phalloidin (green) staining showing normal HC patterning in the cochlear sensory epithelium. (D) Sox2 immunostaining of E14.5 whole cochlea to identify progenitor domains (arrows). (E) Sox2 and pHH3 co-immunostaining of E12.5 cochlea sections. (F, G) Measurement of HC number (F) and length of cochleae (G) of E18.5 control, Fgfr1−/f::Twist2Cre/+ (Fgfr1−/f;Fgfr2+/f::Twist2Cre/+), Fgfr2−/f::Twist2Cre/+ (Fgfr1+/f;Fgfr2−/f::Twist2Cre/+), and Fgfr1−/f;Fgfr2−/f::Twist2Cre/+ embryos. (H) Measurement of Sox2+ sensory progenitor (green) proliferation (red, pHH3) in E12.5 embryo sections. All samples were compared with controls. *p < 0.001. Sample numbers (n) are indicated in data bars. See also Figure 5—figure supplement 1.
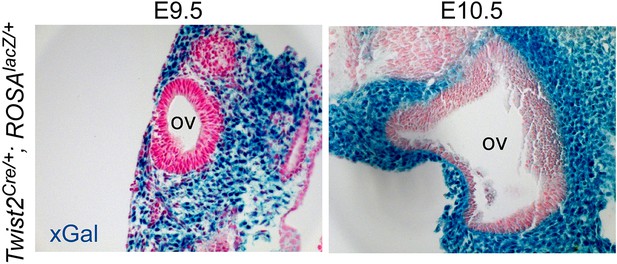
Twist2Cre targeting of periotic mesenchyme.
Twist2Cre/+;ROSA26lacZ/+ (ROSAlacZ/+) double transgenic mice showing βGal activity (xGal, blue) in periotic mesenchymal cells at E9.5 and E10.5. OV, otic vesicle.
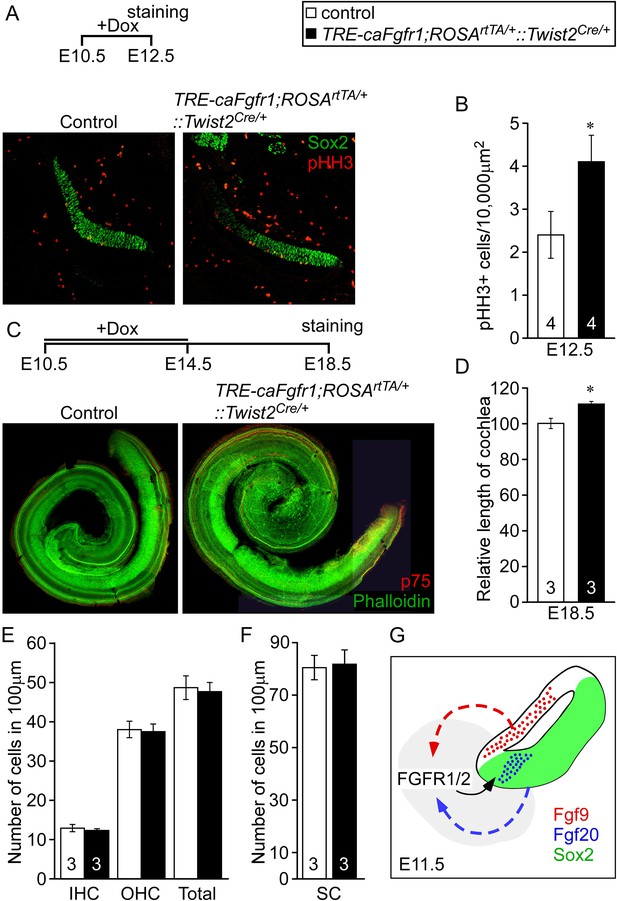
Ectopic activation of FGFR signaling in mesenchyme increases sensory progenitor proliferation and cochlear length.
(A) Sox2 (green) and pHH3 (red) co-immunostaining of E12.5 control and TRE-caFgfr1;ROSArtTA/+::Twist2Cre/+ embryo sections. (B) Measurement of Sox2+ sensory progenitor proliferation at E12.5. (C) Phalloidin (green) and p75 immunostaining (red) showing the patterning of HCs and pillar cells in the cochlear duct of control and TRE-caFgfr1;ROSArtTA/+::Twist2Cre/+ embryos. Measurement of the length of the cochleae (D), HC number (E), and SC number (F) of E18.5 control and TRE-caFgfr1;ROSArtTA/+::Twist2Cre/+ embryos. (G) Schematic diagram indicating the requirement for epithelial FGF9/20 signaling to mesenchymal FGFR1/2 to induce sensory progenitor proliferation. *p < 0.01 in B and *p < 0.001 in D. Sample numbers (n) are indicated in data bars. See also Figure 6—figure supplement 1.
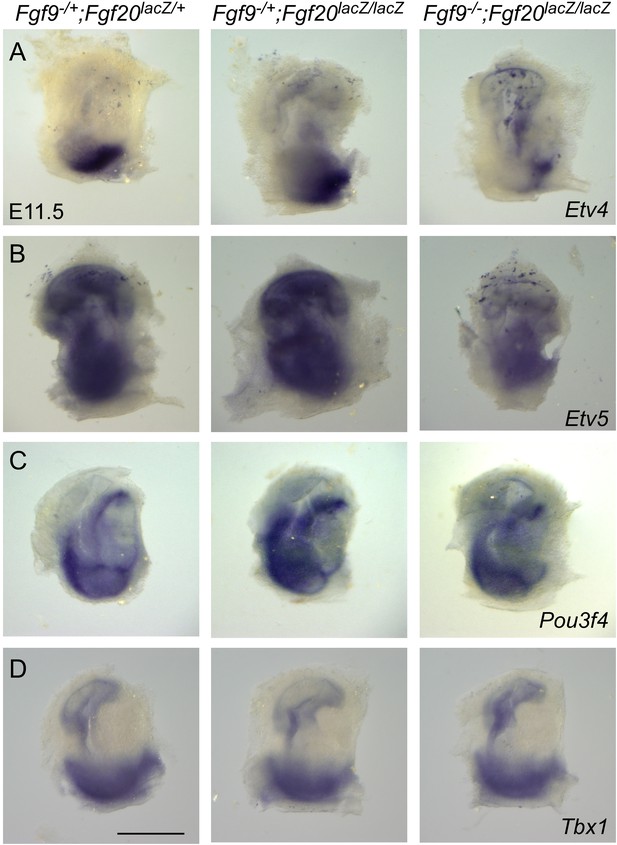
Fgf9 and Fgf20 regulate the expression of Etv4 and Etv5, but not Pou3f4 or Tbx1.
(A–D) Wholemout mRNA in situ staining of Etv4 (A), Etv5 (B), Pou3f4 (C), and Tbx1 (D) genes on otic vesicles from E11.5 embryos. The genotypes of embryos analyzed are: Fgf9−/+;Fgf20lacZ/+, Fgf9−/+;Fgf20lacZ/lacZ and, Fgf9−/−;Fgf20lacZ/lacZ. Images are representative of at least three embryos for each probe and genotype. Scale bar, 500 μm.





