High throughput in vivo functional validation of candidate congenital heart disease genes in Drosophila
Figures
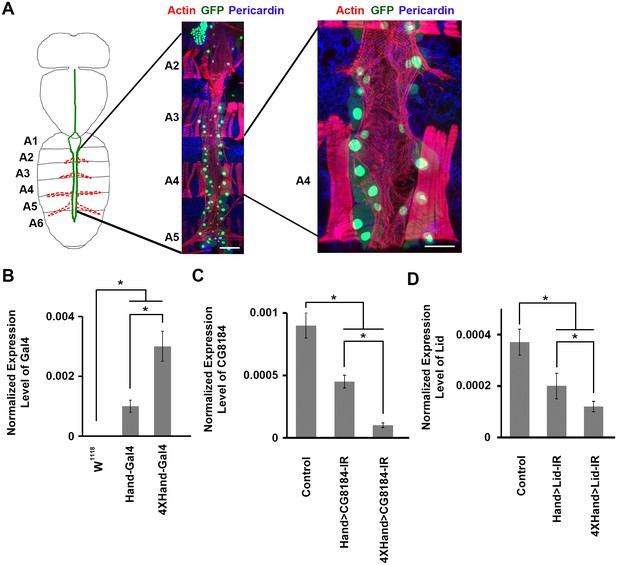
Drosophila adult heart structure and tissue visualization, evaluation of 1X vs. 4X Hand enhancer constructs driving heart specific silencing of gene expression.
(A) Drosophila heart structure and visualization. Left: the adult heart is depicted schematically in green. Supporting lateral alary muscles are depicted in red. Middle: fluorescence microscopy of dissected heart tissue spanning abdominal segments A2–A5. Myocardial actin filaments (cardiac myofibers) were visualized by Phalloidin staining (red), which also stained somatic muscle fibers in segments A2, A3, and A4 and alary muscle fibers in A5. GFP (green) labels cardiomyocytes and pericardial nephrocytes. Expression of a nuclear-localized GFP transgene was controlled by the cardioblast specific Hand gene enhancer element. Scale bar = 100 µ. Right: higher magnification of segment A4 heart tissue. Scale bar = 50 µ. In this example, dissection and preparation for microscopy involved removal of a layer of longitudinal muscle, which resulted in the loss of some cardiomyocytes and pericardial nephrocytes. In situ, the heart tube is composed of parallel, symmetrical rows of cardiomyocytes (small nuclei), flanked by pericardial nephrocytes (large nuclei). (B) The 4XHand enhancer promotes significantly greater Gal4 mRNA production than the single Hand enhancer. The Gal4 mRNA level in the dissected adult heart was determined by qRT-PCR. Statistical significance (*) was defined as p<0.05. w1118 was used as a negative control since it does not express any Gal4. (C) Compared to the single Hand enhancer, 4XHand induced significantly greater knockdown of CG8184 (the homolog of human HUWE1) mRNA when driving expression of the CG8184-IR RNAi silencing transgene. The CG8184 mRNA level in the dissected adult heart was determined by qRT-PCR. Statistical significance (*) was defined as p<0.05. (D) Compared to the single Hand enhancer, 4XHand induced significantly greater knockdown of Lid (the homolog of human KDM5A and KDM5B) mRNA when driving expression of the Lid-IR RNAi silencing transgene. The Lid mRNA level in the dissected adult heart was determined by qRT-PCR. Statistical significance (*) was defined as p<0.05. In C and D, Control flies were the progeny of a cross between homozygous 4XHand-Gal4 and w1118, which has one copy of 4xHand-Gal4 but does not carry a UAS-RNAi silencing construct.
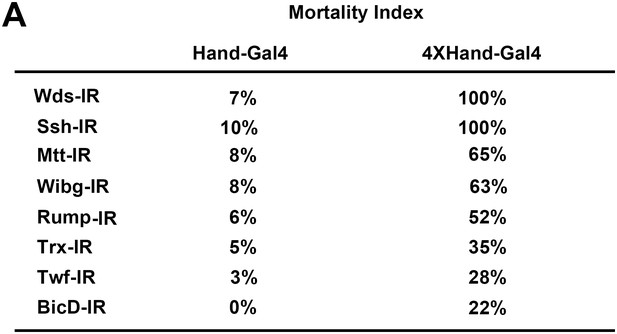
4XHand-Gal4 efficiently silences cardiac gene expression.
(A) Mortality Index, expressed as the percentage of flies of the indicated genotype that die during pre-adult stages. Male and female flies of the appropriate genotypes were crossed to produce progeny flies carrying the indicated UAS-gene silencing (i.e. RNAi) interfering RNA (IR) construct, the expression of which is directed specifically in cardioblast cells by the indicated Hand-Gal4 driver. Hand-Gal4 and 4X-Hand-Gal4 constructs incorporate either one copy or four tandem copies of a Hand gene enhancer element, respectively. Significant developmental lethal effects of heart-specific gene silencing were only observed in progeny flies carrying the 4XHand-Gal4 driver.
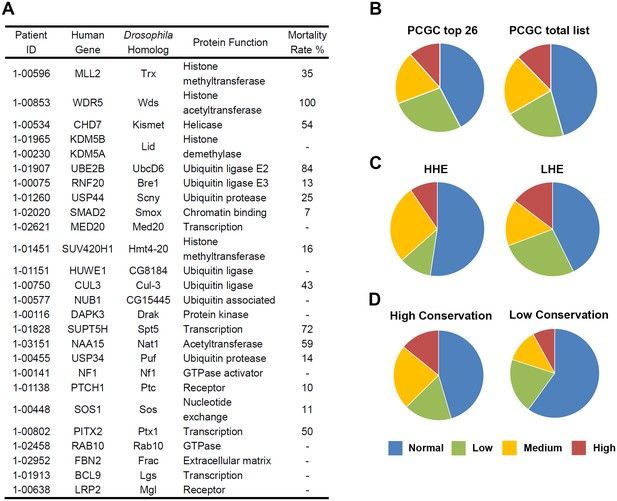
Top 26 candidate CHD genes and developmental lethality induced by heart specific RNAi-based silencing of Drosophila gene homologs.
(A) 26 de novo mutated genes from CHD study participants selected as being of particular interest (Zaidi et al., 2013) based upon sequence quality, mutation type, the expression level of the mouse homolog during heart development on embryonic day 14.5, and previously reported involvement in CHD or heart development. The 26 corresponding Drosophila homologs are shown with protein function (Flybase), and Mortality Rate (Mortality Index). (B–D) The proportions of Drosophila gene homologs that, when silenced by cardiac cell specific RNAi expression, induce developmental lethality at normal/background levels (blue); low levels (green); medium levels (orange); high levels (red) based on Mortality Index values. The Mortality Index is determined by crossing homozygous UAS-RNAi transgenic flies with flies bearing a 4XHand-Gal4 ‘driver’ (four repeats of the cardioblast cell-specific Hand enhancer element 5’ of Gal4) balanced over CyO. Progeny flies that emerge as adults with curly wings (CyO, no transgene expression) vs. straight wings (expressing 4XHand-Gal4 driven UAS-RNAi transgene in cardioblasts) are recorded and the developmental mortality attributable to RNAi heart expression (Mortality Index) is calculated as (Curly – Straight) / Curly X 100. Divergence from 1:1 ratio (Normal, blue) ≥ 7% was considered a lethal phenotype. A Normal range of divergence from a 1:1 ratio of <6% based on analysis of 400 progeny from control crosses. Varying degrees of phenotype severity were observed (Low = 7–30%, green; Medium = 31–60%, orange; High = 61–100%, red). (B) Left: chart (PCGC top 26) shows proportions of RNAi silencing effects on lethality for Drosophila homologs of 26 genes identified as being of particular interest based exclusively on bioinformatics-based criteria. Right: chart (PCGC total list) shows the results of 134 fly homologs of all de novo mutated genes (with available RNAi silencing lines) identified in pediatric CHD study participants (Zaidi et al., 2013) (Supplementary file 1). (C) Comparison of silencing-induced lethality for Drosophila homologs of 134 candidate CHD-associated genes as a function of high (HHE) versus low (LHE) levels of expression of murine homologs in embryonic mouse heart. (D) Comparison of silencing-induced lethality for Drosophila homologs of 134 candidate CHD-associated genes as a function of fly-to-human gene conservation (High Conservation, score 6 to 10; Low Conservation, score 2 to 5).
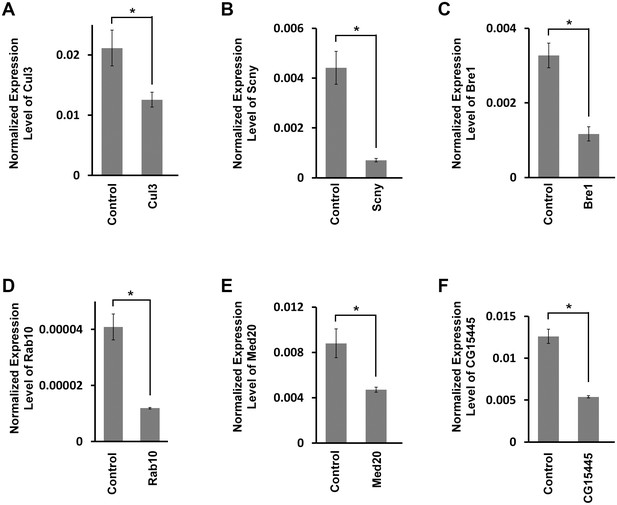
Target gene mRNA levels in adult fly heart tissues.
(A) Drosophila Cul3 homolog of human CUL3 encoding ubiquitin ligase (Mortality Index 43%). (B) Drosophila Scny homolog of human USP44 encoding ubiquitin protease (Mortality Index 25%). (C) Drosophila Bre1homolog of human RNF20 encoding ubiquitin ligase E3 (Mortality Index 13%). (D) Drosophila Rab10 homolog of human RAB10 encoding GTPase (Mortality Index normal). (E) Drosophila Med20 homolog of human MED20 (Mortality Index normal). (F) Drosophila CG15445 homolog of human NUB1 (Mortality Index normal). mRNA levels in dissected adult hearts were determined by qRT-PCR. Statistical significance (*) was defined as p<0.05. Control flies were the progeny of a cross between homozygous 4XHand-Gal4 and w1118, which has one copy of 4xHand-Gal4 but does not carry a UAS-RNAi silencing construct.
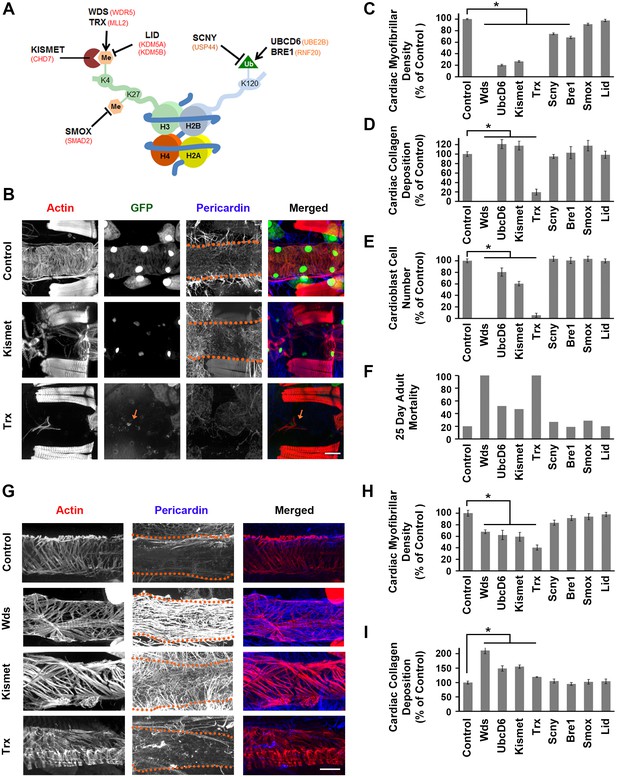
Genes involved in H3K4 and H3K27 methylation, mutated in CHD patients, affect heart structure, developmental mortality, and adult survival.
(A) Depiction of nucleosome showing H3K4 and H3K27 methylation, and ubiquitination of H2BK120 (required for H3K4 methylation). Drosophila homologs involved in the production, removal, or interpretation of modifications are shown (human genes shown in red). (B) Adult heart phenotype induced by cardioblast-specific expression (driven by 4XHand-Gal4) of UAS-RNAi transgenes targeting Kismet and Trx. Cardiac actin (myofibers) was visualized by Phalloidin staining. Hand-GFP expression (nuclear) labels cardioblast cells. Pericardin was immune-labeled. Dotted lines delineate the heart tube outline. Red arrow points to remnant cardioblast cell in Trx-silenced heart. Scale bar = 50 µ. (C) Quantitation of adult heart cardiac myofibrillar density (as % of control; N = 10 for Control, N = 6 for indicated silenced gene). Statistical significance (*) was defined as p<0.05. Scale bar = 50 µ. (D) Quantitation of adult heart cardiac collagen (Pericardin) deposition (as % of control; N = 10 for Control, N = 6 indicated silenced gene). Statistical significance (*) was defined as p<0.05. (E) Quantitation of adult heart cardioblast cell numbers (cardioblasts expressing nuclear GFP; N = 10 for Control, N = 10 for indicated silenced gene). Statistical significance (*) was defined as p<0.05. (F) Percentage of adult male flies dead at day 25 post-emergence (N = 50 flies per genotype). (G) Larva (third instar) heart phenotypes induced by cardioblast-specific expression (driven by 4XHand-Gal4) of UAS-RNAi transgenes targeting Wds, Kismet, and Trx. Cardiac actin (myofibers) was visualized by Phalloidin staining. The pericardin was immune-labeled. Dotted lines delineate heart tube outline. (H) Quantitation of larval heart cardiac myofibrillar density (as % of control; N = 10 for Control, N = 6 for indicated silenced gene). Statistical significance (*) was defined as p<0.05. (I) Quantitation of larva heart cardiac collagen (Pericardin) deposition (as % of control; N = 10 for Control, N = 6 for indicated silenced gene) Statistical significance (*) was defined as p<0.05. Control flies were the progeny of a cross between homozygous 4XHand-Gal4 and w1118, which has one copy of 4xHand-Gal4 but does not carry a UAS-RNAi silencing construct.
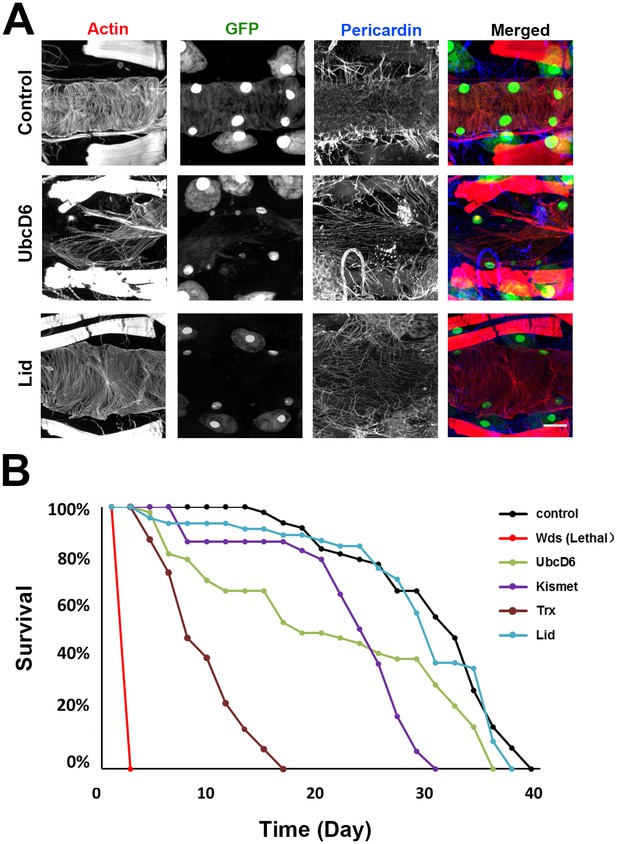
Genes involved in H3K4 and H3K27 methylation, mutated in CHD patients.
(A) Adult heart phenotype induced by 4XHand-Gal4 driven expression of UAS-RNAi transgenes targeting UbcD6 and Lid. Left to right panels: cardiac myofibers visualized by Phalloidin staining, cardioblast cells labeled by Hand-GFP expression (nuclear), Pericardin (Type IV collagen) immuno-labeled, merged images. Scale bar = 50 µ. (B) Adult fly survival curves (N = 50 flies per genotype). Control flies were homozygous for w1118 and carried the 4XHand-Gal4 transgene, but not a UAS-RNAi silencing construct.
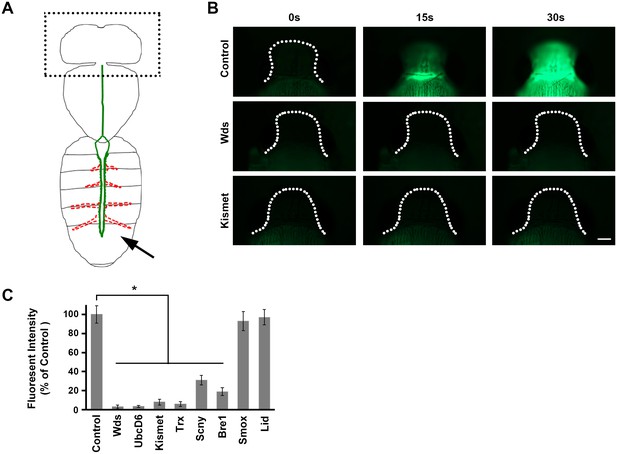
Direct measurement of heart function.
(A) The adult heart is depicted schematically in green. Supporting lateral alary muscles are depicted in red. Fluorescent dye injected into the posterior body cavity (arrow) enters the heart tube lumen and is pumped anteriorly into the brain region. (B) Time course of injected fluorescent dye entering the brain region (dotted outline). In normal control flies dye is easily detectable 15 s (s) after injection, and the brain is highly fluorescent within 30 s. In flies expressing heart-specific Wds or Kismet gene targeting RNAi silencing constructs, by contrast, dye does not reach the brain by 30 s after injection. Scale bar = 100 µ. (C) Quantitative analysis of brain region fluorescence, relative to control fly levels, as a function of heart-specific gene silencing. Experiments were performed in triplicate (3 independent dye injection experiments). Statistical significance (*) was defined as p<0.05. Control flies were the progeny of a cross between homozygous 4XHand-Gal4 and w1118, which has one copy of 4xHand-Gal4 but does not carry a UAS-RNAi silencing construct.
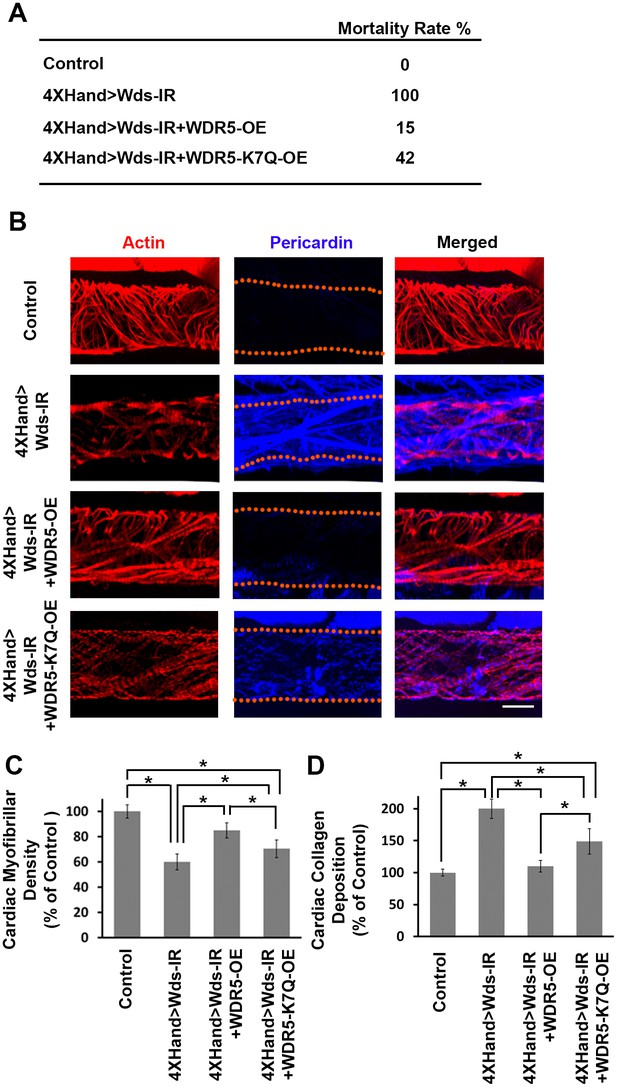
Wds silencing-induced lethality and heart phenotypes rescued by wild type human WDR5 but not by a patient derived WDR5-K7Q mutant allele.
(A) Developmental lethality (Mortality Index) for flies in which endogenous Wds heart expression was silenced, and attempted rescue by either wild type WDR5 or mutant WDR5-K7Q overexpression (OE). (B) Larva (third instar) heart phenotype induced by cardioblast-specific expression (driven by 4XHand-Gal4) of UAS-RNAi transgene targeting Wds, and attempted rescue by WDR5 or WDR5-K7Q overexpression. Cardiac actin (myofibers) visualized by Phalloidin staining. Pericardin was immune-labeled. Dotted lines delineate the heart tube outline. (C) Quantitation of larval heart cardiac myofibrillar density (as % of control; N = 10 for Control, N = 6 for Wds-IR, Wds-IR+WDR5-OE, or Wds-IR+WDR5-K7Q-OE). Statistical significance (*) was defined as p<0.05. For each strain, fiber density was significantly lower than control. Rescue by wild type WDR5 significantly rescued fiber density, to levels significantly greater than achieved by the overexpression of the WDR5-K7Q mutant allele. (D) Quantitation of larva heart cardiac collagen (Pericardin) deposition (as % of control; N = 10 for Control, N = 6 for Wds-IR, Wds-IR+WDR5-OE, or Wds-IR+WDR5-K7Q-OE) Statistical significance (*) was defined as p<0.05. Wild type WDR5-OE fully rescued up-regulated collagen deposition induced by Wds-IR. Mutant WDR5-K7Q-OE partially rescued the Wds-IR phenotype, but collagen deposition levels remained upregulated compared to the control. The control flies were the progeny of a cross between homozygous 4XHand-Gal4 and w1118, which has one copy of 4xHand-Gal4 but does not carry a UAS-RNAi silencing construct.
Additional files
-
Supplementary file 1
134 de novo mutated genes from CHD study participants with corresponding Drosophila homologs showing conservation score (Hu et al., 2011), function (from Flybase) and mortality index (determined by crossing homozygous UAS-RNAi transgenic flies with flies bearing a 4XHand-Gal4 driver balanced over CyO, progeny flies that emerge as adults with curly wings (CyO, no transgene expression) vs. straight wings (4XHand-Gal4 driven UAS-RNAi transgene expression in cardioblasts) are recorded and the lethal rate attributable to RNAi heart expression is calculated as (Curly – Straight / Curly) X 100 = % Mortality).
Patient identification number, human gene, mutation type and category (DH, damaging mutation in gene with homolog expression high in embryonic day 14.5 mouse heart (emh); CH, mutation in phylogenetically conserved region of gene with homolog expression high in emh; NH, mutation in phylogenetically nonconserved region of gene with homolog expression high in emh; DL, damaging mutation in gene with homolog expression low in emh; CL, mutation in phylogenetically conserved region of gene with homolog expression low in emh; NL, mutation in phylogenetically nonconserved region of gene with homolog expression low in emh) are from (Zaidi et al., 2013).
- https://doi.org/10.7554/eLife.22617.011
-
Supplementary file 2
Drosophila lines used to silence expression of genes.
Flies were obtained from either the Bloomington Drosophila Stock Center (BDSC) or the Vienna Drosophila Resource Center (VDRC). Individual fly lines are identified by source ID numbers. Both the human genes and their corresponding Drosophila homologs are listed. Each line carries an inhibitory RNA transgene designed to silence the indicated Drosophila gene, cloned downstream of a UAS DNA element. The RNAi transgene is only expressed in cells producing the yeast Gal4 transcription factor.
- https://doi.org/10.7554/eLife.22617.012
-
Supplementary file 3
Gene specific PCR primers.
- https://doi.org/10.7554/eLife.22617.013





