Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2
Figures
Dorsal versus ventral DG.
Areas of dorsal (A) and ventral (B) dentate gyrus used for cell proliferation quantification are highlighted in red. Images are adapted from brainatlas.org.
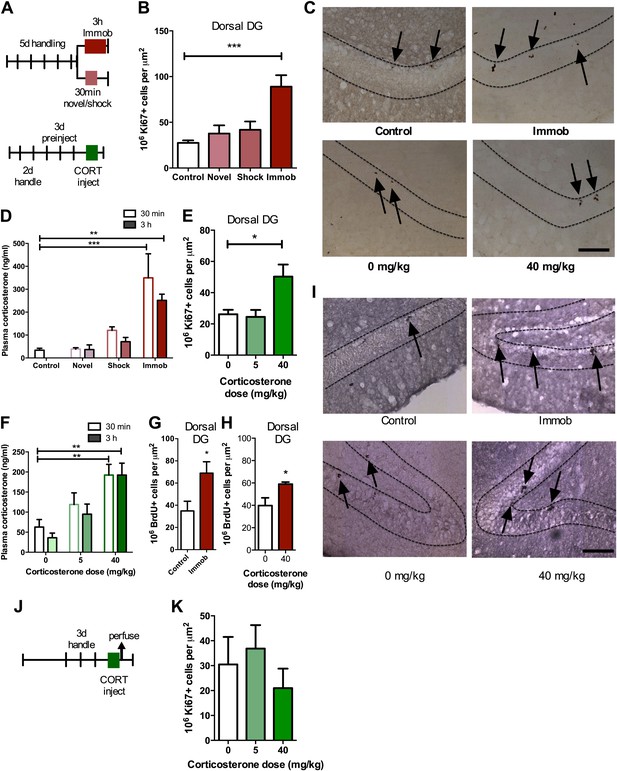
Acute stress increases adult cell proliferation in dorsal hippocampus.
(A) Experimental timeline. (B) Acute immobilization increased Ki67+ cell count in the adult dorsal DG while exposure to novel environment or footshock did not significantly change Ki67+ cell count. One-way ANOVA, p<0.0001; ***q = 5.975, p<0.0001. (C) Representative images of Ki67+ cells (black arrows) in the dorsal DG (dashed outline) of control, immobilized, 0 mg/kg and 40 mg/kg CORT-injected rats. (D) Acute immobilization increased plasma CORT levels 30 min and 3 hr after the stressor began. CORT elevations caused by novel environment and footshock were not significant. One-way ANOVA, p<0.0001; ***q = 5.56, p<0.0001; **q = 4.02, p<0.001. (E) Acute injection of 40 mg/kg CORT increased Ki67+ cell count in the adult dorsal DG compared to 0 mg/kg oil control while 5 mg/kg CORT did not significantly alter Ki67+ cell count. One-way ANOVA, p=0.007. *q = 3.15, p<0.05. (F) 40 mg/kg CORT injection led to a sustained increase in plasma CORT 30 min and 3 hr after injection. The change in plasma CORT following 5 mg/kg CORT injection was not significantly different from oil injection. Two-way ANOVA, effect of CORT dose p<0.0001. **q = 3.62 and 3.61, p<0.001, 40 mg/kg 30 min and 3 hr, respectively. (G) The number of BrdU-labeled newborn cells surviving 24 hr after the end of immobilization was greater in immobilized rats than controls. *p=0.03 (H) the number of BrdU-labeled newborn cells surviving 24 hr after CORT/oil injection was greater in rats given 40 mg/kg CORT compared to 0 mg/kg CORT. *p=0.04. (I) Representative images of BrdU+ cells (black arrows) in the dorsal DG (dashed outline) of control, immobilized, 0 mg/kg and 40 mg/kg CORT-injected rats. (J) Experimental timeline. Rats were handled for 3 days, injected with CORT or oil vehicle then perfused 3 hr later. (K) No difference in Ki67+ cell number was found in the adult dorsal DG with increasing CORT dose. All values are average ± SEM. Scale bar is 100 μm.
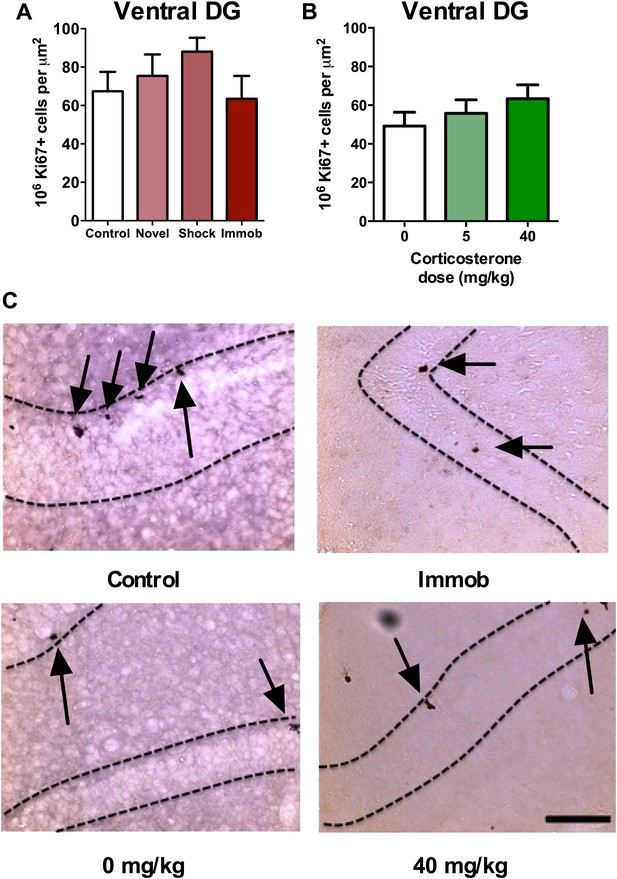
Acute stress does not increase adult cell proliferation in ventral hippocampus.
(A) None of the stressors affected Ki67+ cell count in the ventral DG. (B) CORT did not affect Ki67+ cell count in the ventral DG. (C) Representative images of Ki67+ cells (black arrows) in the ventral DG (dashed outline) of control, immobilized, 0 mg/kg and 40 mg/KG CORT-injected rats. All values are average ± SEM. Scale bar is 100 μm.
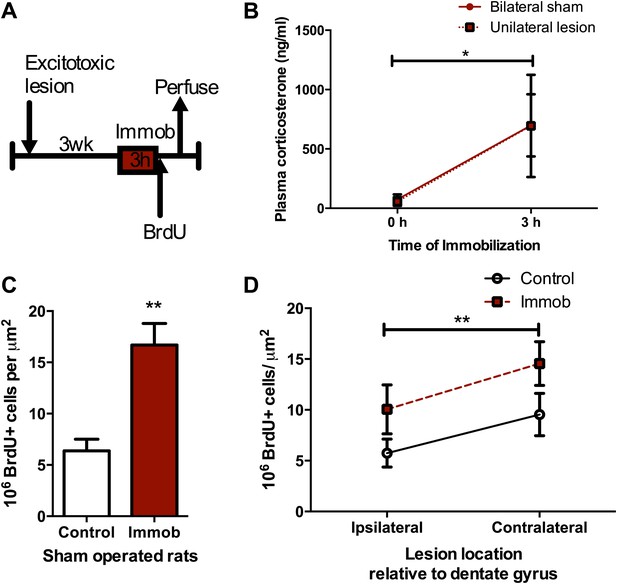
Acute stress increases cell proliferation independent of BLA input.
(A) Experimental timeline. (B) Plasma CORT elevation after immobilization was similar between sham-operated and unilaterally BLA-lesioned rats. Two-way ANOVA effect of time, *p=0.04. (C) In sham-operated rats, acute immobilization increased the number of BrdU+ cells in the adult DG. **p=0.001. (D) Unilateral excitotoxic lesion of the BLA decreased the number of BrdU+ cells in the ipsilateral DG, but did not interact with stress. Two-way ANOVA, effect of lesion, **p=0.002. All values are average ± SEM.
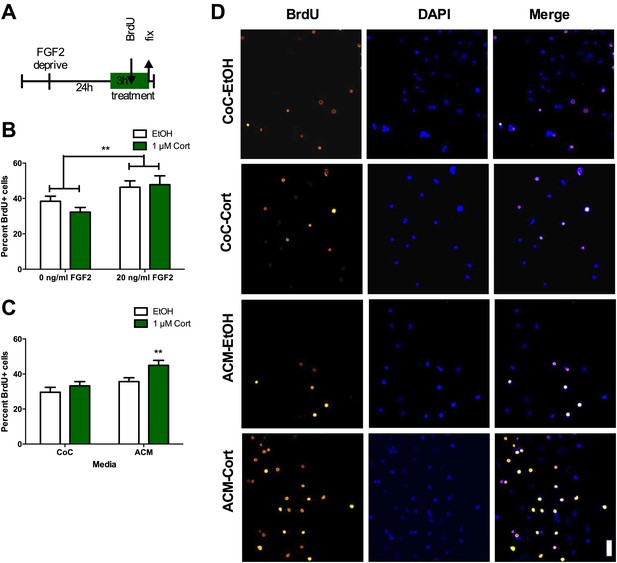
ACM from CORT-treated astrocytes increases NPC proliferation.
(A) Experimental timeline. (B) Treatment of isolated hippocampal NPCs with 1 µM CORT for 3 hr did not alter the percent of proliferating BrdU+ cells compared to EtOH vehicle. 3 hr of treatment with 20 ng/ml human recombinant FGF2 increased the percent of proliferative BrdU+ cells. Two-way ANOVA, effect of FGF2, **p=0.005. (C) ACM was extracted from astrocytes treated with 1 µM CORT or EtOH vehicle. Treatment of NPCs with ACM from CORT-treated astrocytes increased the percent of proliferative BrdU+ cells compared to EtOH, CoC-treated control NPCs. Two-way ANOVA, effect of CORT, p=0.02; effect of media, p=0.0024. **q = 4.23, p<0.001 vs EtOH-CoC. (D) Representative images of NPCs treated with CoC or ACM, EtOH or CORT. BrdU+ cells are orange and DAPI is blue. All values are average ± SEM. Scale bar is 10 μm.
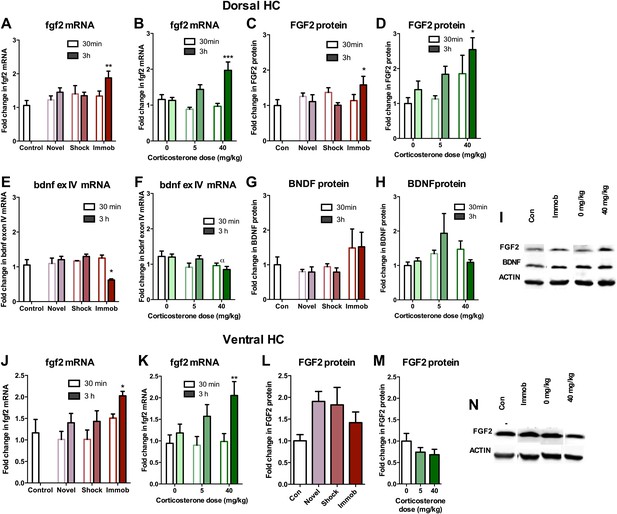
Acute stress increases FGF2 expression in dorsal hippocampus.
(A) 3 hr of immobilization increased fgf2 mRNA expression over control in dorsal hippocampus. Other groups did not significantly differ from control. One-way ANOVA, p=0.05. **q = 3.54, p<0.01. (B) 40 mg/kg CORT increased fgf2 mRNA expression in the dorsal hippocampus 3 hr after CORT injection compared to 30 min after 0 mg/kg CORT injection. Other groups did not significantly differ from oil-injected controls. Two-way ANOVA, effect of CORT, p=0.03; effect of time, p<0.0001; interaction, p=0.0021. ***q = 4.34, p<0.001. (C) FGF2 protein levels in dorsal hippocampus increased with 3 hr of immobilization over control. Other groups did not significantly differ from control. One-way ANOVA, p>0.05. *q = 2.79, p<0.05. (D) FGF2 protein levels in dorsal hippocampus increased 3 hr after 40 mg/kg CORT injection compared to 30 min after 0 mg/kg vehicle injection. Two-way ANOVA, effect of CORT, p=0.01; effect of time, p=0.03. *q = 3.18, p<0.05. (E) 3 hr of immobilization decreased bdnf exon IV expression over control in dorsal hippocampus. Other groups did not significantly differ from controls. One-way ANOVA, p=0.0007. *q = 3.05, p<0.05. (F) There was an overall significant decrease in bdnf exon IV mRNA expression with increasing CORT dose in dorsal hippocampus. Two-way ANOVA, effect of CORT, *p=0.02. (G) BDNF protein levels in dorsal hippocampus did not change with immobilization, novel environment or shock compared to control. (H) BDNF protein levels did not change compared to 0 mg/kg vehicle with increasing CORT dose. (I) Representative western bands of FGF2, BDNF and ACTIN from the 3 hr time point in dorsal hippocampus. (J) 3 hr of immobilization increased fgf2 mRNA expression over control in ventral hippocampus. Other groups did not significantly differ from controls. One-way ANOVA, p=0.03. *q = 2.87, p<0.05. (K) 40 mg/kg CORT increased fgf2 mRNA expression in the ventral hippocampus 3 hr after CORT injection compared to 30 min after 0 mg/kg CORT injection. Other groups did not significantly differ from oil-injected controls. Two-way ANOVA, effect of time, p=0.002. **q = 3.34, p<0.01. (L) Immobilization, novel environment and shock did not alter FGF2 protein levels in ventral hippocampus. (M) CORT did not alter FGF2 protein levels in ventral hippocampus 3 hr after injection. (N) Representative western bands of FGF2 and ACTIN from the 3 hr time point in ventral hippocampus. All values are average ± SEM.
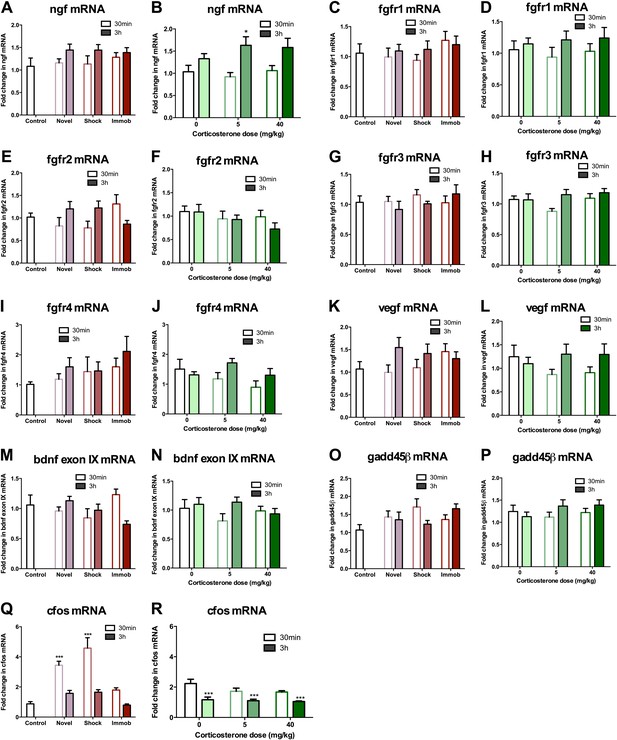
mRNA expression levels in dorsal hippocampus following acute stressors.
(A) There was no change in ngf mRNA with novel environment, shock or immobilization. (B) 5 mg/kg CORT significantly increased ngf mRNA at 3 hr over 0 mg/kg CORT, 30 min *q = 2.79, p<0.05. There was no change in fgfr1 (C, D), fgfr2 (E, F), fgfr3 (G, H), fgfr4 (I, J), vegf (K, L), bdnf exon IX (M, N) Or gadd45β (O, P) mRNA expression in dorsal hippocampus. (Q) Exposure to novel environment or footshock increased cfos expression in the dorsal hippocampus. One-way ANOVA, p<0.0001. ***q = 5.92 and q = 8.54, novel and shock, respectively, p<0.001. All values are average ± SEM. (R) All injection conditions showed a decrease in cfos mRNA over time. Two-way ANOVA, effect of time, p<0.0001. ***q = 4.42, 4.68, 4.91, 0 mg/kg 3 hr, 5 mg/kg 3 hr, 40 mg/kg 3 hr, respectively, p<0.001. All values are average ± SEM.
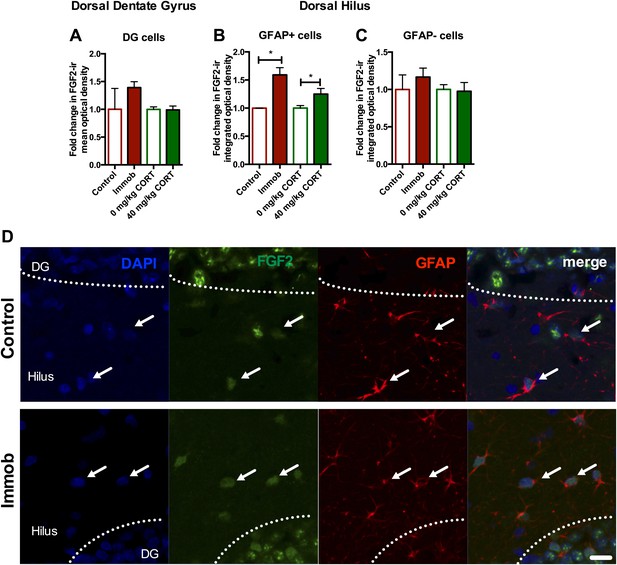
Acute stress increases FGF2 expression in GFAP+ astrocytes in the dorsal hilus.
(A) There was no change in mean optical density of FGF2-ir in the DG following 3 hr immobilization or CORT injection. (B) Both immobilization and 40 mg/kg CORT injection significantly increased integrated optical density of FGF2-ir in GFAP+ cells in the hilus. One-way ANOVA, p=0.0024. *p=0.04 and 0.05, con v immob and 0 V 40 mg/kg CORT, respectively. (C) FGF2-ir integrated optical density in GFAP- cells of the hilus did not change. (D) Representative images of FGF2+ cells (green) that are GFAP+ (red; white arrows) or GFAP- in DG and hilus of a control and an immobilized rat. DAPI is blue. Scale bar = 10 μm.
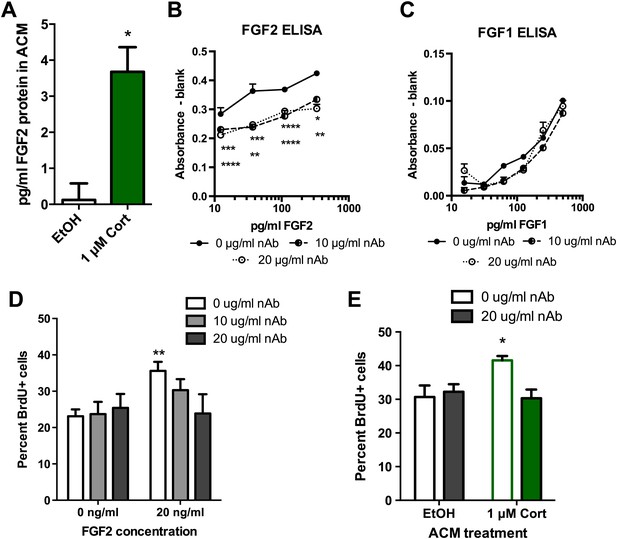
Blocking FGF2 prevents CORT-ACM induced increase in NPC proliferation.
(A) ACM from EtOH-treated primary astrocytes had no FGF2 protein (relative to blank) while CORT-treated ACM contained 3.5 pg/ml FGF2. *p=0.01. (B) Availability of rat FGF2 was dramatically reduced by pretreating FGF2 protein with an FGF2 neutralizing antibody. Two-way ANOVA, p<0.0001 main effects of nAb and FGF2 concentration. Post-hoc Dunnett's multiple comparison tests with 0 μg/ml as control shown for 10 μg/ml (upper row *s) and 20 μg/ml (lower row *s): *p<0.05, ** p<0.01, ***p<0.001, ****p<0.0001. (C) Availability of FGF1 was not affected by pretreating FGF1 protein with the same FGF2 neutralizing antibody. (D) Isolated NPCs were treated with the FGF2 nAb in either high (20 ng/ml) or low (0 ng/ml) FGF2 conditions. 20 μg/ml nAb effectively blocked the FGF2-induced increased in percent BrdU+ proliferating cells. Two-way ANOVA, effect of FGF2, p=0.04. **q = 3.37, p<0.01. The nAb did not affect proliferation in low FGF2 conditions. (E) Pretreatment with FGF2 nAb prevented the increase in percent BrdU+ proliferating NPCs caused by ACM from CORT-treated astrocytes. Two-way ANOVA, interaction, p=0.02. *q = 3.06, p<0.05. All values are average ± SEM.
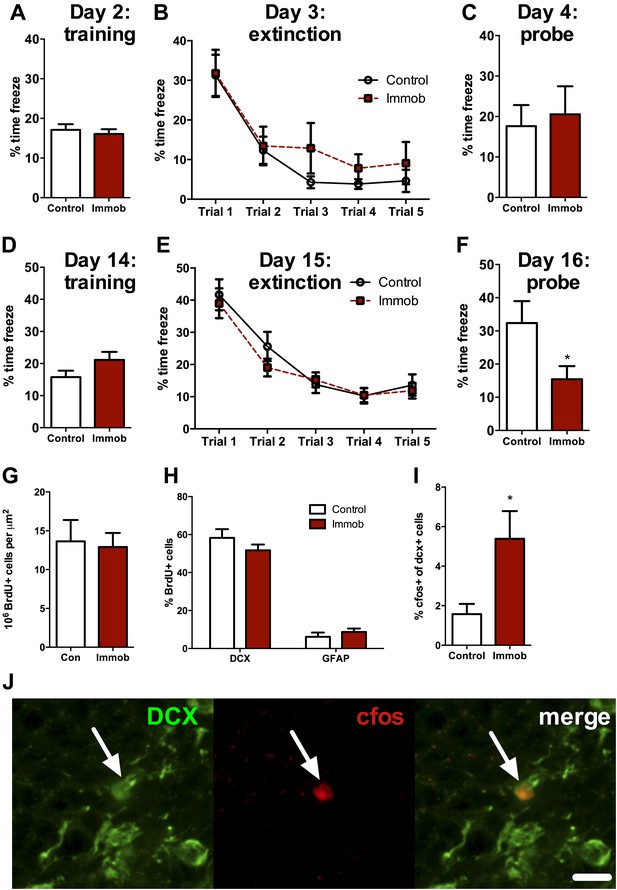
Acute stress causes delayed enhancement of contextual fear extinction retention.
Acute immobilization 2 days prior to contextual fear conditioning did not change percent time freezing during training (A), extinction (B) or 24-hr extinction probe (C) compared to control. Two-way ANOVA for extinction, effect of trial, p<0.0001. Acute immobilization 2 weeks prior to contextual fear conditioning did not change percent time freezing during training (D) or extinction (E) compared to control. Two-way ANOVA for extinction, effect of trial, p<0.0001. (F) Immobilized rats froze significantly less than controls in the 24-hr extinction probe. *p=0.04. (G) The number of surviving BrdU+ cells in the dorsal DG of immobilized rats did not differ from controls. (H) Immobilization does not alter the percent of BrdU+ cells co-expressing doublecortin (DCX) or glial fibrillary acidic protein (GFAP). (I) The percent of DCX+ immature neurons expressing cfos was greater in immobilized rats compared to controls. *p=0.02. All values are average ± SEM.
Tables
List of primers
| Gene | Direction | Sequence |
| bdnfexonIV | + | GGAGTGGAAAGGGTGAAACA |
| − | GGATTCAGTGGGACTCCAGA | |
| bdnfexonIX | + | GAGAAGAGTGATGACCATCCT |
| − | TCACGTGCTCAAAAGTGTCAG | |
| cfos | + | GGCAAAGTAGAGCAGCTATCTCCT |
| − | TCAGCTCCCTCCTCCGATTC | |
| fgf2 | + | CGGTACCTGGCTATGAAGGA |
| − | CTCCAGGCGTTCAAAGAAGA | |
| fgfr1 | + | ACCTGAGGCATTGTTTGACC |
| − | GTGAGCCACCCAGAGTGAAT | |
| fgfr2 | + | GGCCTCTCTGAATGCTAACG |
| − | ACGAGACAATCCTCCTGTGG | |
| fgfr3 | + | TCTGGTCCTTTGGTGTCCTC |
| − | TGAGGATGCGGTCTAAATCC | |
| fgfr4 | + | GTGGCTGTGAAGATGCTGAA |
| − | GAGGAATTCCCGAAGGTTTC | |
| gadd45β | + | GTCACCTCCGTCTTCTTGGA |
| − | GAGGCGGTGGGACTTACTTT | |
| ngf | + | GGACGCAGCTTTCTATCCTGG |
| − | CCCTCTGGGACATTGCTATCTG | |
| rplp | + | CCAAAGGTTTGGGAGAACAA |
| − | GGGTCATGGCATAGAGCAAT | |
| vegf | + | GAGGAAAGGGAAAGGGTCAAA |
| − | CACAGTGAACGCTCCAGGATT |





