The Ki-67 and RepoMan mitotic phosphatases assemble via an identical, yet novel mechanism
Figures
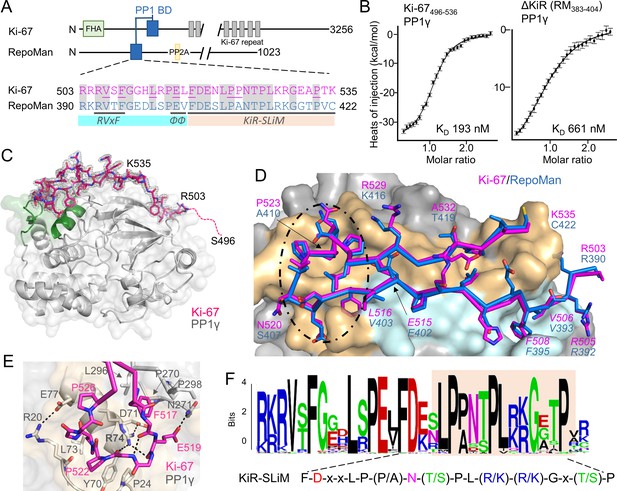
The RepoMan:PP1 holoenzyme complex.
(A) Cartoon depicting Ki-67 and RepoMan domains. The only region of homology between the two proteins is indicated in blue. The sequences corresponding to the homologous regions are shown below, with conserved residues highlighted in grey. Ki-67 residues that interact directly with PP1 are underlined. The sequences corresponding to the RVxF and ΦΦ SLiMs (blue highlight) and the newly discovered KiR-SLiM (orange) are shown. (B) left, binding isotherm of Ki-67496-536 with PP1γ7–323 (KD, 193 ± 16 nM; the KD of the corresponding domain of RepoMan383-423 with PP1γ7–323 is 133 ± 16 nM); right, binding isotherm of the ΔKiR-SLIM, RepoMan383-404, with PP1γ7–323 (KD, 661 ± 160 nM). (C) Crystal structure of the Ki-67:PP1γ holoenzyme. PP1γ is in grey and Ki-67496-536 is in pink with the 2Fo–Fc electron density map contoured at 1σ (2.0 Å); no electron density was observed for Ki-67 residues 496–503 (pink dotted line) and 536. PP1 residues in green correspond to PP1 secondary structure elements helix A’, loop 1 and helix B. (D) Close-up of the Ki-67 (pink) and RepoMan (blue) interaction with PP1. Ki-67 residues 503–516 and RepoMan residues 390–403 bind the PP1 RVxF and ΦΦ binding pockets (cyan surface; the Ki-67 and RepoMan RVxF and ΦΦ SLiM residues are labeled and in italics). Ki-67 residues 517–535 and RepoMan residues 404–422 bind the newly defined KiR-SLiM binding pocket (beige surface). The black dotted line highlights the area shown in E. (E) The hydrophobic and polar interactions between Ki-67 (pink sticks) and PP1γ (grey sticks; surface). Hydrogen bonds and salt bridge interactions are indicated by dotted lines with the interacting residues labeled. (F) HMMER-derived sequence logo of the Ki-67/RepoMan PP1 binding domain, with the KiR-SLIM highlighted in beige (hydrophobic residues, black; acidic residues, red; basic residues blue; glycine/serine/threonine, green; asparagine/glutamine, pink).
-
Figure 1—source data 1
Data collection and refinement statistics.
- https://doi.org/10.7554/eLife.16539.003
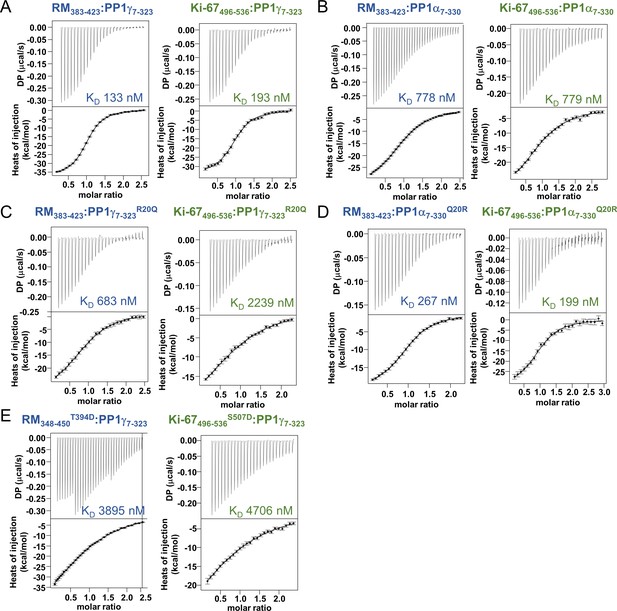
Isothermal titration calorimetry of Ki-67 and RepoMan with PP1.
(A) Ki-67496-536:PP1γ7–323 and RepoMan383-423:PP1γ7–323; (B) Ki-67496-536:PP1α7–330 and RepoMan383-423:PP1α7–330; (C) Ki-67496-536:PP1γ7-323R20Q and RepoMan383-423:PP1γ7-323R20Q; (D) Ki-67496-536:PP1α7-330Q20R and RepoMan383-423:PP1α7-330Q20R; (E), Ki-67496-536:PP1γ7-323S507D and RepoMan348-450T394D: PP1γ7–323. All measurements n = 2–4.
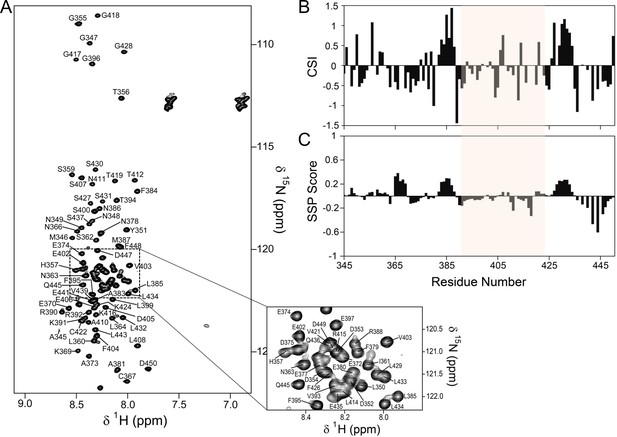
The PP1 binding domain of RepoMan is an intrinsically disordered protein (IDP).
(A) The 2D [1H,15N] HSQC spectrum of RepoMan348-450 (0.25 mM; 20 mM Tris-acetate pH 6.5, 150 mM NaCl, 0.5 mM TCEP, 10% D2O; 298 K; 500 MHz 1H Larmor). The sequence specific resonance assignments of RepoMan are annotated (~93% of backbone HN are assigned). The lack of HN chemical shift dispersion demonstrates the lack of a hydrogen bond network in secondary structure elements and shows that RepoMan is intrinsically disordered. (B) RepoMan chemical shift index (CSI; [Cαexp-Cαref]–[Cβexp-Cβref]). (C) CSI translated into a secondary structure propensity (SSP) indicates lowly populated secondary structures (SSP > 0 for α-helix and SSP < 0 for β-sheet; RefDB database; 5 residue averaging). The residues that correspond to the minimal PP1 binding domain of RepoMan (390–422) are highlighted in beige in (B) and (C).
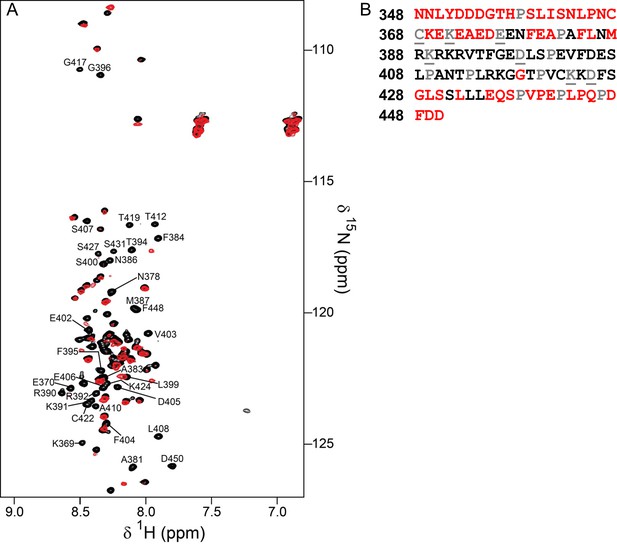
Identification of the RepoMan minimal PP1 binding domain.
(A) Overlay of the 2D [1H,15N] HSQC spectra of RepoMan348-450 in the absence (black) and presence (red) of PP1α7–330. The disappearance of RepoMan peaks in the presence of PP1α indicates residues that are involved in the protein:protein interaction (peaks are annotated; ~0.1 mM holoenzyme concentration, 20 mM Tris-acetate pH 6.5, 150 mM NaCl, 0.5 mM TCEP, 10% D2O; 298 K; 500 MHz 1H Larmor). (B) RepoMan348-450 residues that disappear upon binding PP1 in shown in black (prolines are in grey; unassigned residues are in grey and underlined). The data shows that the RepoMan PP1-binding domain includes residues 383–435.
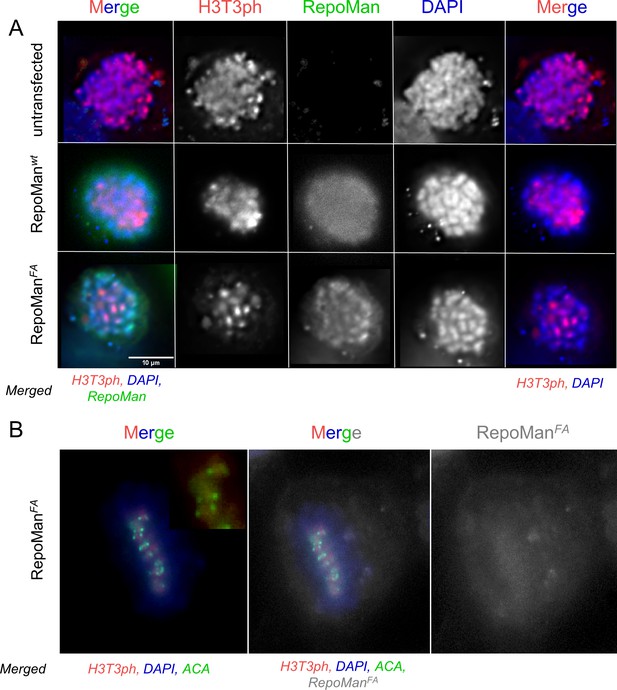
RepoMan F404A variant (RepoManFA) behaves like wt RepoMan (RepoManwt) in cells.
(A) HeLa cells were transfected with RepoMan Si Oligos and the oligo-resistant rescue GFP-RepoMan fusion constructs, as indicated. At 24 hr, the cells were fixed and immunostained for H3T3ph (red) and DAPI stained (blue). In RepoMan depleted cells (untransfected; top panels), the H3T3 phosphorylation signal is diffuse on all the chromosome arms. Both the RepoMan wt (middle panels) and the FA mutant (bottom panels) are able to restrict the phosphorylation to the centromeric region, thus rescuing the phenotype, suggesting that the complex functions normally at this stage of the cell cycle. Scale bar 10 μm. (B) Same experiment as in A showing a cell in mitosis transfected with RepoManFA (grey), stained for H3T3ph (red), DNA (DAPI), but now also stained with an anticentromeric antibody (ACA, green). The ACA antibody was a gift from Prof. Earnshaw (The University of Edinburgh).
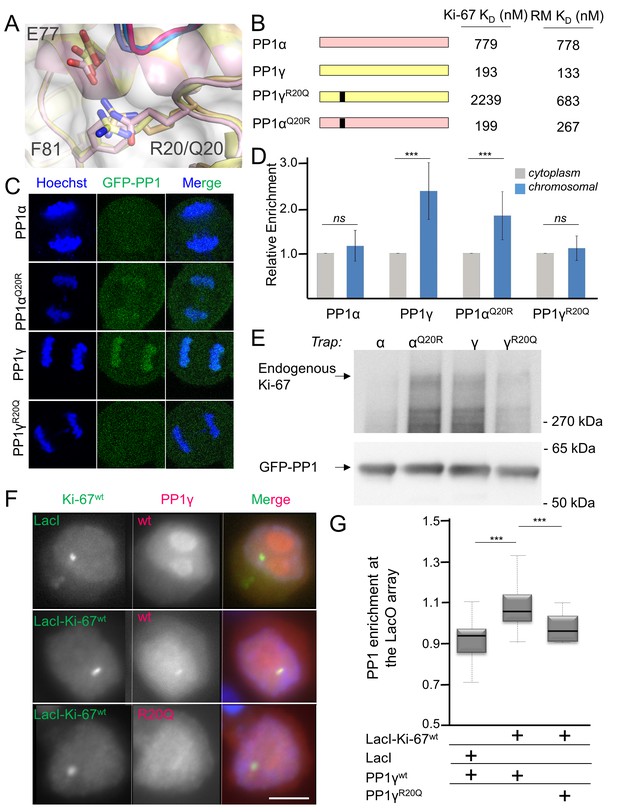
Ki-67/RepoMan isoform specificity is defined by PP1 residue 20.
(A) Overlay of PP1 from the Ki-67496-536:PP1γ7–308 (magenta:yellow), RepoMan383-423:PP1γ7–308 (blue:orange) and RepoMan383-423:PP1α7–300 (lavender:pink) complexes. R20 (PP1γ), Q20 (PP1α), E77 (PP1α/PP1γ) and F81 (PP1α/PP1γ) are shown as sticks. (B) Cartoon illustrating the PP1 variants generated for this study; colored as in A. The resulting KD values of Ki-67 and RepoMan titrated with the different PP1 variants are shown. (C) Subcellular distributions of GFP-PP1 fusions. HeLa cells were transfected with the indicated GFP-PP1 variants. The green fluorescence was visualized by confocal microscopy. Anaphase chromosomes were detected using Hoechst and live imaging. (D) Quantification of the relative enrichment of PP1 variants on chromosomes in C. Average and standard deviation are shown (T-test using Welch’s correction). (E) HEK293T cells were transfected with GFP-PP1 variants. The micrococcal-nuclease-treated cell lysates from nocodazole arrested cells were used for GFP trapping. The traps were processed using immunoblotting. (F) Chicken DT40 cells carrying a LacO array inserted in a single locus were transfected with GFP:LacI or GFP:LacI:Ki-67301-700wt constructs (green) and with RFP:PP1wt or RFP:PP1R20Q (red). GFP:LacI:Ki-67301-700wt and RFP:PP1wt both accumulate at the LacO array, however the RFP:PP1R20Q fails to accumulate together with GFP:LacI:Ki-67wt at the locus. Scale bar 5 μm. (G) Quantification of the enrichment of PP1 at the locus from the experiment in (F) (Mann-Whitney test) between: 1) GFP:LacI/RFP:PP1wt and GFP:LacI:Ki-67wt/RFP:PP1wt or 2) GFP:LacI:Ki-67wt/RFP:PP1wt and GFP:LacI:Ki-67wt/RFP:PP1R20Q.
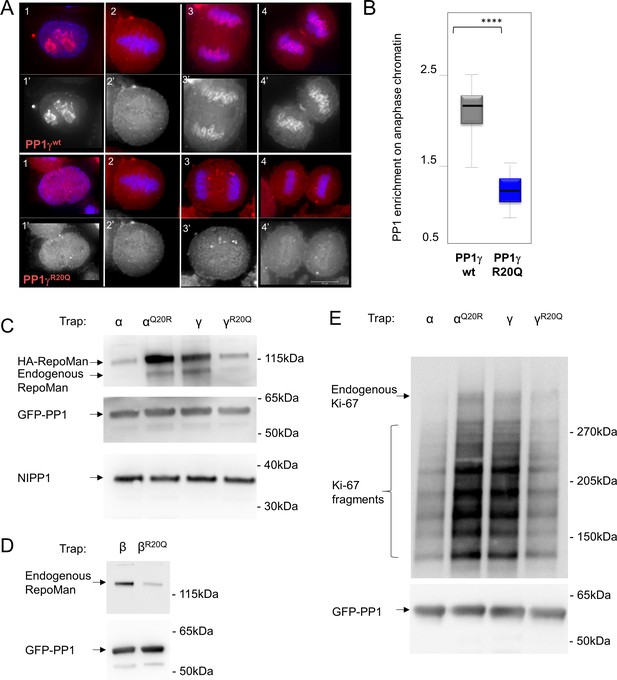
RepoMan and Ki-67 isoform specificity is defined by PP1 residue 20 throughout the cell cycle.
(A) HeLa cells were transfected with RFP-PP1γwt or RFP-PP1γR20Q (red) and cells at different stages of the cell cycle were analyzed. In interphase (1–1’), the PP1γR20Q mutant is still nuclear but does not accumulate in nucleoli; in metaphase (2–2’) both constructs show the same enrichment on the mitotic spindle; in anaphase and telophase PP1γR20Q fails to accumulate on the chromosomes (3–3’, 4–4’). Scale bar 10 μm. (B) Quantification of PP1 enrichment on the anaphase chromatin from the experiment in A. (C) HEK293T cells were co-transfected with GFP-PP1 variants and HA-RepoMan. The micrococcal-nuclease-treated cell lysates from nocodazole arrested cells were used for GFP trapping. The traps were processed using immunoblotting. (D) HEK293T cells were transfected with GFP-PP1β variants. The micrococcal-nuclease-treated cell lysates from nocodazole arrested cells were used for GFP trapping. The traps were processed using immunoblotting. (E) HEK293T cells were transfected with GFP-PP1 variants. The micrococcal-nuclease-treated cell lysates from nocodazole arrested cells were used for GFP trapping. The traps were processed using immunoblotting. This is the full blot of that shown in Figure 2E.
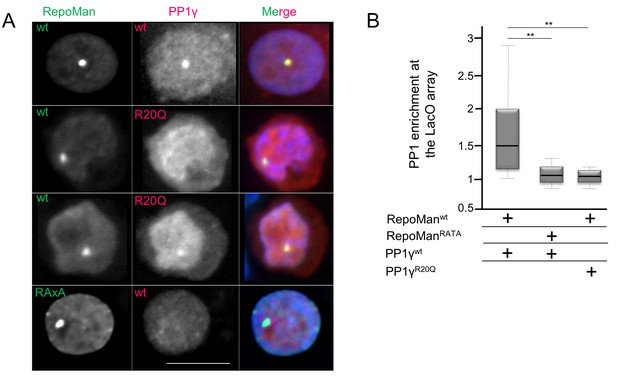
PP1 residue 20 is also critical for tethering by RepoMan.
(A) Chicken DT40 cells carrying a LacO array inserted in a single locus were transfected with GFP:LacI:RepoManwt or GFP:LacI:RepoManRATA constructs (green) and with RFP:PP1wt or RFP:PP1R20Q (red). GFP:Laci:RepoManwt and RFP:PP1wt both accumulate at the LacO array, however the RFP:PP1R20Q fails to accumulate together with GFP:LacI:RepoManwt at the locus. Scale bar 10 μm. (B) Quantification of the enrichment of PP1 at the locus from the experiment in (A) (Mann-Whitney test).
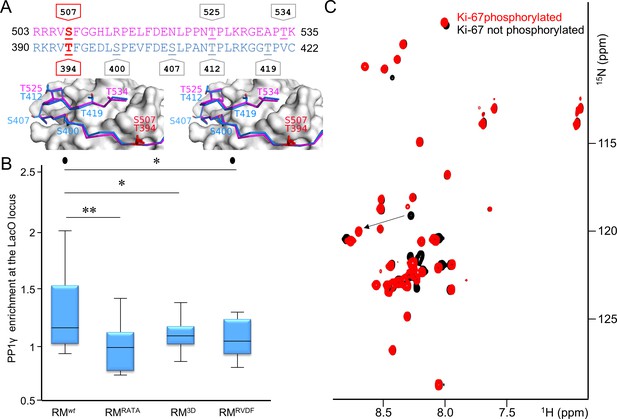
Aurora B kinase phosphorylates Ki-67 S507 and RepoMan T394 to inhibit holoenzyme formation.
(A) Stereo image of Ki-67 (magenta) and RepoMan (blue) bound to PP1 (grey) with serine and threonine residues shown as sticks and labeled. The ‘x’ residues of the RVxF motifs, S507Ki-67 and T394RM, are shown and highlighted in red. (B) Chicken DT40 cells carrying a LacO array inserted in a single locus were transfected with different GFP:LacI:RepoMan constructs (green) and with RFP:PP1 (red) and the enrichment of PP1 at the locus was calculated. Both the CDK-1 (3D) and the Aurora B (RVDF) phosphomimetic mutants cause a significant decrease in PP1 accumulation at the locus although less pronounced than the RATA mutant (Mann-Whitney test). (C) Overlay of the 2D [1H,15N] HSQC spectrum of Ki-67496-536(black) and Aurora B kinase phosphorylated Ki-67496-536(red). Peaks that correspond to S507 (black) and phosphorylated pS507 (red) are shown by an arrow.
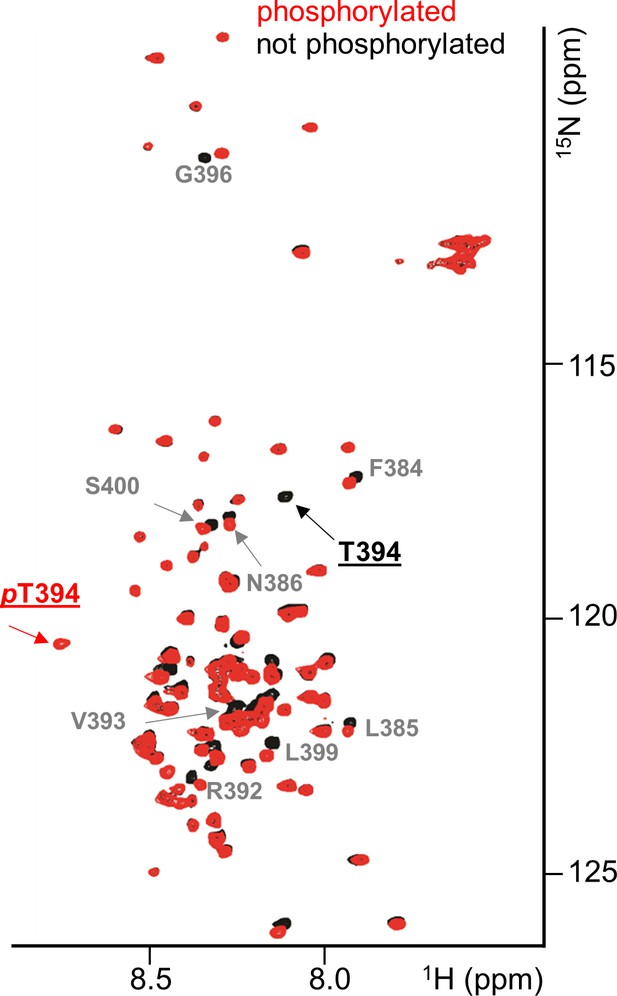
RepoMan RVxF residue T394 is specifically phosphorylated by Aurora B kinase.
Overlay of the 2D [1H,15N] HSQC spectrum of RepoMan348-450(black) and Aurora B kinase phosphorylated RepoMan348-450 (red). Residues with chemical shift perturbations upon Aurora B kinase phosphorylation are labeled. Peaks that correspond to T394 (black) and phosphorylated pT394 (red) are underlined.
Tables
Isothermal titration calorimetry (ITC) measurements.
| Titrant | PP1 | KD (nM) | repeats |
|---|---|---|---|
| Ki-67496–536 | |||
| wt | α7-330 | 779 ± 142 | 3 |
| wt | γ7-323 | 193 ± 16 | 2 |
| wt | α7-330Q20R | 199 ± 46 | 3 |
| wt | γ7-323R20Q | 2239 ± 124 | 3 |
| S507D | γ7-323 | 4706 ± 228 | 2 |
| RepoMan303–515 | |||
| wt | γ7-323 | 77 ± 12 | 4 |
| RepoMan348–450 | |||
| wt | γ7-323 | 124 ± 6 | 4 |
| T394D | γ7-323 | 3895 ± 265 | 3 |
| RepoMan383–441 | |||
| wt | γ7-323 | 117 ± 10 | 3 |
| RepoMan383–423 | |||
| wt | α7-330 | 778 ± 65 | 4 |
| wt | γ7-323 | 133 ± 5 | 3 |
| wt | γ7-308 | 123 ± 24 | 4 |
| wt | α7-330Q20R | 267 ± 28 | 3 |
| wt | γ7-323R20Q | 683 ± 91 | 3 |
| wt | α7-330Q20R/R23K | 232 ± 26 | 4 |
| RepoMan383–404 | |||
| wt | γ7-323 | 661 ± 160 | 3 |





