Evolution: To plasticity and back again
It was the Danish botanist Wilhelm Johannsen who coined the terms ‘genotype’ and ‘phenotype’. He thought that the genotype, or genetic makeup, of an organism referred to its ‘fundamental constitution’, whereas an organism's individual characters or traits, i.e. its phenotype, arose from a complicated interaction between the genotype and the environment (Johannsen, 1913).
Phenotypes can vary in response to external factors such as nutrition and temperature (Woltereck, 1909); this can be simply an accidental consequence of the fact that cellular and developmental processes that produce the phenotype run at different rates under different environmental conditions. And because environments are seldom, if ever, stable, it is very common for there to be some natural variation in phenotypes around some ‘ideal’ value, even between individuals with identical genotypes.
A natural question to ask is—does this variation matter? In terms of evolution, what matters are things that affect fitness: that is, things that affect the ability of an individual, compared to other individuals, to pass its genes to the next generation. If the phenotypic variation reduces fitness, then selection will favor the evolution of mechanisms that make the phenotype more robust, or less sensitive to environmental variation. On the other hand, if some of the phenotypes are well adapted to the environmental factors that induce them, evolution will favor mechanisms that stabilize the production of each phenotype in its best-suited environment.
When one genotype can produce more than one phenotype in different environments, this is generally referred to as ‘phenotypic plasticity’. As this ability can increase the diversity of phenotypes in a population, it is natural to ask whether populations of organisms with phenotypic plasticity are more able to evolve than those without. To date, there are various theories that suggest they should be, but there is little experimental evidence to support this view (Moczek et al., 2011; West-Eberhard, 2003). Now, in eLife, Ralf Sommer and Erik Ragsdale from the Max Planck Institute for Developmental Biology and co-workers—including Vladislav Susoy as first author—report that phenotypic plasticity is associated with evolutionary diversification in nematode worms (Susoy et al., 2015).
Susoy et al. examined a large group of nematode worms, including the model species Caenorhabditis elegans and some species with adults that have so-called ‘polyphenic’ mouthparts. Polyphenism is an extreme, but common, form of phenotypic plasticity in which two (or more) completely different phenotypes develop in response to specific environmental cues. Each of the alternative phenotypes is an adaptation to a different environment (Nijhout, 2003), and natural selection acts to ensure that they are stably and reliably produced to best match the environmental conditions.
Susoy et al. tested 90 species of nematodes for their ability to develop polyphenic mouthparts in the laboratory. In 23 species, some worms developed small smooth mouths specialized for feeding on bacteria, whereas others of the same species developed mouths with hooks and teeth. The toothed phenotype is used to feed on fungi and on other nematodes, and is induced by overcrowding and by starvation during the first larval stage (Bento et al., 2010; Sommer and Ogawa, 2011).
Analysis of the evolutionary relationships between the nematodes revealed that polyphenic mouthparts evolved only once in a common ancestor of the 23 species. Susoy et al. then asked if there was greater diversification in the shapes of mouthparts after polyphenism had evolved compared to the lineages of the ‘nematode family tree’ that had not evolved polyphenism. A sophisticated statistical analysis showed that this was indeed the case. This is some of the best empirical evidence to date for the theory that phenotypic plasticity enhances the ability of a population to evolve new forms (also known as evolvability).
Moreover, although polyphenism evolved only once, it appears to have been lost at least 10 times during the subsequent evolution of the lineage. Unexpectedly, the loss of polyphenism was followed by an even stronger rate of subsequent evolution of new traits and phenotypes. Thus the loss of phenotypic plasticity was also associated with an increase in evolvability. But how can this be explained?
One possible explanation is that a polyphenism requires developmental mechanisms that stabilize two alternative phenotypes, each in a different environment. If they work well, such stabilizing mechanisms buffer the two phenotypes against moderate changes to the organism's genetic makeup. This means that many mutations will not effect the phenotype and therefore will not be selected against. Such mutations will gradually accumulate in a population (Figure 1). Then, when the polyphenism is lost, the need to stabilize one of the two phenotypes disappears. Thus some of the accumulated genetic variation is no longer buffered and can cause the phenotype to vary more. This new phenotypic variation can now come under selection and lead to diverse adaptations in different lineages.
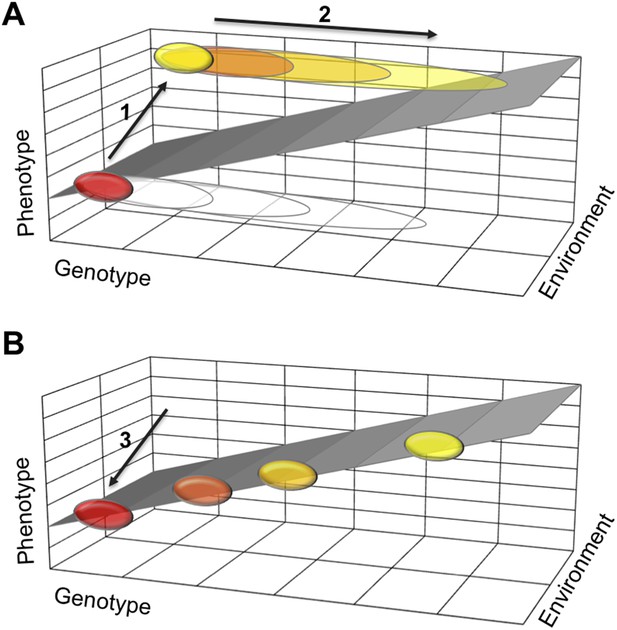
How a loss of phenotypic plasticity could increase evolvability.
Changes in the genetic makeup, or genotype, of an organism can lead to changes in its traits and characteristics, also known as its phenotype. The sloped surface represents the hypothetical ideal relationship between genotype and phenotype in different environments, but in the absence of stabilizing mechanisms. (A) As part of a thought experiment, consider a population where at first all individuals have the same phenotype (shown as a red ellipse). This phenotype is stabilized by developmental mechanisms, which allow some genetic variation to accumulate (depicted as open ellipses expanding to the right). The evolution of a polyphenism (arrow 1) establishes a new phenotype (yellow ellipse) in a different environment, but with the same genotype. More genetic variation will accumulate (arrow 2) that has no effect on the phenotypes but improves stabilization of the alternative phenotypes in different environmental conditions. (B) When the polyphenism is lost (arrow 3), the mechanisms that stabilized the second phenotype are also lost. The accumulated genetic variation can now produce new phenotypes that (initially at least) fall on the ideal sloped surface, and that establish the foundation for the evolution of new traits and characteristics.
It is important to note that this explanation is, of course, a thought experiment that could be supported by statistical analyses. But the great challenge for the future will be to establish whether it is possible to devise experiments that can prove whether such a mechanism exists in nature.
References
-
The role of developmental plasticity in evolutionary innovationProceedings of the Royal Society B: Biological Sciences 278:2705–2713.https://doi.org/10.1098/rspb.2011.0971
-
Development and evolution of adaptive polyphenismsEvolution & Development 5:9–18.https://doi.org/10.1046/j.1525-142X.2003.03003.x
-
Weitere experimentelle Untersuchungen über Artveränderung, speziell über das Wesen quantitativer Artunterschiede bei DaphnienVerhandlungen der Deutschen Zoologischen Gesellschaft 19:110–173.
Article and author information
Author details
Publication history
- Version of Record published: March 12, 2015 (version 1)
Copyright
© 2015, Nijhout
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,651
- views
-
- 250
- downloads
-
- 8
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Evolutionary Biology
The RNA world hypothesis proposes that during the early evolution of life, primordial genomes of the first self-propagating evolutionary units existed in the form of RNA-like polymers. Autonomous, non-enzymatic, and sustained replication of such information carriers presents a problem, because product formation and hybridization between template and copy strands reduces replication speed. Kinetics of growth is then parabolic with the benefit of entailing competitive coexistence, thereby maintaining diversity. Here, we test the information-maintaining ability of parabolic growth in stochastic multispecies population models under the constraints of constant total population size and chemostat conditions. We find that large population sizes and small differences in the replication rates favor the stable coexistence of the vast majority of replicator species (‘genes’), while the error threshold problem is alleviated relative to exponential amplification. In addition, sequence properties (GC content) and the strength of resource competition mediated by the rate of resource inflow determine the number of coexisting variants, suggesting that fluctuations in building block availability favored repeated cycles of exploration and exploitation. Stochastic parabolic growth could thus have played a pivotal role in preserving viable sequences generated by random abiotic synthesis and providing diverse genetic raw material to the early evolution of functional ribozymes.
-
- Ecology
- Evolutionary Biology
Seasonal animal dormancy is widely interpreted as a physiological response for surviving energetic challenges during the harshest times of the year (the physiological constraint hypothesis). However, there are other mutually non-exclusive hypotheses to explain the timing of animal dormancy, that is, entry into and emergence from hibernation (i.e. dormancy phenology). Survival advantages of dormancy that have been proposed are reduced risks of predation and competition (the ‘life-history’ hypothesis), but comparative tests across animal species are few. Using the phylogenetic comparative method applied to more than 20 hibernating mammalian species, we found support for both hypotheses as explanations for the phenology of dormancy. In accordance with the life-history hypotheses, sex differences in hibernation emergence and immergence were favored by the sex difference in reproductive effort. In addition, physiological constraint may influence the trade-off between survival and reproduction such that low temperatures and precipitation, as well as smaller body mass, influence sex differences in phenology. We also compiled initial evidence that ectotherm dormancy may be (1) less temperature dependent than previously thought and (2) associated with trade-offs consistent with the life-history hypothesis. Thus, dormancy during non-life-threatening periods that are unfavorable for reproduction may be more widespread than previously thought.






