Parasitic Relationships: Trapped in time
Fossils trapped in amber can reveal astonishing insights about the lives of ancient organisms. Amber can preserve life forms in incredible detail, from their three-dimensional anatomy down to cellular level. It also often catches animals in the middle of an action, like copulation, feeding or pollination, thus providing valuable information about the behavior of a species.
Fossils may also reveal insights into the relationship between parasites and their hosts and how they coevolved. Parasitic relationships, where one species benefits from another – and usually to the other’s detriment – are widespread and crucial for ecosystems. A better understanding of how these relationships have changed over time is thus vital for predicting future changes to diversity (Farrell et al., 2021).
Scientists often rely on phylogenetic analysis and host-distribution data to study these interactions. However, the potential of fossils has so far been overlooked when studying the association between parasites and their hosts (De Baets et al., 2021). Now, in eLife, Bo Wang and colleagues from various research institutes in China and the United States – including Cihang Luo as first author – report how they used fossil records to study the coevolution of nematodes and their invertebrate (Luo et al., 2023).
Nematodes, also known as roundworms, can be found in most habitats across the globe and multiple lineages have independently acquired a parasitic lifestyle. Some parasitic roundworms using plants as hosts have existed at least since the Devonian period around 408 million years ago (Figure 1). The oldest lineages exploiting animals can be found as eggs in Triassic vertebrate coprolites, which may indicate that lineages exploiting invertebrates might have evolved by that time. However, fossils of intact soft-bodied worms are rare, as they usually decay before fossilization sets in. Luo et al. therefore decided to focus their attention on the Mermithidae family, a group of nematodes that eventually kill their insect hosts once they emerge, making it easy to study their life histories.
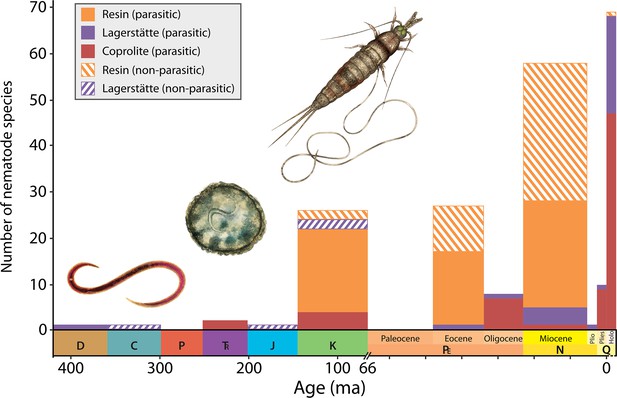
Number of reported parasitic and nonparasitic nematode fossil species from the Devonian through to Holocene.
Nematodes are roundworms that can form parasitic relationships (solid bars) with their hosts. One of the oldest known examples – nematodes that parasitize plants – dates back to the Devonian period, around 408 million years ago (ma). The figure shows reconstructions (not to scale) of the fossil nematodes Palaeonema phyticum (left) from early land plants found in well preserved fossil beds known as Lagerstätte (purple bars); Ascarites priscus (middle) preserved in Cretaceous vertebrate coprolite ca. 125 ma (red bars); and Cretacimermis incredibilis (right) exiting a bristletail preserved in Cretaceous amber described by Luo et al., 2023 (orange bars). Data updated from De Baets et al., 2021. Abbreviations: D: Devonian, C: Carboniferous, P: Permian, T: Triassic, J: Jurassic, K: Cretaceous, PE: Paleocene, N: Neogene, Q: Quaternary, Plio: Pliocene, Pleis: Pleistocene, Holo: Holocene.
Illustrations provided by Franz Anthony (CC BY 4.0).
By analyzing several fossil samples, the team could augment the number of described mermithid species from Cretaceous amber to 13, identifying nine species of mermithids with an age around 100 million years which had not been previously recorded. This doubles the currently known diversity of parasitic nematodes alive during the Cretaceous from nine to 18 species and suggests that parasitism by mermithids was rather widespread during this time, which probably helped regulate insect populations.
Luo et al. further provided the first fossil records of seven host associations, including three arthropod groups that are not recognized hosts today: bristletails, bark lice and extinct planthoppers. Additional amber samples further revealed four modern mermithid-host associations not yet known from the fossil record including dragonflies, earwigs, crickets and cockroaches.
To further explore the evolutionary relationship between nematodes and their hosts, Luo et al. looked at data records from three well-known amber periods: the mid-Cretaceous, the Eocene and the Miocene. This revealed a major shift in host preference over time. Nematodes in the Cretaceous period preferred insects that lacked complete metamorphosis, while roundworms from the Eocene and Miocene mainly used insects with a complete metamorphosis (80%).
Similar to many other parasitic worms, mermithids prefer aquatic or moist environments but have evolved a strategy to infect land-living arthropods (which would also frequent water sources) as juveniles; the nematodes then kill their hosts when approaching adulthood, so they can continue developing and reproducing in aquatic or humid soil environments (Ni et al., 2021). Due to this parasitoid strategy, mermithids have a tight relationship with their host, but their initial host preferences remain poorly known.
The study of Luo et al. adds to previous research indicating important changes in the diversity of strategies of parasitoids exploiting insects from the Cretaceous period to present day (Labandeira and Li, 2021). It is tempting to attribute this shift to a change in the diversity of host species, which saw some major arthropod lineages becoming extinct and ones appearing during the Cretaceous and the Paleogene period. However, several other factors could also contribute to host preferences, including the variety of sampled paleoenvironments or differences in the quality of preservation or collection practices (Penney, 2016; Solórzano Kraemer et al., 2018).
In the past, researchers have mainly studied amber fossils with the aim of discovering new insect species, leaving nematodes – including mermithids – and other potential hosts undersampled or understudied (Schmidt et al., 2010; Stilwell et al., 2020; Košulič and Mašová, 2019). Further investigations will require systematic sampling and global collaboration among all relevant communities and research institutions, along with sufficient funding. Moreover, since nematodes are less likely to remain fully accessible in amber, it is challenging to study morphological traits that could identify different species. Recent advancements in microscopy and tomography techniques offer promising opportunities to overcome this challenge and to visualize the full morphology of nematodes (Penney, 2016).
Mermithid nematodes, with their distinct physique and the characteristic damage they cause when exiting their hosts, serve as an intriguing model to study evolutionary host-parasite dynamics. Luo et al. show the potential of studying amber deposits to document ancient shifts in host preferences, from the Mesozoic to the present, and demonstrate the advantages of investigating ancient host-parasite relationships to better understand evolutionary patterns and estimate the extinction risk of modern species (Ni et al., 2021; Mulvey et al., 2022).
References
-
The fossil record of parasitism: Its extent and taphonomic constraintsTopics in Geobiology 50:1–50.https://doi.org/10.1007/978-3-030-52233-9
-
The ghost of hosts past: impacts of host extinction on parasite specificityPhilosophical Transactions of the Royal Society of London. Series B, Biological Sciences 376:20200351.https://doi.org/10.1098/rstb.2020.0351
-
The history of insect parasitism and the mid-mesozoic parasitoid revolutionTopics in Geobiology 49:377–533.https://doi.org/10.1007/978-3-030-42484-8
-
Where traditional extinction estimates fall flat: using novel cophylogenetic methods to estimate extinction risk in platyhelminthsProceedings of the Royal Society B: Biological Sciences 289:20220432.https://doi.org/10.1098/rspb.2022.0432
-
Convergent patterns of body size variation in distinct parasite taxa with convergent life cyclesGlobal Ecology and Biogeography 30:2382–2392.https://doi.org/10.1111/geb.13389
Article and author information
Author details
Publication history
- Version of Record published: July 14, 2023 (version 1)
Copyright
© 2023, De Baets et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 521
- views
-
- 55
- downloads
-
- 2
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Ecology
Almost all herbivorous insects feed on plants and use sucrose as a feeding stimulant, but the molecular basis of their sucrose reception remains unclear. Helicoverpa armigera as a notorious crop pest worldwide mainly feeds on reproductive organs of many plant species in the larval stage, and its adult draws nectar. In this study, we determined that the sucrose sensory neurons located in the contact chemosensilla on larval maxillary galea were 100–1000 times more sensitive to sucrose than those on adult antennae, tarsi, and proboscis. Using the Xenopus expression system, we discovered that Gr10 highly expressed in the larval sensilla was specifically tuned to sucrose, while Gr6 highly expressed in the adult sensilla responded to fucose, sucrose and fructose. Moreover, using CRISPR/Cas9, we revealed that Gr10 was mainly used by larvae to detect lower sucrose, while Gr6 was primarily used by adults to detect higher sucrose and other saccharides, which results in differences in selectivity and sensitivity between larval and adult sugar sensory neurons. Our results demonstrate the sugar receptors in this moth are evolved to adapt toward the larval and adult foods with different types and amounts of sugar, and fill in a gap in sweet taste of animals.
-
- Ecology
- Epidemiology and Global Health
Zoonotic disease dynamics in wildlife hosts are rarely quantified at macroecological scales due to the lack of systematic surveys. Non-human primates (NHPs) host Plasmodium knowlesi, a zoonotic malaria of public health concern and the main barrier to malaria elimination in Southeast Asia. Understanding of regional P. knowlesi infection dynamics in wildlife is limited. Here, we systematically assemble reports of NHP P. knowlesi and investigate geographic determinants of prevalence in reservoir species. Meta-analysis of 6322 NHPs from 148 sites reveals that prevalence is heterogeneous across Southeast Asia, with low overall prevalence and high estimates for Malaysian Borneo. We find that regions exhibiting higher prevalence in NHPs overlap with human infection hotspots. In wildlife and humans, parasite transmission is linked to land conversion and fragmentation. By assembling remote sensing data and fitting statistical models to prevalence at multiple spatial scales, we identify novel relationships between P. knowlesi in NHPs and forest fragmentation. This suggests that higher prevalence may be contingent on habitat complexity, which would begin to explain observed geographic variation in parasite burden. These findings address critical gaps in understanding regional P. knowlesi epidemiology and indicate that prevalence in simian reservoirs may be a key spatial driver of human spillover risk.






