Protein Tracking: Everything, everywhere, almost at once
Proteins play a role in almost all cellular processes and are essential for maintaining life across species and organisms. This means that their aberrant function is a major cause of disease. If one could look directly inside cells, they would see a seemingly chaotic scene of proteins continuously moving around. The motion of each protein is heterogeneous in time and space, and linked to its role within the cell. It is also heavily influenced by the local cellular environment and interactions with other molecules (Lippincott-Schwartz et al., 2001; Kusumi et al., 2014).
Conventional research techniques average the behavior of a large number of unsynchronized molecules, and thus fail to account for these variable factors, which are essential for understanding the biology of proteins. This is where single-molecule tracking methods come into play (Chenouard et al., 2014 ). Traditional ways for tracking individual molecules rely on advanced fluorescence microscopy, single-particle tracking and super-resolution imaging to directly observe the movement and interactions of proteins (Kusumi et al., 2014; Sahl et al., 2017; Shen et al., 2017).
These approaches provide the required spatiotemporal resolution, but typically can only analyze a few cells under limited conditions, offering a narrow glimpse of the vast and dynamic world of proteins. Although high-throughput microscopy has become much refined, scaling single-particle tracking remains a challenge (Park et al., 2023; Malle et al., 2022). A method that could record the performance of every single protein inside millions of individual cells, as well as thousands of molecular compounds, and analyze how they move, interact and respond to therapeutics, would be a major scientific breakthrough – one that may soon be a reality.
Now, in eLife, Hilary Beck and colleagues at Eikon Therapeutics and University of California Berkeley – including David McSwiggen as first author – report a high-throughput tracking (htSMT) platform that makes it possible to observe and analyze the behavior and movement of single proteins and molecules on an unprecedented scale (McSwiggen et al., 2023). The platform involves a robotic system capable of autonomously handling reagents and collecting sequential microscopy movies that are then computationally processed to obtain the trajectories of individual proteins within cells and even cellular compartments. This system allows users to image over a million cells, track thousands of individual proteins per cell, and screen thousands of compounds in a single day.
McSwiggen et al. then tested the platform on estrogen receptors and investigated how over 5,000 compounds affected their motion, analyzing hundreds of thousands of cells twice in a single day (Figure 1). This revealed a new correlation between the dynamics of estrogen receptors and the ability of their antagonists to suppress the growth of cancer cells, which conventual methods have failed to detect previously. Moreover, the htSMT platform also revealed whether the tested molecules affect estrogen receptors directly or indirectly through other biological targets that are known to modify the receptor. This provides an unprecedented and unbiased analysis of a complex biological pathway.
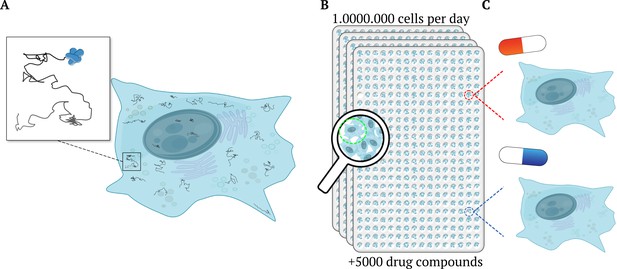
High-throughput single-molecule tracking of proteins across millions of cells.
(A) Schematic illustration of a cell (blue shape) as taken from a high-throughput single-molecule tracking (htSMT) assay (inset), which tracks the motion of multiple proteins within individual cells (squiggly lines). (B) The platform can record the movement of thousands of individual, heterogeneous proteins per cell in over a million cells per day. It does this by automatically collecting a series of images from 384-well plates mounted on a microscope. Each well contains multiples cells and constitutes an independent experiment, enabling researchers to investigate different cell types, and test the effects of various drugs and other molecules and/ or proteins. (C) The htSMT results from cells treated with different drugs (indicated as a red or blue pill) can then be used to assess which treatment is likely to work best.
Image credit: Jacob Kæstel-Hansen and Nikos S. Hatzakis. The figure was – with large modifications – generated with elements from Servier Medical Art and Scidraw.io (10.5281/zenodo.3926549; CC BY 4.0).
Overall, the htSMT platform paves the way for a new era in cellular biology and pharmacology, enabling large-scale, automated observations of how proteins move and interact across millions of cells within 24 hours – a feat that until recently remained in the realm of fantasy. This profound increase in scale, together with advanced analytic tools (Muñoz-Gil et al., 2021; Pinholt et al., 2021), promises to unlock even more unresolved information about complex biological pathways, such as those associated with the estrogen receptor. Adapting htSMT to other proteins and cell systems could help construct unique libraries that ultimately link movement to function. Exploiting the full potential of htSMT will further our understanding of the intricate processes occurring within cells and how protein motion contributes to – and depends on – cellular function. Ultimately this could help researchers design new pharmaceutical treatments for controlling certain diseases.
References
-
Objective comparison of particle tracking methodsNature Methods 11:281–289.https://doi.org/10.1038/nmeth.2808
-
Tracking single molecules at work in living cellsNature Chemical Biology 10:524–532.https://doi.org/10.1038/nchembio.1558
-
Studying protein dynamics in living cellsNature Reviews. Molecular Cell Biology 2:444–456.https://doi.org/10.1038/35073068
-
Objective comparison of methods to decode anomalous diffusionNature Communications 12:6253.https://doi.org/10.1038/s41467-021-26320-w
-
Fluorescence nanoscopy in cell biologyNature Reviews Molecular Cell Biology 18:685–701.https://doi.org/10.1038/nrm.2017.71
-
Single particle tracking: from theory to biophysical applicationsChemical Reviews 117:7331–7376.https://doi.org/10.1021/acs.chemrev.6b00815
Article and author information
Author details
Publication history
- Version of Record published: January 29, 2024 (version 1)
Copyright
© 2024, Kæstel-Hansen and Hatzakis
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,192
- views
-
- 148
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
- Developmental Biology
The generation of distinct cell fates during development depends on asymmetric cell division of progenitor cells. In the central and peripheral nervous system of Drosophila, progenitor cells respectively called neuroblasts or sensory organ precursors use PAR polarity during mitosis to control cell fate determination in their daughter cells. How polarity and the cell cycle are coupled, and how the cell cycle machinery regulates PAR protein function and cell fate determination is poorly understood. Here, we generate an analog sensitive allele of CDK1 and reveal that its partial inhibition weakens but does not abolish apical polarity in embryonic and larval neuroblasts and leads to defects in polarisation of fate determinants. We describe a novel in vivo phosphorylation of Bazooka, the Drosophila homolog of PAR-3, on Serine180, a consensus CDK phosphorylation site. In some tissular contexts, phosphorylation of Serine180 occurs in asymmetrically dividing cells but not in their symmetrically dividing neighbours. In neuroblasts, Serine180 phosphomutants disrupt the timing of basal polarisation. Serine180 phosphomutants also affect the specification and binary cell fate determination of sensory organ precursors as well as Baz localisation during their asymmetric cell divisions. Finally, we show that CDK1 phosphorylates Serine-S180 and an equivalent Serine on human PAR-3 in vitro.
-
- Cancer Biology
- Cell Biology
Philadelphia chromosome-positive (Ph+) leukemia is a fatal hematological malignancy. Although standard treatments with tyrosine kinase inhibitors (TKIs) have achieved remarkable success in prolonging patient survival, intolerance, relapse, and TKI resistance remain serious issues for patients with Ph+ leukemia. Here, we report a new leukemogenic process in which RAPSYN and BCR-ABL co-occur in Ph+ leukemia, and RAPSYN mediates the neddylation of BCR-ABL. Consequently, neddylated BCR-ABL enhances the stability by competing its c-CBL-mediated degradation. Furthermore, SRC phosphorylates RAPSYN to activate its NEDD8 E3 ligase activity, promoting BCR-ABL stabilization and disease progression. Moreover, in contrast to in vivo ineffectiveness of PROTAC-based degraders, depletion of RAPSYN expression, or its ligase activity decreased BCR-ABL stability and, in turn, inhibited tumor formation and growth. Collectively, these findings represent an alternative to tyrosine kinase activity for the oncoprotein and leukemogenic cells and generate a rationale of targeting RAPSYN-mediated BCR-ABL neddylation for the treatment of Ph+ leukemia.






