Knockdown of PHOX2B in the retrotrapezoid nucleus reduces the central CO2 chemoreflex in rats
Abstract
PHOX2B is a transcription factor essential for the development of different classes of neurons in the central and peripheral nervous system. Heterozygous mutations in the PHOX2B coding region are responsible for the occurrence of Congenital Central Hypoventilation Syndrome (CCHS), a rare neurological disorder characterised by inadequate chemosensitivity and life-threatening sleep-related hypoventilation. Animal studies suggest that chemoreflex defects are caused in part by the improper development or function of PHOX2B expressing neurons in the retrotrapezoid nucleus (RTN), a central hub for CO2 chemosensitivity. Although the function of PHOX2B in rodents during development is well established, its role in the adult respiratory network remains unknown. In this study, we investigated whether reduction in PHOX2B expression in chemosensitive neuromedin-B (NMB) expressing neurons in the RTN altered respiratory function. Four weeks following local RTN injection of a lentiviral vector expressing the short hairpin RNA (shRNA) targeting Phox2b mRNA, a reduction of PHOX2B expression was observed in Nmb neurons compared to both naive rats and rats injected with the non-target shRNA. PHOX2B knockdown did not affect breathing in room air or under hypoxia, but ventilation was significantly impaired during hypercapnia. PHOX2B knockdown did not alter Nmb expression but it was associated with reduced expression of both Task2 and Gpr4, two CO2/pH sensors in the RTN. We conclude that PHOX2B in the adult brain has an important role in CO2 chemoreception and reduced PHOX2B expression in CCHS beyond the developmental period may contribute to the impaired central chemoreflex function.
eLife assessment
This important study utilizes a viral-mediated short hairpin RNA (shRNA) approach to investigate in a novel way the role of the wild-type PHOX2B transcription factor expressed in critical chemosensory neurons in the brainstem retrotrapezoid nucleus (RTN) region for maintaining normal CO2 chemoreflex control of breathing in adult rats. The convincing results show blunted ventilation during elevated inhaled CO2 (hypercapnia) with knockdown of PHOX2B, accompanied by a reduced expression of Gpr4 and Task2 mRNA for the proposed RTN neuron proton sensor proteins GPR4 and TASK2. These results indicate that maintained expression of wild-type PHOX2B affects respiratory control in adult animals, complementing previous studies showing that PHOX2B-expressing RTN neurons may be critical for chemosensory control throughout the lifespan, and with implications for neurological disorders involving the RTN, which will be of interest to neuroscientists studying respiratory neurobiology as well as the neurodevelopmental control of motor behavior.
https://doi.org/10.7554/eLife.94653.3.sa0Introduction
The Paired Like Homeobox 2B (PHOX2B) is a transcription factor essential for embryonic differentiation and for the maintenance of the neuronal phenotype of different classes of neurons in both the central and peripheral nervous systems (Brunet and Pattyn, 2002; Pattyn et al., 1997; Pattyn et al., 1999; Pattyn et al., 2000; Stanke et al., 1999; Tiveron et al., 1996). In recent years, the PHOX2B gene has been extensively investigated, not only for its neurodevelopmental role, but also because its heterozygous mutation leads to Congenital Central Hypoventilation Syndrome (CCHS, OMIM 209880; Amiel et al., 2003), Hirschsprung’s disease (HSCR; OMIM 142623; Berry-Kravis et al., 2006; Trochet et al., 2005), and neuroblastoma (Bourdeaut et al., 2005; van Limpt et al., 2004). CCHS is a rare (1:200,000 births in France Trang et al., 2005; 1:148,000 births in Japan Shimokaze et al., 2015) disorder characterised by a failure to respond to both hypercapnia and hypoxia (Amiel et al., 2003; Di Lascio et al., 2020; Weese-Mayer et al., 2017). The most frequent PHOX2B mutation is an expansion of a polyalanine repeat, ranging from +5 to +13 residues on the C-terminal of PHOX2B, an area that regulates DNA binding affinity and protein solubility (Amiel et al., 2003; Di Lascio et al., 2016; Weese-Mayer et al., 2003). CCHS symptoms present with varying degrees of severity depending on the expansion of the mutation (Berry-Kravis et al., 2006; Di Lascio et al., 2018b; Matera et al., 2004). In most mild cases, patients display normal ventilation during wakefulness, hypoventilation during sleep leading to hypercarbia and hypoxemia, as well as a lack of sensitivity and discomfort to these challenges. In the most severe of cases, hypoventilation may also be present while awake.
Respiratory disturbances resulting from the PHOX2B mutations appear to stem from its widespread expression in respiratory-related neurons both during development and into adulthood. Although PHOX2B is absent in respiratory rhythmogenic neurons of the preBötzinger complex, it is highly expressed in adult neurons that are responsible for CO2 and O2 chemoreflexes (Kang et al., 2007). Among those are the neurons of the retrotrapezoid nucleus (RTN), the locus coeruleus, the carotid bodies and the nucleus of the solitary tract that, together with the dorsal motor nucleus of the vagus nerve and the area postrema, make up to the dorsal vagal complex, a structure that provides the major integrative centre for the mammalian autonomic nervous system (Cutsforth-Gregory and Benarroch, 2017; Guyenet et al., 2019; Kang et al., 2007; Zoccal et al., 2014). Previous work by our group and others specifically investigated the role of PHOX2B-expressing RTN neurons by either selective lesions or inactivation of neuronal activity of these neurons (Abbott et al., 2011; Basting et al., 2015; Marina et al., 2010; Nattie and Li, 1994; Souza et al., 2018; Souza et al., 2023; Janes et al., 2024) and showed that RTN neurons have a key role in central CO2 chemoreception.
Defects observed in the CO2-chemoreflex of CCHS transgenic rodent models are primarily caused by the improper development or function of PHOX2B-expressing neurons in the RTN (Dubreuil et al., 2008; Ramanantsoa et al., 2011), although anatomo-pathological studies in CCHS patients suggest that the respiratory impairment may be caused by a more widespread brain dysfunction (Harper et al., 2015; Nobuta et al., 2015). Transgenic mice born with PHOX2B knockout fail to develop the RTN, the locus coeruleus (Pattyn et al., 1999) and catecholaminergic groups in A1, A2, A5, and A7 (Pattyn et al., 2000), as well as neurons of the nucleus of the solitary tract and the carotid bodies. Mice harbouring heterozygous PHOX2B knockout develop apparently normal but have depressed responses to hypoxia and hypercapnia (Dauger et al., 2003) and sleep disordered breathing in the perinatal period (Durand et al., 2005). Furthermore, the expression of the most common heterozygous PHOX2B mutation found in CCHS patients (PHOX2B+7 Ala expansion) in mice results in a near complete loss of RTN neurons, impaired CO2 response and consequent death in the newborn period (Dubreuil et al., 2008; Madani et al., 2021; Ramanantsoa et al., 2011; Takakura et al., 2008). More selective expression of the mutant PHOX2B in the RTN neurons allowed for survival in the neonatal period and partial recovery of the CO2 response in adulthood (Ramanantsoa et al., 2011).
Multiple potential cellular mechanisms have been implicated in the aetiology of CCHS, from loss of PHOX2B function in development due to heterozygous mutant PHOX2B expression, to gain of function of the mutated protein that may alter transcriptional activity in neurons, as well as aggregate formation and premature cell death (Bachetti et al., 2005; Di Lascio et al., 2013; Dubreuil et al., 2008; Durand et al., 2005; Goridis et al., 2010; Trochet et al., 2005). While its role during embryonic neurodevelopment is established, PHOX2B is still expressed in selected neuronal populations in the adult brain (Kang et al., 2007; Stornetta et al., 2006) and its function, with the exception of the contribution to the maintenance of the noradrenergic phenotype (Fan et al., 2011), is not yet fully understood. Considering the fundamental role of PHOX2B in CCHS pathogenesis and its expression in key structures for CO2 chemoreflex responses, it is essential to understand how both wild-type and mutant PHOX2B proteins impact CO2 homeostasis and ventilation not only across development, but also in adulthood.
In order to have a better understanding of the role of PHOX2B in the CO2 homeostatic processes, we used a non-replicating lentivirus vector of two short-hairpin RNA (shRNA) clones targeting selectively Phox2b mRNA to knockdown the expression of PHOX2B in the RTN of adult rats and tested ventilation and chemoreflex responses. In parallel, we also determined whether knockdown of PHOX2B in adult Nmb neurons of the RTN negatively affected their cell survival. Finally, we sought to provide a mechanistic link between PHOX2B expression and the chemosensitive properties of Nmb neurons of the RTN, which have been attributed to two proton sensors, the proton-activated G-protein-coupled receptor (GPR4) and the proton-modulated potassium channel (TASK-2; Gestreau et al., 2010; Guyenet et al., 2016; Kumar et al., 2015). The expression of these sensors is partially overlapping in Nmb neurons of the RTN and the genetic deletion of either one impairs the central respiratory chemoreflex with maximal impairment when both sensors are eliminated (Gestreau et al., 2010; Guyenet et al., 2016; Kumar et al., 2015).
Our results indicate that progressive knockdown of PHOX2B in RTN causes a reduction in the chemoreflex response that was proportional to the decrease in the fraction of Nmb/PHOX2B expressing RTN neurons. This effect was associated with a reduction in the expression of Gpr4 and Task2 mRNA in Nmb expressing cells of the RTN in which PHOX2B was knocked down, suggesting a role for PHOX2B in the transcription of the proton sensors in RTN neurons.
Results
To directly investigate the role of PHOX2B in RTN neurons, we performed selective knockdown of this protein in adult rats through local injection of a non-replicating lentiviral vector of two short-hairpin RNA (shRNA) clones targeting two different sequences of the Phox2b mRNA (n=25) or non-target shRNA as control (NT-shRNA; n=23). Initial experiments utilized 200 nl/injection at each of four stereotaxic coordinates (1x109 VP/ml; n=14). Four weeks post injection, we assessed ventilatory parameters in room air, hypercapnia, and hypoxia. Interestingly, a small but significant impairment in the CO2 chemoreflex was observed not only in PHOX2B-shRNA but also in NT-shRNA rats compared to pre-surgical baseline (allometric VE in 7% CO2: –21% and –24%, respectively; Figure 1—figure supplement 1). Because histological analysis also showed significant reduction of Nmb+/PHOX2B+ RTN cells in NT-shRNA and PHOX2B-shRNA rats (33% and 19% cells remaining, respectively; Figure 1—figure supplement 1; Supplementary file 1), we concluded that the volume and titre of the shRNA viral constructs triggered off-target effects (McBride et al., 2008; van Gestel et al., 2014) that cause cell death in RTN neurons.
PHOX2B expression is modestly reduced two weeks following infection without respiratory impairment
Due to the off-target effects obtained with large volume injections, we reduced injection volume of both PHOX2B shRNA (n=17) and NT-shRNA viruses (n=17; 4x100 nl/injection; 1x109 VP/ml).
Two weeks post-surgery we assessed respiratory function during exposure to room air, hypercapnia and hypoxia. Overall, we observed little change in respiration at this time point. During room air, as well as in 5% and 7.2% CO2, frequency (ƒR), was reduced in every experimental group (naive, NT-shRNA, PHOX2B-shRNA) compared to baseline (Air: p=0.014; 5% CO2: p<0.001; 7.2% CO2 p<0.001; Figure 1A; Supplementary file 1) but no effect on treatment was observed, suggesting that these changes were likely due to the increase in age and body weight during the long-term experiment (naive:+32.2%; NT-shRNA:+27.8%; PHOX2B-shRNA:+28.6% change in body weight). Tidal volume was reduced in the PHOX2B-shRNA rats at 7.2% CO2 compared to both naive rats (naive: 9.6±1.2 ml·kg–1; PHOX2B-shRNA: 8.5±0.9 ml·kg–1; –11%; p=0.022) and baseline pre-surgery conditions (baseline: 9.6±1.3 ml·kg–1; –11%; p<0.001; Figure 1B) but it was not different from NT-shRNA.
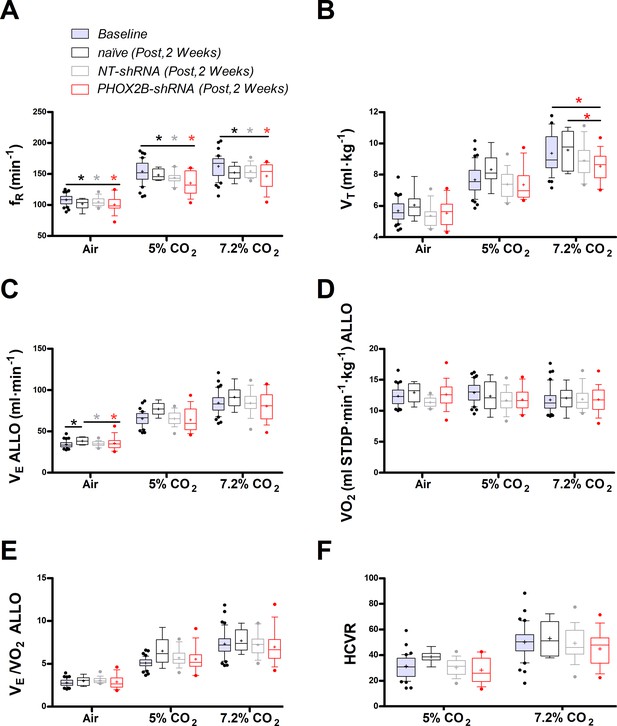
Respiratory data 2 weeks post viral PHOX2B shRNA injection.
(A) Breathing frequency (ƒR,), (B) tidal volume (VT), (C) allometric minute ventilation (VE ALLO), (D) oxygen consumption (VO2 ALLO), (E) convective requirement ratio (VE/VO2 ALLO), (F) hypercapnic ventilatory response (HCVR absolute change in VE ALLO vs. corresponding room air) of baseline (pre-surgery, grey filled box), naive (black n=8), non-target control shRNA (NT-shRNA, grey n=17) and PHOX2B shRNA (PHOX2B-shRNA, red n=17) rats 2 weeks post injection during room air, hypercapnia 5% and 7.2% CO2. ƒR was equally reduced in all experimental groups compared to baseline but no treatment effect was observed (A). VT was significantly impaired following RTN injection in PHOX2B-shRNA group compared to naive animals (p=0.022) and baseline (p<0.001) at 7.2% CO2 (B). VE ALLO was increased in naive rats compared to the other treatment groups (p=0.005) in room air (C). Boxplots: median, 1st – 3rd quartiles and 10th – 90th percentiles, outliers = dots, ‘+’ indicates arithmetic mean. Bonferroni post-hoc as indicated. Black*, different from naive; Grey*, different from NT-shRNA; Red*, different from PHOX2B- shRNA.
-
Figure 1—source data 1
Respiratory data 2 weeks post viral PHOX2B shRNA injection.
- https://cdn.elifesciences.org/articles/94653/elife-94653-fig1-data1-v1.xlsx
Despite similar changes in body weight across treatment groups, allometric ventilation (VE ALLO) was higher in naive rats compared to the other treatment groups in room air (naive: 38.4±4.5 ml·min–1; NT-shRNA: 34.7±2.8 ml·min–1; PHOX2B-shRNA: 34.0±4.5 ml·min–1; p=0.005; Figure 1C), but no changes in VE ALLO were observed in hypercapnia (7.2% CO2) across treatments (naive: 91.5±13.2 ml·min–1; NT-shRNA: 84.4±13.7 ml·min–1; PHOX2B-shRNA: 80.5±17.2 ml·min–1). Oxygen consumption (VO2 ALLO) was not different between experimental conditions or treatment groups (Figure 1D), and VE/VO2 ALLO indicated that ventilation was matched with metabolism for each treatment across all gas compositions (naive: 7.7±1.3; NT-shRNA: 7.2±1.3; PHOX2B-shRNA: 6.9±2.0; Figure 1E). These results suggest that despite a reduction in hypercapnic VT in the PHOX2B-shRNA rats, overall ventilation was not impaired. Since we observed differences in room air VE ALLO between treatment groups, we normalized these data to show the hypercapnic ventilatory response (HCVR, i.e. absolute change in VE from room air; Figure 1F). The HCVR was also not affected by the PHOX2B shRNA treatment two weeks post-injection (naive: 53.1±13.4 ml·min–1; NT-shRNA: 49.6±13.2 ml·min–1; PHOX2B-shRNA: 46.5±14.8 ml·min–1).
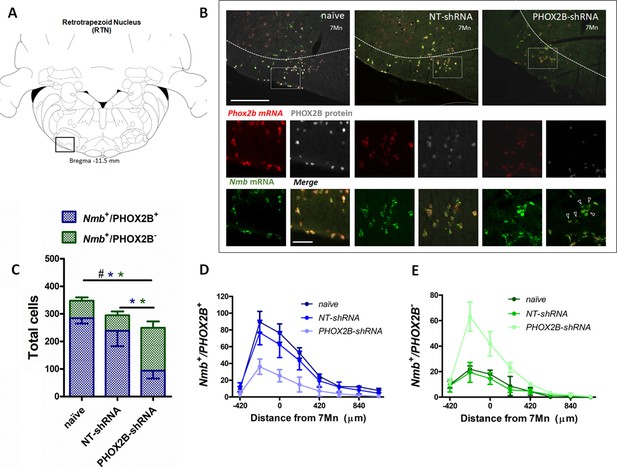
To determine the extent of PHOX2B knockdown in RTN neurons, we combined RNAScope and immunohistochemistry assays to quantify RTN neurons expressing Nmb and PHOX2B (Figure 2A and B). We reasoned that by assessing the fraction of RTN neurons that express only Nmb (compared to Nmb+/PHOX2B+), we would be able to determine the level of PHOX2B knockdown in our experiments. Two weeks post viral injection, we observed a small decrease (15.2%) in the total number of Nmb RTN neurons (i.e. Nmb+/PHOX2B+ plus Nmb+/ PHOX2B-) in NT-shRNA rats compared to naive rats (naive; 337.6±11.95; n=4; NT-shRNA: 286.0±10.44; n=4; p<0.001; Figure 2C; Supplementary file 1). The total number of Nmb cells of the RTN in PHOX2B-shRNA rats was further reduced compared to both naive rats (PHOX2B-ShRNA 218.8±7.8; n=4; –35.2%; p<0.001) and to NT-shRNA (–23.5%; p<0.001).

PHOX2B and Nmb expression and total cell count within the RTN area in naive, NT-shRNA and PHOX2B-shRNA injected rats two weeks post viral shRNA injection.
(A) Schematic and representative image of a transverse brainstem section at the level of the RTN (–11.5 mm distance from Bregma) showing the area of investigation containing RTN neurons. (B) Expression of Phox2b mRNA (red), PHOX2B protein (white) and Nmb mRNA (green) in RTN Nmb+/PHOX2B+ and Nmb+/PHOX2B- neurons in naive (left), NT-shRNA (middle), and PHOX2B-shRNA (right) rats (magnified view inserts below). Arrowheads indicate absence of PHOX2B protein. Scale bar = 400 μm (top figures), 150 μm (inserts below). (C) The number of total cells (Nmb+/PHOX2B+ plus Nmb+/PHOX2B-) comprising the RTN were reduced in PHOX2B-shRNA rats (n=4) as compared to naive (n=4) and NT-shRNA (n=4), and in NT-shRNA rats as compared to naive rats (black#, One-way ANOVA, p<0.001). The number of Nmb+/PHOX2B+ cells were reduced in PHOX2B-shRNA rats as compared to naive and NT-shRNA (blue*, One-way ANOVA, p<0 0.001). The number of Nmb+/PHOX2B- cells was unchanged across groups. (D,E) Rostral-caudal distribution (distance from the caudal tip of the facial nucleus, 7Mn) of Nmb+/PHOX2B+ (D) and Nmb+/PHOX2B- (E) neurons along the RTN.
-
Figure 2—source data 1
PHOX2B and Nmb expression and total cell count within the RTN area in naive, NT-shRNA and PHOX2B-shRNA injected rats two weeks post viral shRNA injection.
- https://cdn.elifesciences.org/articles/94653/elife-94653-fig2-data1-v1.xlsx
We then quantified the number of neurons in the RTN expressing either Nmb and PHOX2B (Nmb+/PHOX2B+) or Nmb only (Nmb+/PHOX2B-). The number of Nmb+/PHOX2B+ neurons in PHOX2B-shRNA rat was reduced by 36.1% compared to NT-shRNA and by 46.2% compared to naive rats (Nmb+/PHOX2B+ NT-shRNA: 232.3±11.6 vs naive: 275.8±17.1; vs PHOX2B-shRNA: 148.3±12.6; p<0.05 and 0.001, respectively; Figure 2C and D). On the contrary, the fraction of Nmb+/PHOX2B- neurons in PHOX2B-shRNA rats was not different compared to naive and NT-shRNA rats (Nmb+/PHOX2B- NT-shRNA: 53.7±8.6; vs naive: 61.8±13.9; vs PHOX2B-shRNA: 70.5±8.7 cells; Figure 2C and E). These results thus indicate that despite modest loss of Nmb+ neurons in NT-shRNA and a reduction in the proportion of Nmb neurons expressing PHOX2B in PHOX2B-shRNA rats, 2 weeks of PHOX2B shRNA viral infection was not sufficient to impair ventilation.
Four weeks of PHOX2B knockdown impairs the CO2-chemoreflex
Since the shRNAs integrate into the genome of the infected cells and are continuously produced, it is reasonable to expect that knockdown efficiency is time-dependent, and an extended infection time would result in a higher knockdown effect. To test this hypothesis, a subgroup of rats (naive, NT-shRNA and PHOX2B-shRNA) were investigated 4 weeks post-viral injection (Figure 3A and B).
PHOX2B and Nmb expression and total cell count within the RTN area in naive, NT-shRNA and PHOX2B-shRNA injected rats 4 weeks post viral shRNA injection.
(A) Schematic and representative image of a transverse brainstem section at the level of the RTN (–11.5 mm distance from Bregma) showing the area of investigation containing RTN neurons. (B) Expression of Phox2b mRNA (red), PHOX2B protein (white) and Nmb mRNA (green) in naive (left), NT-shRNA (middle), and PHOX2B-shRNA (right) rats (magnified view insert). Arrowheads indicate absence of PHOX2B protein. Scale bar = 400 μm (top figures), 150 μm (inserts below). (C) The number of total cells (Nmb+/PHOX2B+ + Nmb+/PHOX2B-) comprising the RTN were reduced in PHOX2B-shRNA rats (n=6) as compared to naive (n=4) (Black#, One-way ANOVA, p=0.0087) but not to NT-shRNA (n=10). The number of Nmb+/PHOX2B+ cells were reduced in PHOX2B-shRNA rats as compared to naive and NT-shRNA (Blue*, one-way ANOVA, p<0.001). The number of Nmb+/PHOX2B- cells were increased in PHOX2B-shRNA rats as compared to both naive and NT-shRNA (Green*, one-way ANOVA p<0.001). (D,E) Rostral-caudal distribution (distance from the caudal tip of the facial nucleus, 7Mn) of Nmb+/PHOX2B+ (D) and Nmb+/PHOX2B- (E) neurons along the RTN.
-
Figure 3—source data 1
PHOX2B and Nmb expression and total cell count within the RTN area in naive, NT-shRNA and PHOX2B-shRNA injected rats 4 weeks post viral shRNA injection.
- https://cdn.elifesciences.org/articles/94653/elife-94653-fig3-data1-v1.xlsx
The total number of Nmb neurons in the RTN of PHOX2B-shRNA rats (Nmb+/PHOX2B+ plus Nmb+/PHOX2B-; Figure 3C; Supplementary file 1) was significantly reduced compared to naive rats (naive: 347.8±12 cells, n=4; PHOX2B-shRNA: 249.5±33.9 cells; n=6; –28.3%; p=0.009) but no difference was observed compared to NT-shRNA rats (294.9±52.8 cells; n=10; –15.4%;). Furthermore, there was no difference in the total number of Nmb cells in RTN between 2 and 4 weeks post infection for both NT-shRNA (week 2: 286±10.4; week 4: 294.9±52.8, p=0.783) and PHOX2B-shRNA rats (week 2: 218.8±7.8; week 4: 249.5±33,9, p=0.118), suggesting that the progressive PHOX2B knockdown was specific for PHOX2B-shRNA treatment and it was not accompanied by an increased cell death beyond the first 2 weeks.
The number of Nmb+/PHOX2B+ cells in the RTN was significantly reduced in PHOX2B-shRNA rats by 67.0% and 60.7% compared to naive and NT-shRNA rats, respectively (naive: 284.5±19.8; NT-shRNA: 238.7±56; PHOX2B-shRNA: 93.8±11.9 cells, p<0.001; Figure 3D). Moreover, a significant increase in the fraction of Nmb+/PHOX2B- was observed with PHOX2B knockdown (PHOX2B-shRNA: 155.7±22.7; naive: 63.2±12 cells;+146.1% increase vs naive; NT-shRNA: 56.2±13.8 cells;+177% increase vs NT-shRNA, pm<0.001; Figure 3E), confirming the efficiency of the PHOX2B knockdown in the Nmb cells of the RTN.
The respiratory function prior to histological examination (4 weeks post-viral injection) showed significant differences in PHOX2B-shRNA rats (n=6) compared to both pre-surgery baseline, naive (n=8) and NT-shRNA rats (n=10). Similar to what we reported at 2 weeks post-surgery, ƒR at 4 weeks was lower in every treatment group compared to baseline recordings (Air: p=0.001; 5% CO2: p<0.001; 7.2% CO2 p<0.001; Figure 4A; Supplementary file 1).

Respiratory data following 4 weeks post viral shRNA injection.
(A) Breathing frequency (ƒR,), (B) tidal volume (VT), (C) allometric minute ventilation (VE ALLO), (D) convective requirement ratio (VE/VO2 ALLO), (E) hypercapnic ventilatory response (HCVR, absolute change in VE ALLO vs. corresponding room air), (F) HCVR at baseline, week 2 and week 4 post-viral injections in naive (black n=8), non-target control shRNA (NT-shRNA, grey n=10) and PHOX2B-shRNA (PHOX2B-shRNA, red n=6). ƒR was equally impaired in all experimental group compared to baseline but no treatment effect was observed (A). VT was significantly impaired following RTN injection in PHOX2B-shRNA group compared to baseline pre-surgery (p<0.001), naive rats (p<0.001), and NT-shRNA rats (p=0.002) at 7.2% CO2. (B). VE ALLO was impaired in PHOX2B-shRNA rats during exposure to hypercapnia (7.2% CO2) compared to baseline (p=0.0025), naive rats (p=0.007), and NT-shRNA rats (p=0.002). (C). VE/VO2 ALLO was reduced in PHOX2B-shRNA animals compared to NT-shRNA rats both at 5% (p=0.023) and 7.2% (p=0.004) CO2 (D). HCVR during 7.2% CO2 was lower in PHOX2B-shRNA rats compared to baseline (p=0.007), naive rats (p=0.001), and NT-shRNA rats (p=0.016) (E). Boxplots: median, 1st – 3rd quartiles and 10th – 90th percentiles, outliers = dots, ‘+’ indicates arithmetic mean. Bonferroni post-hoc as indicated. HCVR was significantly impaired only in PHOX2B-shRNA rats 4 weeks post-surgery (One-way ANOVA p=0.007) (F). Black*, different from naive; Grey*, different from NT-shRNA; Red*, different from PHOX2B- shRNA.
-
Figure 4—source data 1
Respiratory data following 4 weeks post viral shRNA injection.
- https://cdn.elifesciences.org/articles/94653/elife-94653-fig4-data1-v1.xlsx
During room air, VT was reduced in naive rats compared to baseline (baseline: 5.9±0.5 ml·kg–1; week 4: 5.2±0.6 ml·kg-1; –12%; p=0.001; Figure 4B). Moreover, VT in PHOX2B-shRNA rats was reduced compared to NT-shRNA (NT-shRNA: 5.6±0.4 ml·kg–1; PHOX2B-shRNA: 5.3±0.4 ml·kg–1; –10%; p=0.043) and to the pre-surgery baseline (6.1±0.7 ml·kg–1; –18%; p=0.002). No significant changes were observed at 5% CO2, although a decrease in VT occurred at 7.2% CO2 in PHOX2B-shRNA rats compared to pre-surgery baseline (baseline: 7.7±1.2 ml·kg–1; PHOX2B-shRNA: 6.7±0.6 ml·kg–1; –31%; p<0.001), naive rats (7.9±0.9 ml·kg–1; –26%; p<0.001), and NT-shRNA rats (8.1±0.7 ml·kg–1; –23%; p=0.002).
Decreased VT observed for PHOX2B-shRNA led to a ventilation (VE ALLO) impairment during exposure to hypercapnia (7.2% CO2, PHOX2B-shRNA vs. NT-shRNA: –17%; p=0.021; PHOX2B-shRNA vs. naive; –21%; p=0.007; PHOX2B-shRNA vs. baseline, –18% p=0.002; Figure 4C). Since O2-consumption (VO2 ALLO) did not change (data not shown), a significant reduction in VE/VO2 ALLO confirmed alveolar hypoventilation in PHOX2B-shRNA animals compared to NT-shRNA rats both at 5% (NT-shRNA: 5.8±0.5; PHOX2B-shRNA: 4.8±0.4; –16%; p=0.023) and 7.2% CO2 (NT-shRNA: 7.9±1.2; PHOX2B-shRNA: 6.1±0.8; –24%; p=0.004; Figure 4D). Moreover, the HCVR (Figure 4E) at 7.2% CO2 was lower in PHOX2B-shRNA (40.3±9.4 ml·min–1) rats compared to the pre-surgery baseline (58.1±8.9 ml·min–1; –31%; p=0.015), naive rats (63.6±14.3 ml·min–1; –37%; p=0.008) and NT-shRNA rats (56.0±10.4 ml·min–1; –28%; p=0.016). Analysis of individual rats in each treatment group over time showed that the HCVR was only consistently impaired in PHOX2B-shRNA rats between weeks 2 and 4 (Figure 1F; p=0.007).
Given that previous studies have proposed a role for the RTN in the hypoxic chemoreflex (Barna et al., 2016; Basting et al., 2015; Gourine et al., 2005; Wickström et al., 2004), and we observed impairment of the CO2-chemoreflex at 4 weeks post-infection, we investigated whether PHOX2B knockdown would also affect respiratory responses to hypoxia (10% O2). The 4 week VE ALLO was increased relative to baseline values in naive (baseline: 62.3±5.2 ml·min–1; naive: 71.0±10.1 ml·min–1;+14%), NT-shRNA (baseline: 71.8±11.1 ml·min–1; NT-shRNA: 75.8±12.8 ml·min–1;+6%) and PHOX2B-shRNA rats (baseline: 65.9±9.8 ml·min–1; PHOX2B-shRNA: 79.3±6.4 ml·min–1;+29%; p<0.001; data not shown). However, analysis of the convective requirement ratio (VE/VO2 ALLO) indicates that changes in hypoxic ventilation were due to metabolic adjustments that occurred in all treatment groups (VE/VO2 ALLO naive: 6.5±1.3; NT-shRNA: 6.7±1.7; PHOX2B-shRNA: 6.6±0.6; data not shown), suggesting that the hypoxic ventilatory response was not affected by PHOX2B knockdown.
PHOX2B knockdown does not extend to C1 catecholaminergic neurons and does not affect mRNA expression of Nmb in RTN neurons
To exclude that the observed chemoreflex impairment was due to unintended knockdown of PHOX2B in adjacent areas of the brain, we quantified the total number of TH+/PHOX2B+ catecholaminergic C1 neurons in PHOX2B-shRNA rats compared to naive and NT-shRNA (Figure 5A and B). No differences were observed in either the TH+ cell numbers or in the TH+/PHOX2B+ neurons between the three treatment groups (TH+/PHOX2B+ cells: naive: 378.3±23.81; NT-shRNA: 391.7±33.08; PHOX2B-shRNA: 370.2±33.04 cells; Figure 5B; Supplementary file 1).

PHOX2B knockdown in RTN neurons does not alter TH or Nmb expression but impairs the hypercapnic ventilatory response.
(A) PHOX2B protein (white) and TH (red) expression in C1 neurons of nave (left), NT-shRNA (middle), and PHOX2B-shRNA (right) rats. Magnified view at the bottom. Arrowheads indicate the colocalization of PHOX2B and TH protein in C1 neurones cells. Scale bar = 400 μm (top figures), 150 μm (bottom figures). (B) No differences were observed in the rostral-caudal distribution of TH+/PHOX2B+ catecholaminergic C1 neurons cells caudal to the RTN between naive (black, n=4), NT-shRNA (grey, n=10) and PHOX2B-shRNA (red, n=6) rats. (C) PHOX2B protein (white) and Nmb mRNA (green) expression in NT-shRNA rat at the level of NTS and RTN regions. Magnified view on the left. Arrowheads indicate cells with Nmb mRNA expression. Scale bar = 400 μm (right figures), 150 μm (left figures). (D) Quantification of single cells Nmb mRNA fluorescence intensity along the rostro-caudal extension of RTN in naive (black, n=4), NT-shRNA (grey, n=10) and PHOX2B-shRNA (red, n=6) rat calculated as average ratio between RTN and NTS cells showed no difference between treatment groups. Data are shown as average cell fluorescence value at different rostro-caudal levels. (E-F) X-Y plot of HCVR during 7.2% CO2 exposure relative to the number of Nmb+/PHOX2B+ (E, slope is different from ‘0’ at p<0.001; r2=0.739) and Nmb+/PHOX2B- (F, p<0.001 for difference between slopes; r2=0.482) in the RTN.
-
Figure 5—source data 1
PHOX2B knockdown in RTN neurons does not alter TH or Nmb expression but impairs the hypercapnic ventilatory response.
- https://cdn.elifesciences.org/articles/94653/elife-94653-fig5-data1-v1.xlsx
Neuromedin B is currently considered the most selective anatomical marker for chemosensitive RTN neurons (Shi et al., 2017) and was used in this study to identify RTN location, cell numbers and relative PHOX2B expression. Since changes in the level of the transcription factor PHOX2B could regulate the expression of its target genes, we investigated whether Nmb is a target gene of PHOX2B and it is affected by PHOX2B knockdown. We assessed Nmb expression in individual RTN cells of PHOX2B-shRNA rats and compared its cellular expression to both naive and NT-shRNA rats ( Figure 5 C and D ). Neuromedin B mRNA expression was calculated as total fluorescence intensity (CTCF) ratio in single cells of the RTN relative to the single cell Nmb expression measured at the level of NTS Nmb cells, which served as an internal control (Figure 5D). We observed no changes in relative fluorescence of Nmb in RTN neurons across the three groups (p=0.608), suggesting that Nmb levels in RTN neurons were not affected by PHOX2B knockdown.
To better analyse the effect of PHOX2B knockdown on the attenuation of the HCVR, we evaluated the correlation between the number of Nmb+/PHOX2B+ or Nmb+/PHOX2B- cells in the RTN and the resulting HCVR using linear regression (Figure 5E and F). Our results indicate that there is a significant correlation between HCVR and number of Nmb+/PHOX2B+ (r2=0.739 p<0.001; Figure 5E), and Nmb+/PHOX2B- (r2=0.482 p<0.001 Figure 5F) cells, suggesting that the number of PHOX2B expressing cells in the RTN is a good predictor of the chemoreflex response. Moreover, the nature of the correlation between PHOX2B- cells and HCVR is the opposite of PHOX2B+, another important indicator that PHOX2B protein may contribute to the CO2 sensing and consequently, the reduction of PHOX2B protein impairs the CO2-chemoreflex.
Gpr4 and Task2 mRNAs expression is affected by PHOX2B Knockdown
We next examined the mRNA expression of Gpr4 and Task2, two important pH sensors for the central respiratory chemoreflex response in RTN neurons (Figure 6A and D; Gestreau et al., 2010; Guyenet et al., 2016; Kumar et al., 2015). As previously reported, the two pH sensors are expressed in the Nmb cells of the RTN in partially overlapping populations of chemosensory neurons (Gestreau et al., 2010; Kumar et al., 2015). In naive rats 91.6 ± 11.2% of Nmb cells were Gpr4 positive, and 76.6 ± 14.8% of Nmb cells were also Task2 positive (n=4 rats). These values were not significantly different in either NT-shRNA or PHOX2B-shRNA rats (NT-shRNA: 90.0 ± 15.7% Nmb+/Gpr4+ and + ± 21.8% Nmb+/Gpr4+, n=10 rats; PHOX2B-shRNA: 89.4 ± 18.3% Nmb+/Gpr4+ and + ± 27.8% Nmb+/Gpr4+, n=6 rats), suggesting that the fraction of Nmb cells expressing the two pH sensors were not affected by the PHOX2B shRNA treatment.

Gpr4 and Task2 mRNA expression within the RTN area in naive, and PHOX2B-shRNA injected rats 4 weeks post viral shRNA injection.
(A, D) Nmb (green), Gpr4, Task2 mRNA (red) and PHOX2B protein (grey) expression in RTN neurons in naive (top) and PHOX2B-shRNA (bottom) rats. Scale bar = 150 μm. Arrowheads indicate colocalization of Gpr4 (A) and Task2 (D) with Nmb+/PHOX2B+ neurons. Asterisks indicate colocalization of Gpr4 (A) and Task2 (D) with Nmb+/PHOX2B- neurons. (B, E) Quantification of single cells Gpr4 and Task2 mRNA fluorescence intensity along the rostro-caudal extension of RTN in naive (black), NT-shRNA (grey) and PHOX2B-shRNA (red) rats. Black*, different from naive, p<0.001. Grey*, different from NT-shRNA, p<0.001 (C, F) Quantification of single cells Gpr4 and Task2 mRNA fluorescence staining intensity along the rostro-caudal extension of RTN in Nmb+/PHOX2B+ (red filled dot) and Nmb+/PHOX2B- (red empty dot) neurons in PHOX2B-shRNA rats (C, p=0.022); (F, p=0.029). Data are shown as average cell fluorescence value at different rostro-caudal levels of the RTN. Mean corrected total cell fluorescence (CTCF) value ± SEM combined (naive, n=4; NT-shRNA, n=10; PHOX2B-shRNA, n=6) (see Materials and methods for detail). One-way ANOVA repeated measures.
-
Figure 6—source data 1
Gpr4 and Task2 mRNA expression within the RTN area in naive, and PHOX2B-shRNA injected rats 4 weeks post viral shRNA injection.
- https://cdn.elifesciences.org/articles/94653/elife-94653-fig6-data1-v1.xlsx
Because it is not yet known whether PHOX2B controls the expression of GPR4 AND TASK2, we quantified their single-cell mRNA expression (CTCF) in Nmb cells of the RTN. Averaged cell fluorescence for Gpr4 and Task2 mRNA in PHOX2B-shRNA rats was significantly reduced compared to naive and NT-shRNA rats (Gpr4: –34.4 ± 4.79% vs naive, –27.9 ± 5.71% vs NT-shRNA p<0.001; Task2: –39.0 ± 3.02% vs naive, –34 ± 2.62% vs NT-shRNA p<0.001; Figure 6B and E; Supplementary file 1). To better understand whether this reduction was ascribed to the specific loss of PHOX2B expression, we compared their single cell mRNA expression levels between Nmb+/PHOX2B- and Nmb+/PHOX2B+ neurons in PHOX2B-shRNA rats. Both Gpr4 and Task2 mRNA expression was reduced in Nmb+/PHOX2B- cells compared to Nmb+/PHOX2B+ (Gpr4: –63.8 ± 3.9%; p=0.022; Task2: –63.2 ± 2.77%; p=0.029; Figure 6C and F). Our data suggest that PHOX2B knockdown not only causes a reduction in the HCVR but also a reduction in Gpr4 and Task2 mRNA levels in cells that are affected by PHOX2B knockdown.
Discussion
The role of the transcription factor PHOX2B in the adult brain is still not fully understood. In this study, we reduced the expression of PHOX2B in the key chemosensory structure of the RTN with a selective viral shRNA approach to test whether a knockdown of PHOX2B expression in these neurons negatively impacts ventilation. Two weeks after viral infection, we observed a modest reduction of PHOX2B/Nmb expressing neurons in the RTN but no significant changes in basal VE or in the CO2 chemoreflex. Four weeks after shRNA infection, we observed a further reduction in the expression of PHOX2B in Nmb neurons and a significant reduction of the CO2 chemoreflex, while ventilation in normoxia or hypoxia was unaffected. Furthermore, the level of expression of Gpr4 and Task2 mRNAs, the two proton sensors responsible for the RTN neurons chemosensory properties were significantly reduced in RTN neurons that lacked PHOX2B protein expression, whereas Nmb mRNA expression level was unaffected by PHOX2B knockdown. These results suggest that PHOX2B reduction affects the transcriptional activity of Nmb neurons in the RTN and decreases the CO2 response, possibly by reducing the expression of key proton sensors in the RTN.
The role of PHOX2B in development and in the adult brain
PHOX2B is a transcription factor with an important role in the development and differentiation of neuronal structures in both the central and peripheral nervous system. Genetic loss of Phox2b expression in mice shows that PHOX2B is essential for the correct development of all autonomic ganglia in sympathetic, parasympathetic, and enteric nervous system and the distal ganglia of the facial (VII), glossopharyngeal (IX), and vagus (X) cranial nerves (Pattyn et al., 1999), in addition to carotid bodies and the solitary tract nucleus (Dauger et al., 2003). Furthermore, PHOX2B is involved in the formation of all branchial and visceral hindbrain motor neurons and its absence during the embryonic period impairs the development of motoneurons of the facial, trigeminal, ambiguus and dorsal motor nucleus of the vagal nerve. PHOX2B is also necessary for the specification and differentiation of the noradrenergic phenotype in multiple centres in the brain (Brunet and Pattyn, 2002; Pattyn et al., 1999).
Interestingly, the expression of PHOX2B persists into adulthood in selected neuronal populations, such as the ones involved in peripheral and central chemoreception, that is the carotid bodies, the solitary tract nucleus and the RTN, in addition to neurons in the dorsal motor nucleus of the vagus, the area postrema, the nucleus ambiguus, C1 catecholaminergic neurons and other scattered neurons throughout the brainstem (Stornetta et al., 2006). Such widespread expression within the adult brain suggests an important transcriptional role in neuronal survival and/or function. With the exception of its role in maintaining the noradrenergic phenotype, its function in the postnatal brain is unknown, in part because a full investigation of its transcriptional activity in neurons has not yet been performed.
Heterozygote polyalanine expansion mutations in the exon 3 of PHOX2B are responsible for the majority of cases of CCHS, a rare genetic disease that affect the autonomic nervous system and central CO2 chemoreflexes (Amiel et al., 2003; Di Lascio et al., 2020; Weese-Mayer et al., 2017). While studies in transgenic mice expressing the mutated PHOX2B protein suggest that the RTN may not form or be severely impaired (Dubreuil et al., 2008; Dubreuil et al., 2009; Ramanantsoa et al., 2011), the function of the wild-type protein in these neurons has never been tested beyond the post-natal period. Thus, in order to determine whether PHOX2B is necessary for the survival and/or chemosensory function of adult PHOX2B+/Nmb+ RTN neurons in vivo, we used a shRNA viral approach to progressively knockdown the PHOX2B protein in these neurons.
Modest reduction of PHOX2B in RTN does not impair ventilation
Although the PHOX2B shRNA approach was not selective for Nmb neurons, we used small volumes and localized injections to target the RTN. We identified RTN neurons by the mRNA expression of the neuropeptide NMB (Shi et al., 2017) and observed a reduced number in both total Nmb and Nmb+/PHOX2B+ neurons in PHOX2B shRNA-treated rats compared to naive and NT-shRNA rats two weeks after viral infection. Interestingly, despite the small reduction in total Nmb cells of the RTN in both NT-shRNA and PHOX2B-shRNA injected rats, possibly due to some off-target effects due to activation of innate immunity or saturation of the microRNA pathway (McBride et al., 2008; van Gestel et al., 2014), no significant impairment in ventilation was observed. Even though PHOX2B may be critical for chemosensory function in RTN neurons, the lack of significant ventilatory impairment with a modest reduction of PHOX2B expressing neurons is not surprising. In our previous study (and the ones of others), in which RTN chemosensory neurons were lesioned with Substance P saporin toxin, CO2-chemoreflex impairment became significant (~30% VE Allo reduction vs. pre-surgical baseline) only when Nmb neurons of the RTN (Nmb+/PHOX2B+ and Nmb+/PHOX2B- neurons) were reduced by ~63% compared to naive controls (medium lesion range: 50–81% loss of Nmb neurons; Janes et al., 2024; Souza et al., 2023). Therefore, even though loss of PHOX2B severely altered the chemosensitive function in a fraction of Nmb cells, we would not expect significant changes in ventilation only with a<50% RTN cell impairment or loss.
Four weeks of PHOX2B knockdown impairs central CO2 chemoreception
Four weeks post- shRNA viral injection, the fraction of Nmb+ cells expressing PHOX2B was further reduced by 67% compared to naive and by 61% compared to NT-shRNA rats. PHOX2B knockdown was also restricted to RTN neurons, as adjacent C1 TH+ neurons did not show any change in number of TH+/PHOX2B+ expressing cells, although we cannot exclude that some C1 cells may have been infected, and their relative PHOX2B expression levels were slightly reduced. To support the lack of significant alterations associated with the possible loss of C1 function was the lack of significant changes in the hypoxic response that has been shown to be dependent on C1 neurons (Malheiros-Lima et al., 2017).
Although we did not specifically quantify the relative mRNA expression change in intracellular Phox2b levels within infected neurons, we measured the success of PHOX2B knockdown by assessing the overall change in the number of Nmb cells that had no detectable levels of PHOX2B protein in the nuclei. Thus, it is possible that this method led us to underestimate the success of the overall PHOX2B shRNA knockdown in the RTN, as some RTN neurons may still have detectable levels of PHOX2B protein, albeit decreased.
In PHOX2B-shRNA rats, we did not observe any respiratory function changes in room air or in hypoxia, similar to RTN lesioning studies (Janes et al., 2024; Souza et al., 2023; Guyenet et al., 2019). When tested in hypercapnia though, PHOX2B-shRNA rats displayed a reduced HCVR compared to both baseline (i.e. pre-surgery), naive and NT-shRNA rats. The impairment of VE Allo was primarily a result of blunted VT, and analysis of the convective exchange ratio (i.e. VE/VO2 ALLO) confirmed hypoventilation in 5% and 7.2% CO2 only for PHOX2B-shRNA rats, suggesting a specific impairment of chemosensitive properties in RTN neurons with PHOX2B knockdown. These results are in line with previous studies in which medium RTN lesions (i.e. loss of >60% Nmb+ cells) elicited by saporin toxin injection resulted in a significant CO2-chemoreflex impairment (Janes et al., 2024; Souza et al., 2018). Hence, killing RTN neurons or knocking down PHOX2B in Nmb cells of the RTN gave comparable results.
Changes in the CO2 response following 4 weeks of PHOX2B-shRNA treatment could occur because the reduced expression of the transcription factor PHOX2B causes changes in the transcriptional machinery of RTN neurons and alters transcription of CO2/pH sensing proteins or other key elements for their chemosensitive function. These transcriptional changes could also affect neuronal survival. In fact, a small but significant loss of NMB expressing RTN neurons may contribute to the reduction in the CO2 response, although multiple studies have demonstrated that significant cell loss must occur in the RTN in order to show a respiratory function impairment (Janes et al., 2024; Souza et al., 2018; Nattie and Li, 2002; Takakura et al., 2008; Takakura et al., 2014).
In our experiments, we observed a 35% reduction in total Nmb+ cells compared to naive rats and 23% compared to NT-shRNA, possibly due to cell death within the first 14 days post-infection. Interestingly the loss of Nmb cells did not increase with time (although the fraction of Nmb+/PHOX2B+ neurons decreased) and the central chemoreflex at 2 weeks post-surgery was not impaired, suggesting that: (i) the observed reduction in overall Nmb cell number in RTN occurring in the first 2 weeks is not responsible for the CO2 chemoreflex impairment that emerges 4 weeks post-infection; (ii) PHOX2B expression is most likely not necessary for neuronal survival of adult Nmb neurons of the RTN; (iii) a large reduction in Nmb neurons expressing PHOX2B+ is necessary to impair the HCVR (as we observed at 4 weeks). The interpretation that PHOX2B expression contributes positively to the chemoreflex is further supported by our linear regression analysis showing that Nmb+/PHOX2B+ cell number was the best predictor of the HCVR (r2=0.739). The initial cell loss, which we observed also in NT-shRNA, and more prominently with larger injection volume of virus, may be attributed to some inherent cell death associated with the surgical procedure (although not observed in similar studies performed by the same investigators, Janes et al., 2024) or, more likely, with the potential off-target toxic effects associated with shRNA procedures (McBride et al., 2008; van Gestel et al., 2014).
PHOX2B knockdown reduces mRNA expression of proton sensors in the Nmb cells of the RTN
Current theories on central respiratory chemosensitivity postulate that changes in CO2/pH in the RTN are detected through two pH sensors, the proton-activated receptor GPR4 and the pH-sensitive K+ channel TASK2 that are expressed in partially overlapping populations of NMB expressing RTN neurons (Kumar et al., 2015). Because it is not known whether PHOX2B has any influence on the expression of TASK2, GPR4 or even the expression of the neuropeptide NMB used to anatomically identify RTN neurons, we quantified their mRNA at cellular levels (calculated as CFTC) and observed no changes in relative fluorescence of Nmb mRNA in the RTN relative to Nmb levels in unrelated cells in the NTS, suggesting that Nmb expression is not affected by PHOX2B shRNA knockdown and that Nmb is most likely not a target gene of PHOX2B in adult RTN neurons.
Furthermore, we determined the mRNA expression levels of Task2 and Gpr4 in both Nmb+/PHOX2B+ and Nmb+/PHOX2B- neurons in shRNA rats. Our results indicate that although the fraction of Nmb cells of the RTN expressing Gpr4 and Task2 did not change across treatment, the levels of Gpr4 and Task2 in Nmb neurons of PHOX2B-shRNA rats was reduced compared to the naive and NT-shRNA groups. Furthermore, there was a significant reduction of both Gpr4 and Task2 levels within Nmb+/PHOX2B- cells compared to Nmb+/PHOX2B+ cells. Because no good antibodies are currently available to detect protein levels of either NMB, GPR4 or TASK2, our results are only based on changes in mRNA levels, thus we can only speculate that a reduction in Gpr4 and Task2 mRNA would translate in a reduction in the protein levels and consequent reduction of RTN neurons chemosensitive properties. Direct electrophysiological recordings in RTN neurons will be able to address the changes in CO2/pH sensitivity of RTN neurons at cellular level.
PHOX2B knockdown may impair CO2-sensing through additional transcriptional targets
Since loss of PHOX2B reduces expression of TH and dopamine beta hydroxylase enzymes in catecholaminergic neurons in vivo and in vitro (Fan et al., 2011), it is possible that in NMB cells of the RTN, reduction of PHOX2B alters the transcriptional machinery of other proteins (in addition to GPR4 and TASK2) and impairs their function in central chemoreception. For example, a change in the expression of enzymes, neurotransmitters, receptors, and ion channels in RTN neurons could affect the excitatory signal transmission to preBötzinger Complex and the respiratory network specifically during CO2 challenges (Guyenet et al., 2016). Further studies will be needed to address and identify additional transcriptional changes induced by PHOX2B knockdown in vivo.
An interesting observation from our histological data was the reduction in the overall number of Nmb cells at 2- and 4-weeks following PHOX2B shRNA viral injections. Although some cell death may be associated with either surgical procedures or off-target effects associated with the shRNA approach (McBride et al., 2008; van Gestel et al., 2014), it is intriguing to speculate that loss of PHOX2B expression may have some effects on viability of RTN neurons in vivo. Even though this is a possibility, especially with an even more severe knockdown, we did observe a large proportion of Nmb cells devoid of PHOX2B protein expression, suggesting that absence of PHOX2B is compatible with neuronal survival, at least in our experimental time frame.
Relevance to CCHS and its pathogenic mechanisms
Our results contribute to further our understanding on potential pathogenic mechanisms in CCHS (Di Lascio et al., 2018a). Homozygous knockout mice for PHOX2B die during gestation (Pattyn et al., 1999; Pattyn et al., 2000) demonstrating a key role of this transcription factor in cellular proliferation, migration and differentiation during embryonic development, whereas heterozygotes for PHOX2B survive birth and live into adulthood, but display apneas (Durand et al., 2005) and impairment of the hypoxic and hypercapnic ventilatory responses in the neonatal period (Dauger et al., 2003). Here we show that, independent of the PHOX2B role during development, PHOX2B is still required to maintain proper CO2 chemoreflex responses in the adult brain.
Heterologous expression of the mutant PHOX2B alters development of RTN in mice and impairs their chemoreflex response (Dubreuil et al., 2008; Dubreuil et al., 2009). The effects of the expression of the mutant PHOX2B on wild-type PHOX2B protein and its transcriptional activity is not known in vivo yet, although in vitro data indicates that the mutated PHOX2B protein alters wild-type PHOX2B conformation and its ability to form homo- and hetero-dimers (Di Lascio et al., 2016; Trochet et al., 2005; Trochet et al., 2008), its affinity for DNA and coactivators, as well as its degradation rate (Nagashimada et al., 2012; Wu et al., 2009). It has also been shown that mutant PHOX2B causes the formation of cytoplasmic aggregates (Bachetti et al., 2005; Trochet et al., 2005; Trochet et al., 2008) and fibrils in vitro (Pirone et al., 2019), and dysregulates the transcriptional function of wild-type PHOX2B (Di Lascio et al., 2013; Parodi et al., 2012), thus changing expression of important target genes such as DBH, PHOX2A and TLX2 (Bachetti et al., 2005; Di Lascio et al., 2013; Trochet et al., 2005). Based on this evidence, different CCHS pathogenic mechanisms have been proposed (e.g. PHOX2B loss-of-function, dominant-negative or toxic function of the mutant protein) including gene- and cell-specific transcriptional dysregulation (Di Lascio et al., 2018a; Di Lascio et al., 2020). Interestingly, only a handful of PHOX2B target genes have been identified and the function of PHOX2B, and its mutated forms in respiratory control and CCHS pathogenesis is still under investigation.
Our data suggest that, in addition to potential effect of mutated PHOX2B on development and cellular function in CCHS pathogenesis, the expression of wild-type PHOX2B has an important role in respiratory control that extends the developmental period and its reduction in CCHS may contribute to the respiratory impairment in this disorder.
Materials and methods
Experiments were performed using male Sprague–Dawley rats (starting weight range 250–350 g) born in the University of Alberta animal facility to pregnant dams obtained from Charles River (strain code: 001, Senneville, QC). Rats were housed at the University of Alberta Health Sciences Animal Housing Facility and maintained on a 12 hr dark/light cycle with food and water available ad libitum. Handling and experimental procedures were approved by the Health Science Animal Policy and Welfare Committee at the University of Alberta (AUP#461) and performed in accordance with guidelines established by the Canadian Council on Animal Care.
ShRNA viral injection in the retrotrapezoid nucleus
Request a detailed protocolIn order to knockdown Phox2b expression at the level of RTN neurons, we used a mix of two shRNA clones targeting two different sequences of the Phox2b mRNA carried by a non-replicating lentivirus vector (1x109 VP/ml; TRCN000041283: GCCTTAGTGAAGAGCAGTATG and TRCN0000096437: CCTCTGCCTACGAGTCCTGTA; Sigma Aldrich, St. Louis, MA, USA). The shRNAs were designed against the Phox2b mouse sequence (Ref Seq NM_008888) which shares 100% homology with the Phox2b rat sequence (Ref Seq XM_008770167).
Metacam analgesic was administered 1 hr prior to surgery (2 mg/kg) to reduce post-surgical pain. Rats were anesthetized with a ketamine/xylazine mixture (100 mg/kg+10 mg/kg, respectively; i.p.); the anaesthetic level was assessed by lack of paw pinch reflex and maintenance of a regular breathing rate. Additional anaesthesia was administered as needed. The rat was positioned on a stereotactic apparatus (Kopf Instruments, Tujunga, CA, USA) and a total of four microinjections (200 or 100 nL per injection; two rostro-caudally aligned injections per side) were made lateral to the midline and ventral to the facial motor nucleus under aseptic conditions. Coordinates were as follows in mm from obex (medio-lateral/rostro-caudal/dorso-ventral): ±1.8, +2.0, –3.5; ±1.8, +2.4, –3.6 (Paxinos and Watson, 2007). Injections were made using a glass microelectrode (30 μm diameter tip, Drummond Scientific, PA, USA). Twenty-five rats (8 rats 200 nl/injection; 17 rats 100 nl/injection) underwent shRNA viral injection (PHOX2B-shRNA) and 23 rats (6 rats 200 nl/injection; 17 rats 100 nl/injection) received a non-target shRNA viral injection (NT-shRNA; SHC016: MISSION pLKO.1-puro non-Target shRNA Control Plasmid DNA; Sigma-Aldrich, St. Louis, MA, USA) to determine any effects of surgery and viral constructs on respiratory behaviour. Rats were randomly assigned to treatment group. Eight naive rats, receiving no surgery and maintained in the same housing conditions, were used as additional controls for both respiratory function and anatomical experiments. Following each injection, the glass electrodes were left in place for 3–5 min to minimize backflow of virus up the electrode track. At the end of the surgery, the incision was sutured, and rats received local anaesthetic bupivacaine (0.1 mL, s.c.) and were treated with metacam analgesic for 72 hr. Rats recovered for 7 days before the next respiratory function testing.
Data acquisition and analysis of respiratory measurements
Request a detailed protocolRats were habituated to whole-body plethysmography chambers (Buxco, 5 L) once, 3–4 days before baseline recordings. On the day of the experiment, rats were placed in the chamber and testing commenced once the animals were quietly awake (typically 20–30 min). Gas mixtures were delivered at 1.5 L/min using a GSM-3 (CWE Inc, Ardmore, PA, USA) and monitored using a Gas Analyzer (AD Instruments, Sydney, Australia). Ventilatory parameters were measured during exposure to different air compositions for 8–15 min each. Room air: 21%O2+0%CO2 (balanced with N2); Normoxic Hypercapnia: 21%O2+5%CO2; 21%O2+7.2%CO2; Normocapnic Hypoxia: 10.6%O2+0% CO2. The order of chemoreflex was randomized from one session to the next.
As previously described (Cardani et al., 2022; Janes et al., 2024) respiratory data were analysed from rats during quiet wakefulness in the last 5 min period of each gas composition using the barometric method (open-flow system; Cardani et al., 2022; Janes et al., 2024; Mortola and Frappell, 1998; Seifert et al., 2000). Raw pressure signals were acquired with a Validyne differential pressure transducer connected to a CD15 carrier demodulator (Validyne Engineering, Northridge, CA, USA) and digitized using a Powerlab 8/35 (AD Instruments, Sydney, Australia). Analysis was done using Labchart 8 (v8.1.19, AD Instruments, Colorado Springs, CO, USA) to determine tidal volume (VT) and breathing frequency (ƒR). The amplitude of the pressure signal was converted to VT (ml·kg–1) using the equations of Drorbaugh and Fenn, 1955 (Drorbaugh and Fenn, 1955) and calibrated against 1 mL of dry air injected into the empty chamber using a rodent ventilator (Harvard Rodent Ventilator Model 683, Holliston, MA, USA ƒ=50, 75, 100, 150 beats·min–1). Humidity and chamber temperature were monitored using a RH-300 water vapour analyzer (Sable Systems, Las Vegas, NV, USA) and a HPR Plus Handheld PIT Tag reader (Biomark, Boise, ID, USA) respectively, depending on the setup. Rectal temperature was read through the temperature probes of a Homeothermic Monitor (507222 F, Harvard Apparatus, Holliston, MA, USA). Minute ventilation (V̇E) was calculated as ƒR x VT and expressed as ml·min–1·kg–1. Rats gained, on average, 160±4 g over the experimental paradigm (naive: 158±19 g; NT-shRNA: 202±25 g; PHOX2B-shRNA: 133±7 g); we therefore applied an allometric correction to V̇E to account for non-linear effects of weight gain (VE ALLO) (Mortola et al., 1994).
Metabolic parameters VO2 and VE/VO2 were calculated by pull-mode indirect calorimetry and allometrically scaled to be consistent with ventilation (Mortola et al., 1994). The % composition of dry gas flowing into, and out of the recording chamber was measured by using an AD Instruments Gas Analyzer (Colorado Springs, CO) and applying the equations of Depocas and Hart (Depocas and Sanford Hart, 1957) and Lighton, 2021.
Weight measurements were taken prior to every recording session. Rats breathing function was measured the week prior to surgery (baseline recording) and weekly following shRNA injections to determine the time course of ventilatory impairment.
Respiratory data were compared for naive, NT-shRNA and PHOX2B-shRNA pre- and post-injection using a mixed factor ANOVA (independent factor = treatment, repeated measures factor = pre and post-lesion; Figures 1 and 4). Post-hoc analysis used the Bonferroni correction factor, with p<0.05 considered to be significant. Ventilatory data are reported as mean ± standard deviation (SD).
In Situ hybridization (RNAScope) and immunofluorescence
Request a detailed protocolEither 2 or 4 weeks after viral injection, rats were transcardially perfused with saline 0.9% NaCl followed by 4% paraformaldehyde (PFA) and the brains were post-fixed in 4% PFA overnight and cryoprotected in 30% sucrose in 1 X Phosphate Buffered Saline (PBS). Brains were then frozen in O.C.T compound (Fisher Scientific) and sectioned on a cryostat (MODEL CM1950, Leica Biosystems, Buffalo grove, IL, USA) at 30 µm and stored in cryoprotectant buffer at –20 °C until processing. Sections were mounted on slides for combined RNAScope in situ hybridization (Advanced Cell Diagnostics-ACD Bio, Newark CA, USA) and immunofluorescence assay and processed as previously detailed (Biancardi et al., 2021; Cardani et al., 2022). The mRNA expression for Neuromedin B (Nmb) was used as marker of RTN CO2-sensing neurons (Shi et al., 2017). Slides were incubated with probes for Nmb (Rn-NMB-C2 #494791-C2, ACDBio, Newark, CA, USA), Phox2b (Rn-Phox2b-O1-C1 #1064121-C1, ACDBio), G-protein-coupled receptor 4 (Gpr4) (Mm-Gpr4-C1, #427941, ACDBio), and potassium channel, subfamily K, member 5 (Kcnk5 or Task-2) (Mm-Kcnk5-C3, #427951-C3, ACDBio) for 2 hr at 40 °C. Gpr4 and Kcnk5 probes are design against the mouse mRNA sequence (Ref Seq NM_175668.4 and NM_021542.4, respectively). However, the alignment of the mouse mRNA sequences with those of rat (GPR4: Ref Seq NM_001025680.1; TASK2: Ref Seq NM_1039516.2) showed an identity of 94% and 96%, respectively, at the level of the target region (Base Pairs 1030–150) (BLAST program, National Library of Medicine, National Center for Biotechnology Information, NIH). In parallel, two sections/rat were treated with positive (low copy housekeeping gene), and negative (non-specific bacterial gene) control probes provided by ACDBio. Finally, slides were processed using the RNAScope Multiplex Fluorescent Assay kit V2 (ACDBio) according to the manufacturer’s instructions. The probes were visualized using Opal 520 and Opal 570 reagent (1:500 and 1:1000, respectively; PerkinElmer, Woodbridge, ON, CA). PHOX2B immunoreactivity was detected using mouse monoclonal PHOX2B antibody (B-11: sc-376997, 1:100, Santa Cruz Biotechnology, RRID: AB_2813765) incubated overnight in 0.3% TritonX-100, 1%NDS in PBS followed by donkey CY5-conjugated anti-mouse IgG (1:200; Jackson Immuno Research Laboratories Inc) in PBS +1% NDS for 2 hr. Slides were then washed in PBS and cover-slipped with Fluorosave mounting media (EMD Millipore).
We also performed staining for tyrosine hydroxylase (TH) to identify and quantify C1 cells (TH+/PHOX2B+) following shRNA injection. Briefly, perfusion and tissue fixation/freezing were done as described above. Floating sections were stained with rabbit anti TH antibody (AB152,1:1000, Millipore SIGMA, USA, RRID:AB_390204), and mouse anti PHOX2B (B-11: sc-376997, 1:100, Santa Cruz Biotechnology, RRID: AB_2813765). Antibodies were visualized using donkey CY3 (TH) and CY5 (PHOX2B) conjugated IgG (1:200; Jackson Immuno Research Laboratories Inc). The sections were washed in PBS and mounted on slides and cover-slipped with Fluorosave mounting media.
Cell counting, imaging and data analysis
Request a detailed protocolTo quantify Nmb+/PHOX2B- and Nmb+/PHOX2B+neurons within the RTN region, we analysed one every seven sections (210 µm interval; 8 sections/rat in total) along the rostrocaudal distribution of the RTN on the ventral surface of the brainstem and compared total bilateral cell counts of PHOX2B-shRNA rats with non-target control (NT-shRNA) and naive rats. Cells that expressed Nmb and Phox2b mRNAs but did not show co-localization with PHOX2B protein were considered Nmb+/PHOX2B-.
The Corrected Total Cell Fluorescence (CTCF) signal for Nmb, Gpr4 and Task2 mRNAs was quantified as previously described (Cardani et al., 2022; McCloy et al., 2014). Briefly, a Leica TCS SP5 (B-120G) Laser Scanning Confocal microscope was used to acquire images of the tissue. Exposure time and acquisition parameters were set for the naive group and kept unchanged for the entire dataset acquisition. The collected images were then analysed by selecting a single cell at a time and measuring the area, integrated density and mean grey value (McCloy et al., 2014). For each image, three background areas were used to normalize against autofluorescence. We used 4 sections/rat (210 µm interval) to count Nmb, Gpr4 and Task2 mRNA CTCF in the core of the RTN area where several Nmb cells could be identified. For each section, two images were acquired with a 20×objective, so that at least fifty cells per tissue sample were obtained for the mRNA quantification analysis. To evaluate changes in Nmb mRNA expression levels following PHOX2B knockdown at the level of the RTN, we compared, the fluorescence intensity of each RTN Nmb cell (223.2±37.1 cells/animal) with the average fluorescent signal of Nmb cells located dorsally in the NTS (4.3±1.2 cells/animal) (Nmb CTCF ratio RTN/NTS) as we reasoned that the latter would not be affected by the shRNA infection and knockdown.
To quantify Gpr4 and Task2 mRNA expression in Nmb cells of the RTN, we first quantified single-cell CTCF for either Gpr4 (200.7±13.2 cells/rat) or Task2 (169.6±10.3 cells/rat) mRNA in Nmb cells of the RTN in the three experimental groups (naive, NT shRNA and PHOX2B shRNA) independent of their PHOX2B expression. We then compared CTCF values of Gpr4 and Task2 mRNA between Nmb+/PHOX2B+ and Nmb+/PHOX2B- RTN neurons in PHOX2B-shRNA rats to address changes in their mRNA expression induced by PHOX2B knockdown.
To assess whether shRNA knockdown affected either the number or the expression of PHOX2B in TH expressing C1 neurons, we counted the number of TH+/PHOX2B+ and TH+/PHOX2B- cells along the ventrolateral medulla (8 sections/rat; 240 µm interval) using an EVOS fluorescent microscope (Thermo Fisher Scientific).
Digital colour photomicrographs were acquired using a Leica TCS SP5 (B-120G) Laser Scanning Confocal microscope. Image J (version 1.54 f; National Institutes of Health, Bethesda, MD, USA) and Excel (Microsoft Office 365 for Windows) were used for cell counting and the measurements of fluorescence intensity. Statistical analysis of cell counts, and fluorescent signals was made using one-way ANOVA (Figures 2C and 3C, Figure 1—figure supplement 1) and repeated-measures ANOVA (Figures 5B, D ,, 6) with GraphPad Prism 8 Software (GraphPad Software, Inc, San Diego, CA, USA). Linear regressions to determine the effect of cell counts (Nmb+/PHOX2B+, Nmb+/PHOX2B-, total cells) on chemoreflex magnitude were run using a combined dataset for naive, NT-shRNA and PHOX2B-shRNA rats (SPSS version 13.0; Figure 5E–F). p values of<0.05 were considered significant and data are reported as mean ± standard deviation (SD).
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
References
-
Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious ratsThe Journal of Neuroscience 31:16410–16422.https://doi.org/10.1523/JNEUROSCI.3280-11.2011
-
Hypoxia silences retrotrapezoid nucleus respiratory chemoreceptors via alkalosisThe Journal of Neuroscience 35:527–543.https://doi.org/10.1523/JNEUROSCI.2923-14.2015
-
Congenital central hypoventilation syndrome: PHOX2B mutations and phenotypeAmerican Journal of Respiratory and Critical Care Medicine 174:1139–1144.https://doi.org/10.1164/rccm.200602-305OC
-
Mapping of the excitatory, inhibitory, and modulatory afferent projections to the anatomically defined active expiratory oscillator in adult male ratsThe Journal of Comparative Neurology 529:853–884.https://doi.org/10.1002/cne.24984
-
Phox2 genes - from patterning to connectivityCurrent Opinion in Genetics & Development 12:435–440.https://doi.org/10.1016/s0959-437x(02)00322-2
-
Etonogestrel administration reduces the expression of PHOX2B and its target genes in the solitary tract nucleusInternational Journal of Molecular Sciences 23:4816.https://doi.org/10.3390/ijms23094816
-
BookUse of the Pdzlling Oxygen Analyzer for Memwement of Oxygen Conszlm~Tion of Ahmds Iti Open-Circuit Systems Ad in a Short-Lag, Closed-Cirwit Appma TmlNational Research Laboratories.
-
Alanine expansions associated with congenital central hypoventilation syndrome impair PHOX2B Homeodomain-mediated dimerization and nuclear importThe Journal of Biological Chemistry 291:13375–13393.https://doi.org/10.1074/jbc.M115.679027
-
Research advances on therapeutic approaches to congenital central hypoventilation syndrome (CCHS)Frontiers in Neuroscience 14:615666.https://doi.org/10.3389/fnins.2020.615666
-
A barometric method for measuring ventilation in newborn infantsPediatrics 16:81–87.
-
Sleep-disordered breathing in newborn mice heterozygous for the transcription factor Phox2bAmerican Journal of Respiratory and Critical Care Medicine 172:238–243.https://doi.org/10.1164/rccm.200411-1528OC
-
Phox2b, congenital central hypoventilation syndrome and the control of respirationSeminars in Cell & Developmental Biology 21:814–822.https://doi.org/10.1016/j.semcdb.2010.07.006
-
Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory responseThe Journal of Neuroscience 25:1211–1218.https://doi.org/10.1523/JNEUROSCI.3763-04.2005
-
Proton detection and breathing regulation by the retrotrapezoid nucleusThe Journal of Physiology 594:1529–1551.https://doi.org/10.1113/JP271480
-
The retrotrapezoid nucleus: central chemoreceptor and regulator of breathing automaticityTrends in Neurosciences 42:807–824.https://doi.org/10.1016/j.tins.2019.09.002
-
Central nervous system distribution of the transcription factor Phox2b in the adult ratThe Journal of Comparative Neurology 503:627–641.https://doi.org/10.1002/cne.21409
-
Obstructive apneas in a mouse model of congenital central hypoventilation syndromeAmerican Journal of Respiratory and Critical Care Medicine 204:1200–1210.https://doi.org/10.1164/rccm.202104-0887OC
-
Metabolism and ventilation in hypoxic rats: effect of body massRespiration Physiology 97:225–234.https://doi.org/10.1016/0034-5687(94)90028-0
-
On the barometric method for measurements of ventilation, and its use in small animalsCanadian Journal of Physiology and Pharmacology 76:937–944.https://doi.org/10.1139/cjpp-76-10-11-937
-
Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expressionThe Journal of Clinical Investigation 122:3145–3158.https://doi.org/10.1172/JCI63401
-
Dysregulation of locus coeruleus development in congenital central hypoventilation syndromeActa Neuropathologica 130:171–183.https://doi.org/10.1007/s00401-015-1441-0
-
Breathing without CO(2) chemosensitivity in conditional Phox2b mutantsThe Journal of Neuroscience 31:12880–12888.https://doi.org/10.1523/JNEUROSCI.1721-11.2011
-
Continuous circadian measurements of ventilation in behaving adult ratsRespiration Physiology 120:179–183.https://doi.org/10.1016/s0034-5687(00)00108-0
-
Neuromedin B expression defines the mouse retrotrapezoid nucleusThe Journal of Neuroscience 37:11744–11757.https://doi.org/10.1523/JNEUROSCI.2055-17.2017
-
Genotype-phenotype relationship in Japanese patients with congenital central hypoventilation syndromeJournal of Human Genetics 60:473–477.https://doi.org/10.1038/jhg.2015.65
-
Breathing regulation and blood gas homeostasis after near complete lesions of the retrotrapezoid nucleus in adult ratsThe Journal of Physiology 596:2521–2545.https://doi.org/10.1113/JP275866
-
Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult ratThe Journal of Neuroscience 26:10305–10314.https://doi.org/10.1523/JNEUROSCI.2917-06.2006
-
Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in ratsThe Journal of Physiology 586:2975–2991.https://doi.org/10.1113/jphysiol.2008.153163
-
Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2bAmerican Journal of Medical Genetics. Part A 123A:267–278.https://doi.org/10.1002/ajmg.a.20527
-
Hypoxic response in newborn rat is attenuated by neurokinin-1 receptor blockadeRespiratory Physiology & Neurobiology 140:19–31.https://doi.org/10.1016/j.resp.2004.01.008
Article and author information
Author details
Funding
Canadian Institutes of Health Research (Project Scheme Grant 486764)
- Silvia Pagliardini
Women and Children Health Research Institute (Innovation Grant)
- Silvia Pagliardini
Canadian Lung Association (Post-doctoral Fellowship)
- Silvia Cardani
Canadian Institutes of Health Research (Post-doctoral Fellowship)
- Tara A Janes
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
SC is supported by the Canadian Lung Association BaO Fellowship. TAJ is supported by the Canadian Institutes for Health Research (CIHR) Postdoctoral Fellowships. SP is supported by a Women and Children’s Health Research Institute Innovation Grant and CIHR Project scheme grant.
Confocal images were acquired at the University of Alberta Faculty of Medicine & Dentistry Cell Imaging Core, RRID:SCR_019200, which receives financial support from the Faculty of Medicine & Dentistry, the University Hospital Foundation, Striving for Pandemic Preparedness – The Alberta Research Consortium, and Canada Foundation for Innovation (CFI) awards to contributing investigators
Ethics
Experiments were performed using male Sprague-Dawley rats housed at the University of Alberta Health Sciences Animal Housing Facility. Handling and experimental procedures were approved by the Health Science Animal Policy and Welfare Committee at the University of Alberta (AUP#461) and performed in accordance with guidelines established by the Canadian Council on Animal Care.
Version history
- Sent for peer review: December 13, 2023
- Preprint posted: December 14, 2023 (view preprint)
- Preprint posted: February 14, 2024 (view preprint)
- Preprint posted: April 11, 2024 (view preprint)
- Version of Record published: May 10, 2024 (version 1)
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.94653. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2024, Cardani, Janes et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 256
- views
-
- 19
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
- Neuroscience
In most mammals, conspecific chemosensory communication relies on semiochemical release within complex bodily secretions and subsequent stimulus detection by the vomeronasal organ (VNO). Urine, a rich source of ethologically relevant chemosignals, conveys detailed information about sex, social hierarchy, health, and reproductive state, which becomes accessible to a conspecific via vomeronasal sampling. So far, however, numerous aspects of social chemosignaling along the vomeronasal pathway remain unclear. Moreover, since virtually all research on vomeronasal physiology is based on secretions derived from inbred laboratory mice, it remains uncertain whether such stimuli provide a true representation of potentially more relevant cues found in the wild. Here, we combine a robust low-noise VNO activity assay with comparative molecular profiling of sex- and strain-specific mouse urine samples from two inbred laboratory strains as well as from wild mice. With comprehensive molecular portraits of these secretions, VNO activity analysis now enables us to (i) assess whether and, if so, how much sex/strain-selective ‘raw’ chemical information in urine is accessible via vomeronasal sampling; (ii) identify which chemicals exhibit sufficient discriminatory power to signal an animal’s sex, strain, or both; (iii) determine the extent to which wild mouse secretions are unique; and (iv) analyze whether vomeronasal response profiles differ between strains. We report both sex- and, in particular, strain-selective VNO representations of chemical information. Within the urinary ‘secretome’, both volatile compounds and proteins exhibit sufficient discriminative power to provide sex- and strain-specific molecular fingerprints. While total protein amount is substantially enriched in male urine, females secrete a larger variety at overall comparatively low concentrations. Surprisingly, the molecular spectrum of wild mouse urine does not dramatically exceed that of inbred strains. Finally, vomeronasal response profiles differ between C57BL/6 and BALB/c animals, with particularly disparate representations of female semiochemicals.
-
- Neuroscience
Midbrain dopamine neurons impact neural processing in the prefrontal cortex (PFC) through mesocortical projections. However, the signals conveyed by dopamine projections to the PFC remain unclear, particularly at the single-axon level. Here, we investigated dopaminergic axonal activity in the medial PFC (mPFC) during reward and aversive processing. By optimizing microprism-mediated two-photon calcium imaging of dopamine axon terminals, we found diverse activity in dopamine axons responsive to both reward and aversive stimuli. Some axons exhibited a preference for reward, while others favored aversive stimuli, and there was a strong bias for the latter at the population level. Long-term longitudinal imaging revealed that the preference was maintained in reward- and aversive-preferring axons throughout classical conditioning in which rewarding and aversive stimuli were paired with preceding auditory cues. However, as mice learned to discriminate reward or aversive cues, a cue activity preference gradually developed only in aversive-preferring axons. We inferred the trial-by-trial cue discrimination based on machine learning using anticipatory licking or facial expressions, and found that successful discrimination was accompanied by sharper selectivity for the aversive cue in aversive-preferring axons. Our findings indicate that a group of mesocortical dopamine axons encodes aversive-related signals, which are modulated by both classical conditioning across days and trial-by-trial discrimination within a day.






