Frizzled3 controls axonal development in distinct populations of cranial and spinal motor neurons
Figures
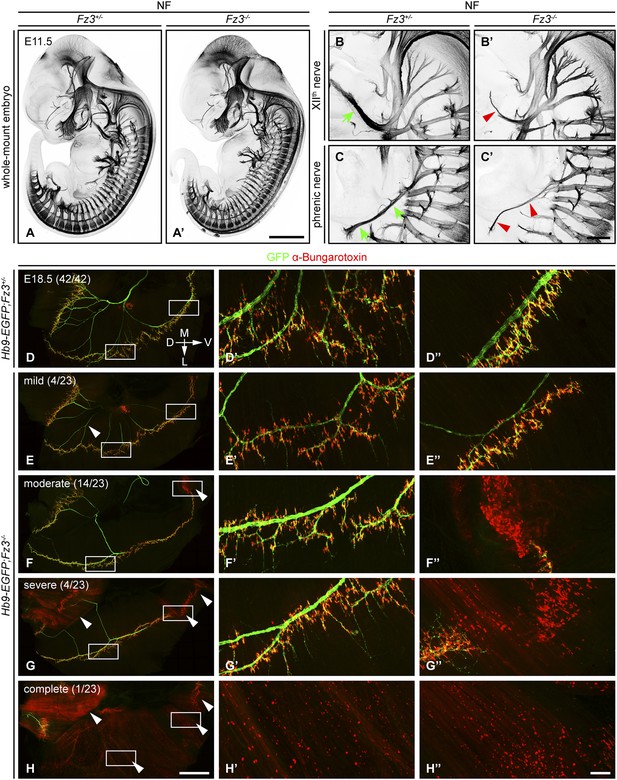
Defects in the XIIth cranial (hypoglossal) and phrenic nerves in Fz3−/− embryos.
(A–C′) Anti-NF immunostaining shows thinned XIIth and phrenic nerves (green arrows in [B] and [C] vs red arrowheads in [B′] and [C′]) in E11.5 Fz3−/− embryos. The nerve roots and the proximal segment of the XIIth nerve appear to be unaffected. Enlarged views of the phrenic and XIIth nerves in (B–C′) are maximum intensity projections from consecutive Z stacks. Scale bars: (A′), 1 mm; (B′) and (C′), 200 µm. (D–H′′) Flatmounts of the diaphragm show variably defective motor innervation in E18.5 Hb9-EGFP;Fz3−/− embryos. Panels (D–H) show half of a diaphragm with the midline at the top. (D′–H′) and (D′′–H′′) show enlargements of the lateral and ventral regions, respectively, boxed in (D–H). D, dorsal; V, ventral; M, medial; L, lateral. (E–H) show examples of mild, moderate, severe, and complete phenotypes, with the fraction of embryos in each class shown in parentheses. Scale bars: (H), 1 mm; (H′′), 100 µm.
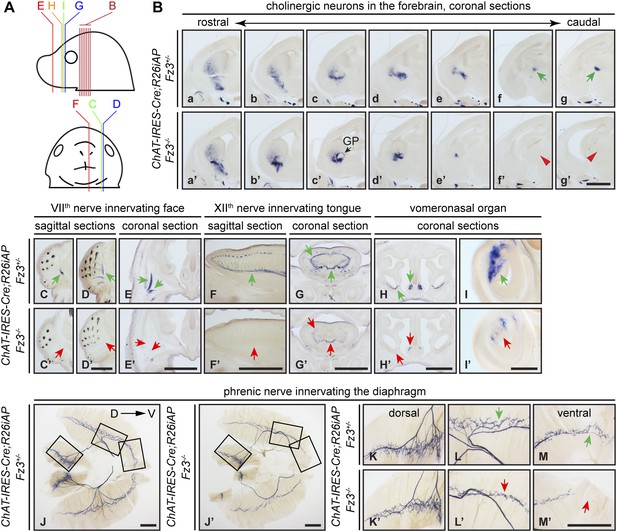
Diverse defects in cholinergic neurons shown by AP histochemisty in ChAT-IRES-Cre;R26iAP;Fz3−/− embryos.
(A) Diagram showing the planes of sections from E18.5 heads in (B–I′). (B) Forebrain cholinergic neurons in consecutive coronal sections. The major cholinergic fiber tract passing through the striatum is missing in the Fz3−/− forebrain [green arrows in [f] and [g] vs red arrowheads in [f′] and [g′]]. GP, globus pallidus. Scale bar, 1 mm. (C–E′) Innervation of facial muscles by the VIIth nerve in sagittal (C–D′) and coronal (E and E′) sections. In the Fz3−/− head, facial muscles are not innervated (green arrows in [C–E] vs red arrows in [C′–E′]). Scale bars, 1 mm. (F–G′) Innervation of tongue musculature by the XIIth nerve in sagittal (F and F′) and coronal (G and G′) tongue sections. In Fz3−/− embryos, the number of axons is reduced (green arrows in [F] and [G] vs red arrows in [F′] and [G′]). Scale bars, 1 mm. (H–I′) Cholinergic neurons in the vomeronasal organ in coronal head sections. In Fz3−/− embryos, these neurons are markedly reduced (green arrows in [H] and [I] vs red arrows in [H′] and [I′]). Scale bars: (H′), 1 mm; (I′), 200 µm. (J–M′) Innervation of the diaphragm by the phrenic nerve visualized by AP histochemistry on flat-mount diaphragms. (K–M), enlarged views of boxed regions in (J); (K′–M′), enlarged views of boxed regions in (J′). Branching is diminished and number of motor terminals is reduced in the moderately affected Fz3−/− diaphragm (Figure 1D–H). D, dorsal; V, ventral. Scale bars, 1 mm.
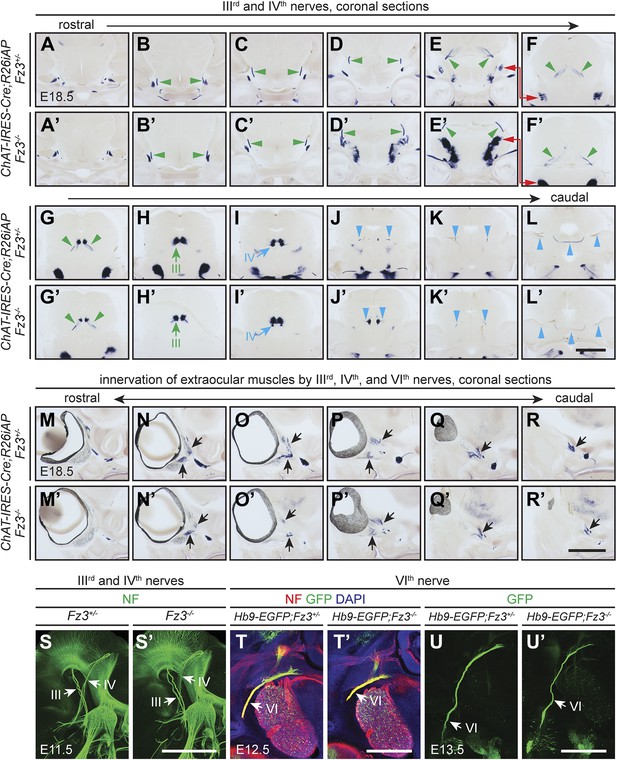
Oculomotor (IIIrd), trochlear (IVth), and abducens (VIth) nerves and their target innervation are not affected by loss of Fz3.
(A–L′) IIIrd and IVth nuclei and nerves visualized by AP staining on continuous coronal sections from E18.5 ChAT-IRES-Cre;R26iAP;Fz3+/− (A–L) and ChAT-IRES-Cre;R26iAP;Fz3−/− (A′–L′) mouse heads. Motor nuclei and axon tracts or nerves are indicated by arrows and arrowheads, respectively. Panels (E/E′) here match panels (a/a’) of Figure 2—figure supplement 2G. Panels (F/F′–K/K′) here match panels (b/b’-g/g’) of Figure 2—figure supplement 2G, but are vertically offset as indicated by the red arrows connecting panels (E/E′) and (F/F′). The locations of the sections in Figure 2—figure supplement 1 are shown schematically in Figure 2—figure supplement 2, panel (J). Scale bar, 1 mm. (M–R′) Innervation of extraocular muscles by IIIrd, IVth, and VIth nerves visualized by AP staining on continuous coronal sections from E18.5 ChAT-IRES-Cre;R26iAP;Fz3+/− (M–R) and ChAT-IRES-Cre;R26iAP;Fz3−/− (M′–R′) heads. Scale bar, 1 mm. (S and S′) IIIrd and IVth nerves visualized in lateral views of NF immunostained whole-mount E11.5 Fz3+/− (S) and Fz3−/− (S′) embryos, as shown in Figure 1A,A′. Scale bar, 500 µm. (T and T′) The VIth nerve visualized by NF and GFP immunostaining on 100 µm-thick horizontal sections from E12.5 Hb9-EGFP;Fz3+/− (T) and Hb9-EGFP;Fz3−/− (T′) heads. Scale bar, 500 µm. (U and U′) The VIth nerve visualized by GFP immunostaining on 700 µm-thick horizontal sections from E13.5 Hb9-EGFP;Fz3+/- (U) and Hb9-EGFP;Fz3−/− (U′) embryos. Scale bar, 500 µm.
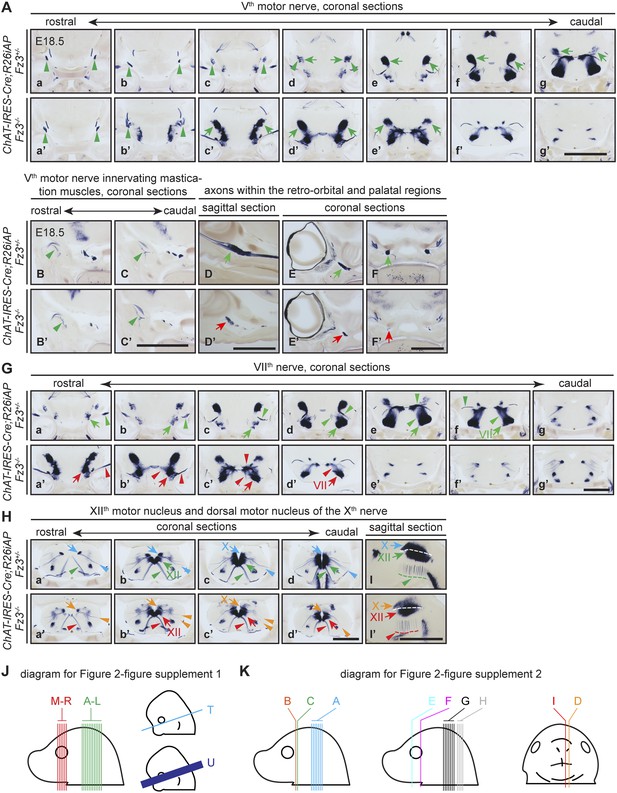
The Vth (trigeminal) motor nerve and its target innervation are not affected by loss of Fz3.
(A) The Vth motor nuclei and nerves visualized by AP staining on continuous coronal sections from E18.5 ChAT-IRES-Cre;R26iAP;Fz3+/− (a–g) and ChAT-IRES-Cre;R26iAP;Fz3−/− (a′–g′) mouse heads. Vth motor nuclei and axon tracts/nerves are indicated by arrows and arrowheads, respectively. Panels (c–g) and (c′–g′) here match panels (a–e) and (a′–e′), respectively, in (G), with a small vertical offset. Scale bar, 1 mm. (B–C′) Innervation of muscles of mastication by the Vth motor nerve visualized by AP staining on coronal sections from E18.5 ChAT-IRES-Cre;R26iAP;Fz3+/− (B and C) and ChAT-IRES-Cre;R26iAP;Fz3−/− (B′ and C′) mouse heads. Scale bar, 1 mm. (D–F′) Cholinergic axons within the retro-orbital and palatal regions in sagittal (D and D′) and coronal (E–F′) sections. In Fz3−/− embryos, the number of axons is reduced (green arrows in [D–F] vs red arrows in [D′–F′]). Scale bars, 1 mm. (G) The VIIth motor nucleus and nerve in consecutive coronal sections. The majority of Fz3−/− VIIth motor neurons fail to migrate cadually to rhombomere 6. Arrows, VIIth motor nuclei; arrowheads, VIIth motor axons. Scale bar, 1 mm. (H–I′) The dorsal motor nucleus of the Xth nerve (blue and orange arrows), the motor nucleus of the XIIth nerve (green and red arrows), and axons from these nuclei (arrowheads, same color scheme) in consecutive coronal (Ha–d′) and sagittal (I and I′) sections. Scale bars, 1 mm. (J and K) Diagrams showing the planes of sections in Figure 2—figure supplement 1 (J) at E18.5 (left) and E12.5 and E13.5 (right), and in Figure 2—figure supplement 2 (K).
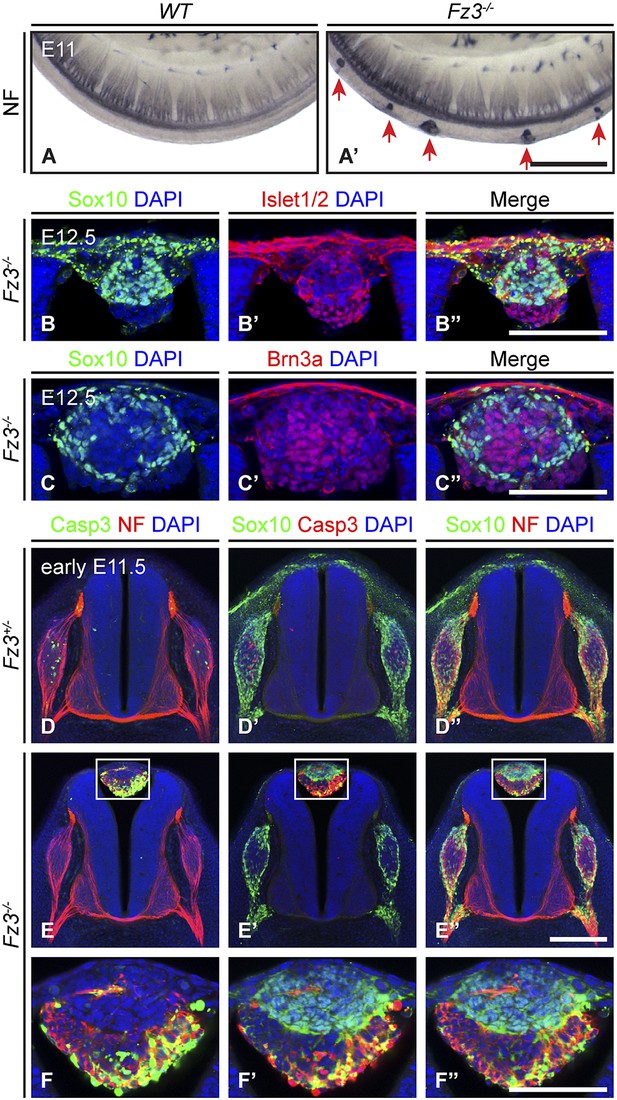
Neural crest cell migration defects in Fz3−/− mice.
(A and A′) NF immunostaining of whole-mount E11 embryos showing aberrant clusters of neurons along the dorsal edge of the caudal neural tube in Fz3−/− embryos. Scale bar, 500 µm. (B–C′′) Islet1/2, Brn3a, and Sox10 immunostaining in cross sections of the NF-rich clusters in Fz3−/− neural tubes at E12.5. Scale bars, 100 µm. (D–F′′) NF, cleaved Caspase3, and Sox10 immunostaining in cross sections of early E11.5 Fz3+/− (D–D′′) and Fz3−/− (E–E′′) spinal cords. The boxed regions in (E–E′′) are enlarged in (F–F′′). Scale bars: (E′′), 200 µm; (F′′), 100 µm.
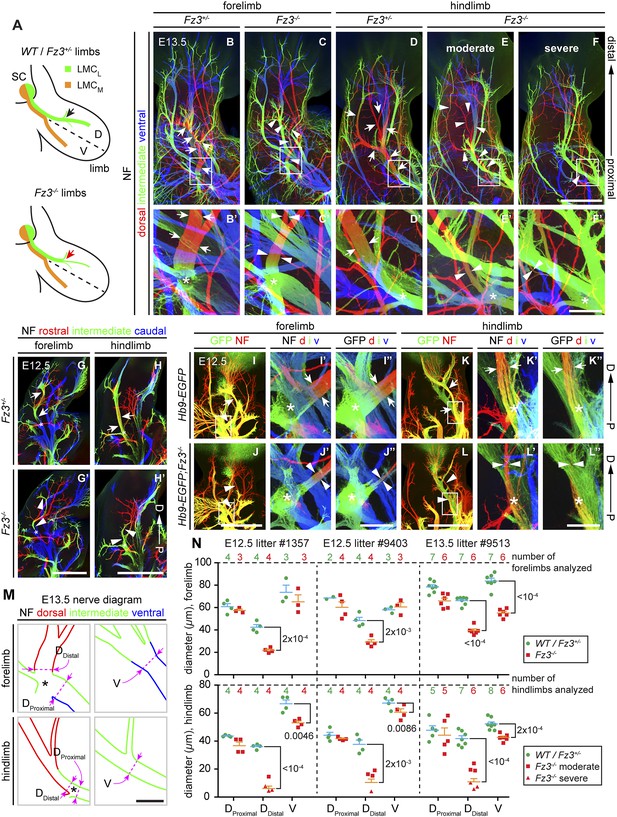
Thinning of the dorsal motor nerve in Fz3−/− limbs.
(A) Diagram showing dorsal (D) and ventral (V) limb innervation by spinal motor nerves in control (WT or Fz3+/−; top) and Fz3−/− (bottom) embryos. In Fz3−/− limbs, the dorsal nerve is thinned distal to the plexus at the base of the limb (highlighted by arrows). SC, spinal cord. (B–F′) NF immunostaining of whole-mount fore- and hindlimbs from E13.5 Fz3+/− and Fz3−/− embryos. (B′–F′), magnified view of boxed regions in (B–F). Arrows (control) and arrowheads (mutant) indicate dorsal nerves, which are moderately thinner in Fz3−/− forelimbs and substantially thinner in Fz3−/− hindlimbs. Asterisks indicate the points at which axons stall in Fz3−/− limbs. The red to blue color code represents depth within the Z-stack, oriented here along the dorsal to ventral axis. Scale bars: (B–F), 500 µm; (B′–F′), 100 µm. (G–H′) NF immunostaining of 1 mm-thick sections in the dorsoventral plane encompassing fore- or hindlimbs from E12.5 Fz3+/− and Fz3−/− embryos. Arrows (control) and arrowheads (Fz3−/−) indicate dorsal nerves, which are thinner in Fz3−/− limbs. Asterisks indicate the points at which axons stall in Fz3−/− limbs. Red to blue color code represents depth within the Z-stack, oriented here along the rostrocaudal axis. P, proximal; D, distal. Scale bars, 500 µm. (I–L′′) GFP (green) and NF (red) double immunostaining of whole-mount forelimbs and hindlimbs from E12.5 Hb9-EGFP;Fz3+/+ and Hb9-EGFP;Fz3−/− embryos (I–L). Motor axons (labeled with GFP) are selectively eliminated from the dorsal nerve in mutant limbs (arrowheads and arrows). (I′–L′) are enlarged views of the NF immunostaining in the boxed regions in (I–L), with color representing depth along the dorsoventral axis. Similarly, (I′′–L′′) are enlarged views of the GFP immunostaining. P, proximal; D, distal; d, dorsal; i, intermediate; v, ventral. Scale bars: (J) and (L), 500 µm; (J′′) and (L′′), 100 µm. (M) Diagrams showing the locations of nerve diameter measurements quantified in (N), traced from images (B′) and (D′). The locations of two dorsal nerve measurements (DDistal, dorsal nerve diameter measured immediately distal to the stalling point; DProximal, dorsal nerve diameter measured immediately proximal to the stalling point) and one ventral nerve measurement (V) are shown. Scale bar, 100 µm. (N) The diameters of motor nerves at the locations shown in (M) were measured from NF immunostained whole-mount forelimbs (top) and hindlimbs (bottom) from two litters at E12.5 and one litter at E13.5. Bars, mean ± SEM. p-values are shown for the indicated pair-wise comparisons (student’s t-test).
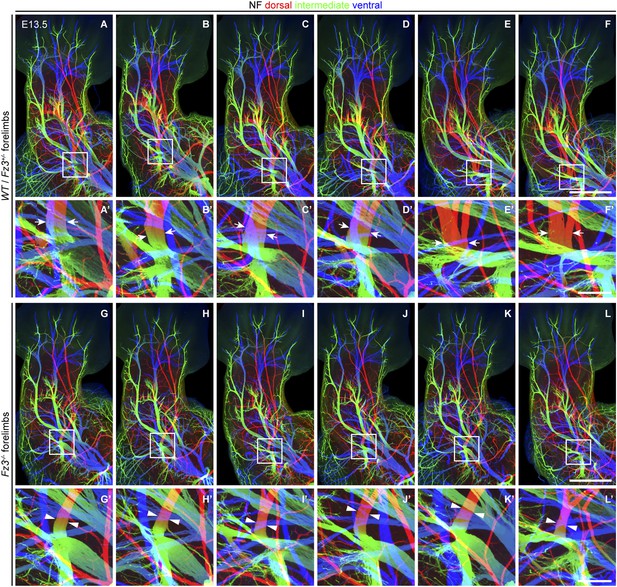
Thinning of spinal motor nerves innervating the dorsal forelimb.
(A–L′) NF immunostaining on whole-mount forelimbs from E13.5 WT or Fz3+/− (A–F) and Fz3−/− (G–L) embryos, with depth coded by colors as in Figure 4B–F′. (A′–L′) are magnified views of the boxed regions in (A–L). Scale bars: (F) and (L), 500 µm; (F′) and (L′), 100 µm.
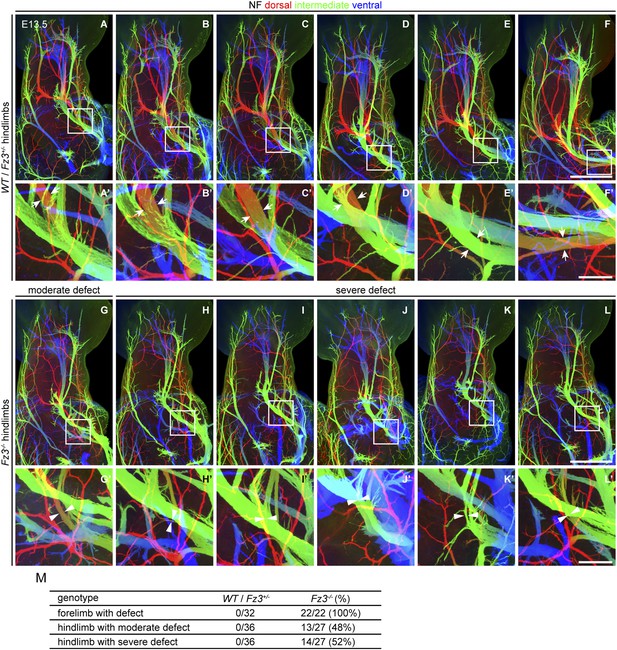
Thinning of spinal motor nerves innervating the dorsal hindlimb.
(A–L′) NF immunostaining on whole-mount hindlimbs from E13.5 WT or Fz3+/− (A–F) and Fz3−/− (G–L) embryos, with depth coded by colors as in Figure 4B–F′. (A′–L′) are a magnified view of the boxed regions in (A–L). Scale bars: (F) and (L), 500 µm; (F′) and (L′), 100 µm. (M) Number of dorsal nerves with different categories of phenotypes in WT and Fz3+/− vs Fz3−/− forelimbs and hindlimbs.
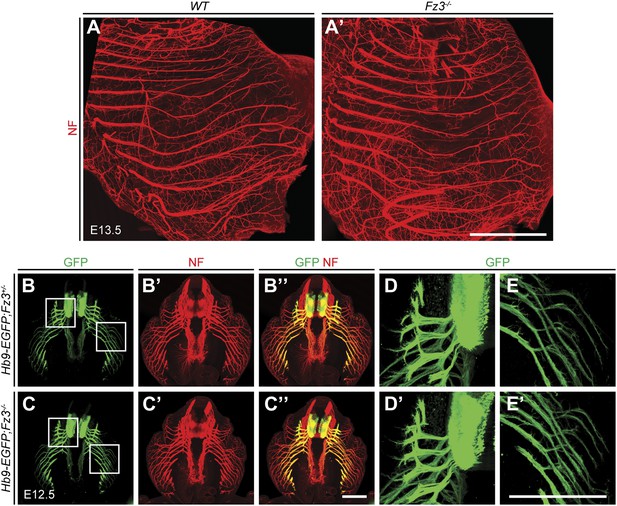
Loss of Fz3 does not affect axial or body wall motor nerve trajectories.
(A and A′) NF immunostaining on whole-mount anterior body wall encompassing the thorax/abdomen junction from E13.5 WT (A) and Fz3−/− (A′) embryos. Scale bar, 1 mm. (B–E′) NF and GFP immunostaining on 700 µm-thick cross sections from E12.5 Hb9-EGFP;Fz3+/− (B–B′′, D, and E) and Hb9-EGFP;Fz3−/− (C–C′′, D′, and E′) embryos. Boxed regions in (B) are enlarged in (D) and (E). Boxed regions in (C) are enlarged in (D′) and (E′). (D) and (D′) show axial motor nerves, and (E) and (E′) show anterior body wall motor nerves. Scale bars, 500 µm.
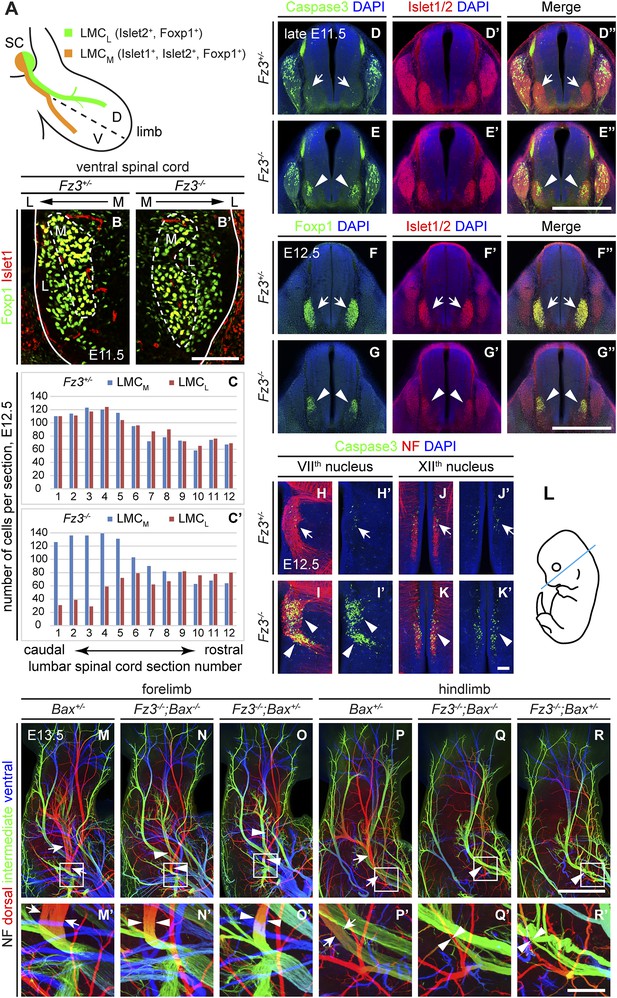
Abundance, differentiation, and apoptosis of motor neurons in Fz3−/− embryos, and the effects of suppressing apoptosis.
(A) Diagram showing the pattern of transcription factor expression in LMCL and LMCM motor neurons. SC, spinal cord; D, dorsal; V, ventral. (B and B′) Islet1 and Foxp1 expression in spinal motor neurons in cross sections of E11.5 Fz3+/− and Fz3−/− lumbar spinal cords. Continuous white line, lateral edge of the spinal cord. The broken white line encircles LMCM motor neurons. L, lateral; M, medial. Scale bar, 100 µm. (C and C′) The number of LMCL (Islet1−/Foxp1+) and LMCM (Islet1+/Foxp1+) motor neurons per 14 µm frozen section in E12.5 Fz3+/− and Fz3−/− lumbar spinal cords. Motor neurons were counted and averaged from 12 serial sections from each of three pairs of embryos, with adjacent counted sections separated by four uncounted sections. (D–E′′) Motor neuron apoptosis visualized with Islet1/2 and cleaved Caspase3 immunostaining in cross sections of E11.5 Fz3+/− (arrows) and Fz3−/− (arrowheads) lumbar spinal cords. Scale bar, 500 µm. (F–G′′) Reduced LMC volume in the Fz3−/− lumbar spinal cord visualized by comparing Islet1/2 and Foxp1 immunostaining in cross sections of E12.5 Fz3+/− (arrows) and Fz3−/− (arrowheads) lumbar spinal cords. Scale bar, 500 µm. (H–L) Cell death in VIIth (H–I′), and XIIth (J–K′) cranial motor nuclei (arrows and arrowheads) visualized with cleaved Caspase3 and NF immunostaining in horizontal sections of E12.5 Fz3+/− and Fz3−/− embryos. (H′–K′) show cleaved Caspase3 immunostaining with DAPI counterstaining for (H–K). (L) planes of section. Scale bar, 100 µm. (M–R′) NF immunostaining of whole-mount forelimbs (M–O′) and hindlimbs (P–R′) from E12.5 Bax+/−, Fz3−/−;Bax−/−, and Fz3−/−;Bax+/− embryos. For each pair of panels, the inset in the upper panel is enlarged in the lower panel. Arrows and arrowheads indicate the dorsal nerve. Depth is color coded as in Figure 4B–F′. Scale bars: (R), 500 µm; (R′), 100 µm.
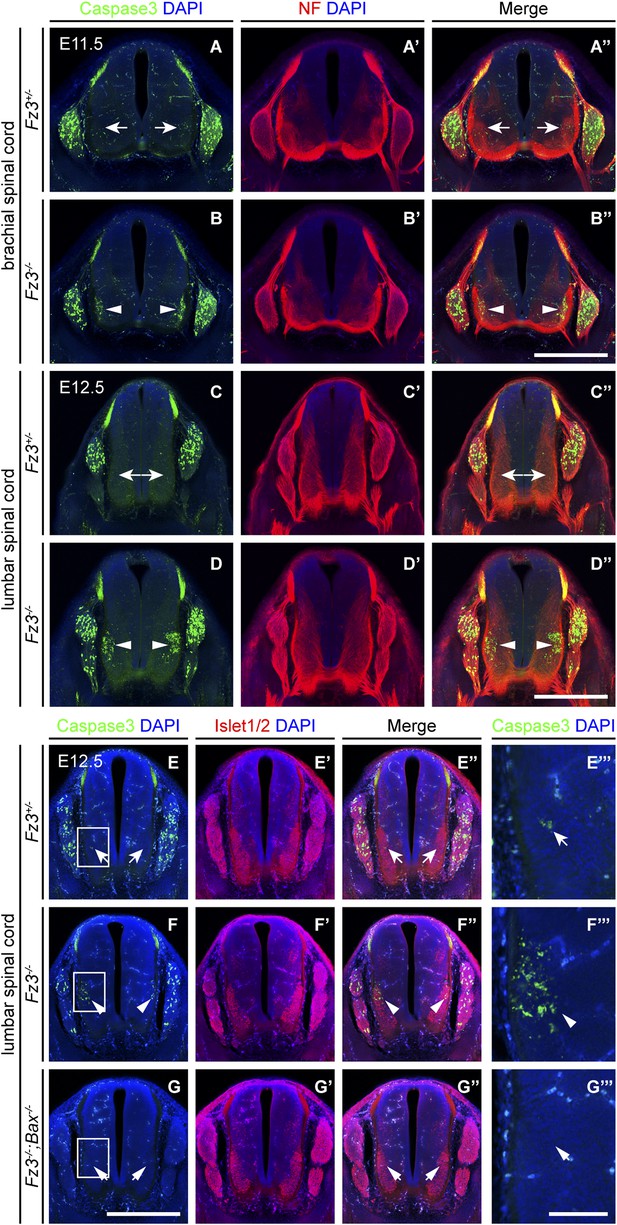
Precocious motor neuron death in Fz3−/− spinal cord is blocked by loss of Bax.
(A–B′′) Cell death in the ventral spinal cord at the brachial level visualized by cleaved Caspase3 and NF immunostaining on cross sections of E11.5 Fz3+/− (A–A′′) and Fz3−/− (B–B′′) embryos. In contrast to the ventrolateral spinal cord, there is no effect of Fz3 loss on cell death in the DRG. Scale bar, 500 µm. (C–D′′) Cell death in the ventral spinal cord at the lumbar level visualized by cleaved Caspase3 and NF immunostaining on cross sections of E12.5 Fz3+/− (C–C′′) and Fz3−/− (D–D′′) embryos. In contrast to the ventrolateral spinal cord, there is no effect of Fz3 loss on cell death in the DRG. Scale bar, 500 µm. (E–G′′′) Caspase3 and Islet1/2 immunostaining on cross sections from E12.5 Fz3+/− (E–E′′′), Fz3−/− (F–F′′′), and Fz3−/−;Bax−/− (G–G′′′) lumbar spinal cords. Boxed regions in (E–G) are enlarged in (E′′′–G′′′). Loss of Bax suppresses cell death in both the DRG and spinal cord. Scale bars: (G), 500 µm; (G′′′), 100 µm.
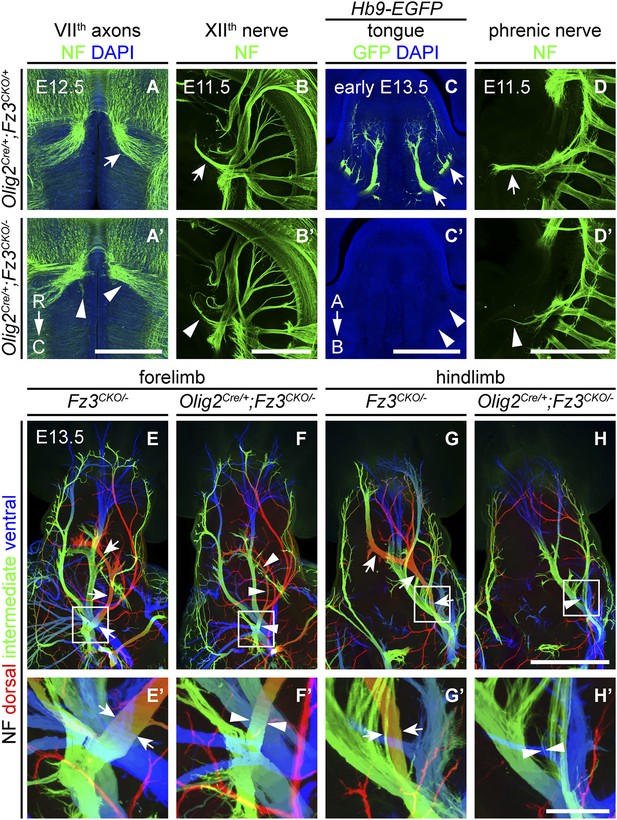
Olig2Cre/+;Fz3CKO/− embryos recapitulate the motor neuron phenotype of Fz3−/− embryos.
(A and A′) The VIIth cranial nerve axons visualized by NF immunostaining of horizontal sections through E12.5 Olig2Cre/+;Fz3CKO/+ (arrow) and Olig2Cre/+;Fz3CKO/− (arrowhead) brainstems. In the Olig2Cre/+;Fz3CKO/− brainstem, the more rostromedial locations of the VIIth nerve cell bodies are apparent, and the VIIth nerve axons fail to loop around the VIth cranial nerve nucleus. C, caudal; R, rostral. Scale bar, 500 µm. (B and B′) The XIIth cranial nerve visualized by NF immunostaining in lateral views of whole-mount E11.5 Olig2Cre/+;Fz3CKO/+ (arrow) and Olig2Cre/+;Fz3CKO/− (arrowhead) embryos. The XIIth nerve has a reduced diameter in Olig2Cre/+;Fz3CKO/− embryos. Scale bar, 500 µm. (C and C′) Innervation of tongue muscles by the XIIth cranial nerve visualized by GFP immunostaining in coronal sections of early E13.5 Hb9-EGFP;Olig2Cre/+;Fz3CKO/+ (arrows) and Hb9-EGFP;Olig2Cre/+;Fz3CKO/− (arrowheads) embryos. The Hb9-EGFP;Olig2Cre/+;Fz3CKO/− tongue shows no motor innervation. A, apical; B, basal. Scale bar, 500 µm. (D and D′) The phrenic nerve visualized by NF immunostaining in lateral views of whole-mount E11.5 Olig2Cre/+;Fz3CKO/+ (arrow) and Olig2Cre/+;Fz3CKO/− (arrowhead) embryos. The phrenic nerve has a reduced diameter in Olig2Cre/+;Fz3CKO/− embryos. Scale bar, 500 µm. (E–H′) NF immunostaining of whole-mount forelimbs (E and F) and hindlimbs (G and H) from E12.5 Fz3CKO/− and Olig2Cre/+;Fz3CKO/− embryos. Boxed regions in (E–H) are enlarged in (E′–H′). Arrows and arrowheads indicate the dorsal nerve. Depth is color coded as in Figure 4B–F′. Scale bars: (H), 500 µm; (H′), 100 µm.
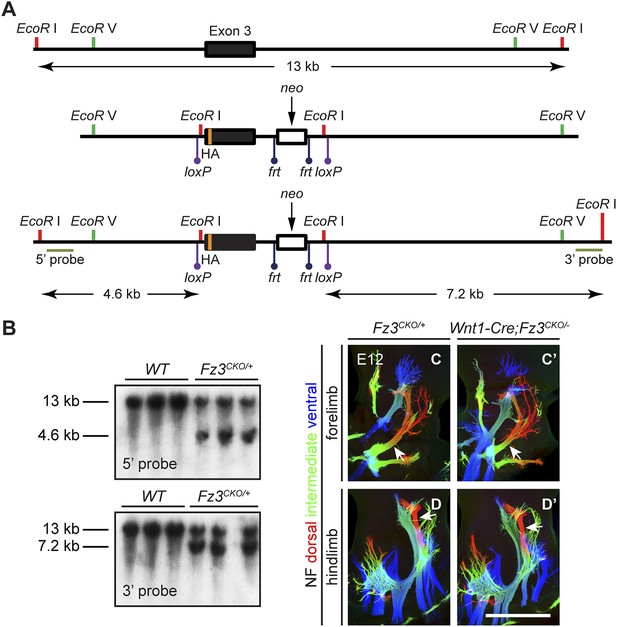
Design of the Fz3CKO allele, and limb innervation in Wnt1-Cre;Fz3CKO/− mice.
(A) Structure of the Fz3CKO allele. Top, partial genomic map of the third Fz3 coding exon. The exon is indicated by a filled rectangle, and cutting sites for EcoR I and EcoR V are shown. Middle, structure of the Fz3CKO targeting construct. Bottom, map of the allele after homologous recombination, and locations of Southern blot hybridization probes. (B) Genotyping of WT and Fz3CKO/+ mice by Southern blotting with the flanking 5′ and 3′ probes shown in (A) after EcoR I digestion. The sizes of genomic fragments generated by WT and targeted alleles are indicated. (C–D′) NF immunostaining on whole-mount forelimbs (C and C′) and hindlimbs (D and D′) from E12 Fz3CKO/+ (C and D) and Wnt1-Cre;Fz3CKO/− (C′ and D′) embryos, with depth coded by color as in Figure 4B–F′. Scale bar, 500 µm.
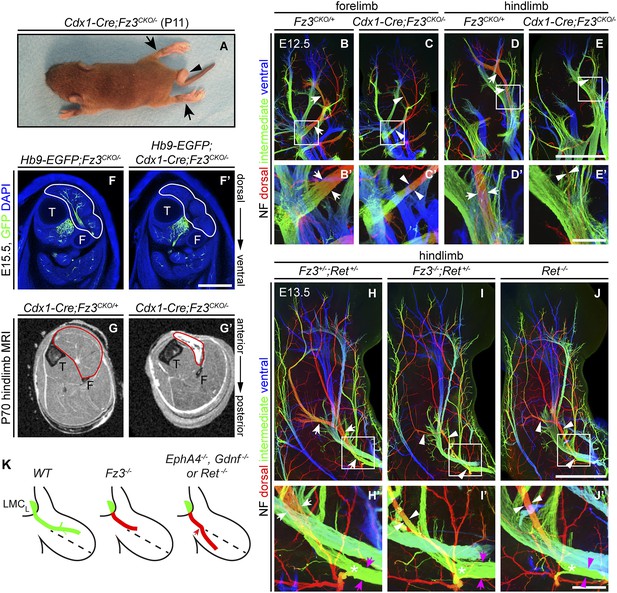
Selective hindlimb muscle atrophy in response to the failure of motor innervation in Fz3−/− mice.
(A) P11 Cdx1-Cre;Fz3CKO/− mouse exhibiting severe plantar flexion of the hind feet (arrows) and a curled tail (arrowhead). (B–E′) NF immunostaining of whole-mount forelimbs and hindlimbs from E12.5 Fz3CKO/+ and Cdx1-Cre;Fz3CKO/− embryos. (B′–E′) are magnified views of the boxed regions in (B–E). Arrows and arrowheads indicate the dorsal nerve. Depth is color coded as in Figure 4B–F′. Scale bars: (E), 500 µm; (E′), 100 µm. (F and F′) In E15.5 Hb9-EGFP;Cdx1-Cre;Fz3CKO/− distal hindlimbs, the anterior compartment musculature (tibialis anterior, extensor hallucis longus, and extensor digitorum longus; delimited by a white border) lacks motor innervation as visualized by GFP immunostaining. Extensive motor innervation is seen in the Hb9-EGFP;Fz3CKO/− littermate control that lacks the Cdx1-Cre transgene. F, fibula; T, tibia. Scale bar, 500 µm. (G and G′) Cross-sectional magnetic resonance images of P70 Cdx1-Cre;Fz3CKO/+ and Cdx1-Cre;Fz3CKO/− hindlimbs show nearly complete degeneration and fibrosis of the anterior compartment musculature in the Cdx1-Cre;Fz3CKO/− distal hindlimb. The anterior muscle compartment is delimited by a red border. The large and small dark territories are the tibia and fibula, respectively. (H–J′) NF immunostaining of whole-mount hindlimbs from E13.5 Fz3+/−;Ret+/−, Fz3−/−;Ret+/−, and Ret−/− embryos. Boxed regions in (H–J) are enlarged in (H′–J′). In (H–J), white arrows and arrowheads indicate the dorsal nerve. (H′–J′) arrow and arrowhead colors: magenta indicates the dorsal nerve diameter proximal and white indicates the dorsal nerve diameter distal to the point where axons stall in Fz3−/− limbs (asterisks). Depth is color coded as in Figure 4B–F′. Scale bars: (J), 500 µm; (J′), 100 µm. (L) Comparison of spinal motor axon growth and guidance defects in different knockout lines. In Fz3−/− embryos, many dorsal motor axons fail to grow beyond a proximal branch point, whereas in EphA4−/−, Gdnf−/−, and Ret−/− embryos, dorsal axons are misrouted to the ventral limb.
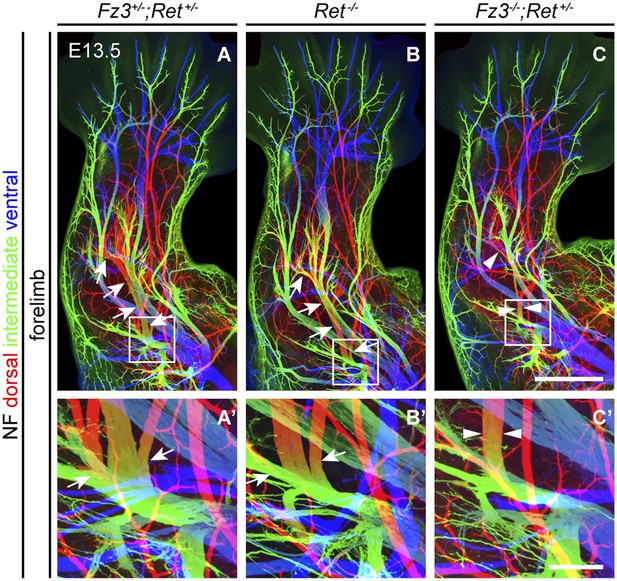
Comparison of dorsal nerve anatomy in forelimbs with various combinations of Fz3 and Ret loss-of-function alleles.
(A–C′) NF immunostaining on whole-mount forelimbs from E13.5 Fz3+/−;Ret+/− (A), Ret−/− (B), and Fz3−/−;Ret+/− (C) embryos, with depth coded by color as in Figure 4B. (A′–C′) are a magnified view of the boxed regions in (A–C). Scale bars: (C), 500 µm; (C′), 100 µm.





