Prefrontal dopamine regulates fear reinstatement through the downregulation of extinction circuits
Figures
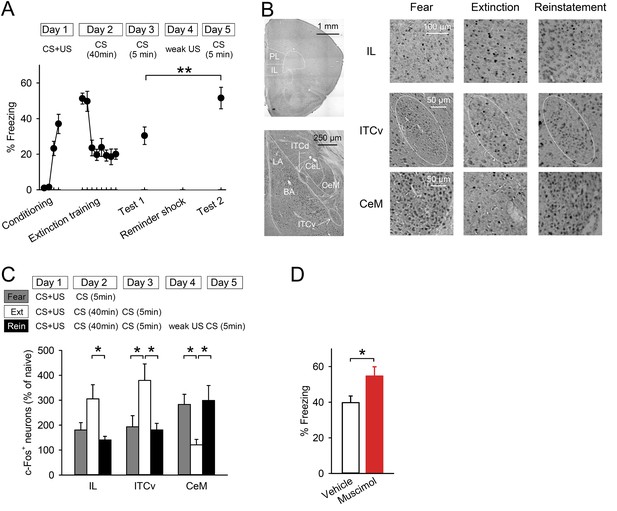
Reinstatement is associated with low IL activity.
(A) A reminder shock reinstated extinguished fear (n = 10 mice; paired t-test, t(9) = 3.6, p = 0.0059). (B) Representative images of the infralimbic cortex (IL), the ventral intercalated amygdala neurons (ITCv), and the central nucleus of the amygdala (CeM) in the Fear, Extinction, and Reinstatement groups. (C) c-Fos+ cell density decreased in the IL and the ITCv and increased in the CeM with reinstatement (n = 8–11 mice; F(2,27) = 4.3, p = 0.023 [IL]; F(2,26) = 4.8, p = 0.0016 [ITCv]; F(2,26) = 6.3, p = 0.0058 [CeM]; Tukey's test, PExtinction vs Reinstatement = 0.029 [IL], 0.035 [ITCv], 0.013 [CeM]). (D) IL muscimol infusions resulted in high freezing (n = 10 mice; t(18) = 2.4, p = 0.030). **p < 0.01, *p < 0.05. Data represent mean ± standard error.
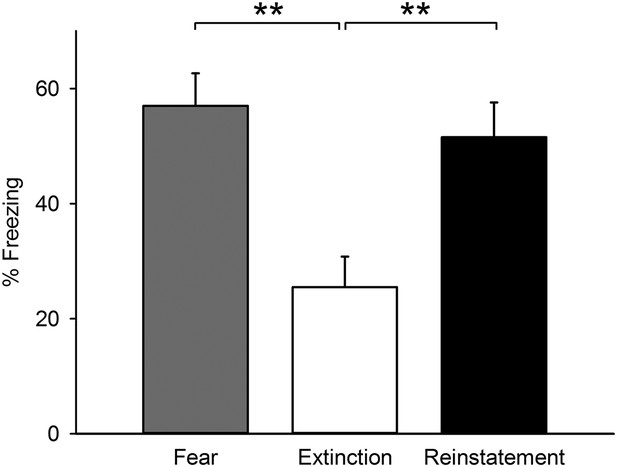
Freezing behaviour of the mice subjected to c-Fos activity mapping.
Mice of the Reinstatement group showed greater freezing behaviour relative to the Extinction group (F(2,25) = 8.7, p = 0.0013; Tukey's test, PFear vs Extinction = 0.0023, PExtinction vs Reinstatement = 0.0073). **p < 0.01. Data represent mean ± standard error.
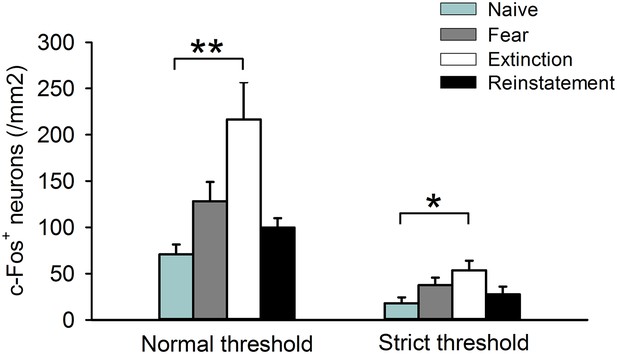
c-Fos+ cell density in the IL calculated in the analysis using a normal threshold and in an additional analysis using a strict threshold.
(n = 8–11 mice; F(3,35) = 5.9, p = 0.0023 [Normal threshold]; F(3,35) = 3.0, p = 0.042 [Strict threshold]; Tukey's test, PNaive vs Extinction = 0.0017 [Normal threshold], 0.032 [Strict threshold]). **p < 0.01, *p < 0.05. Data represent mean ± standard error.
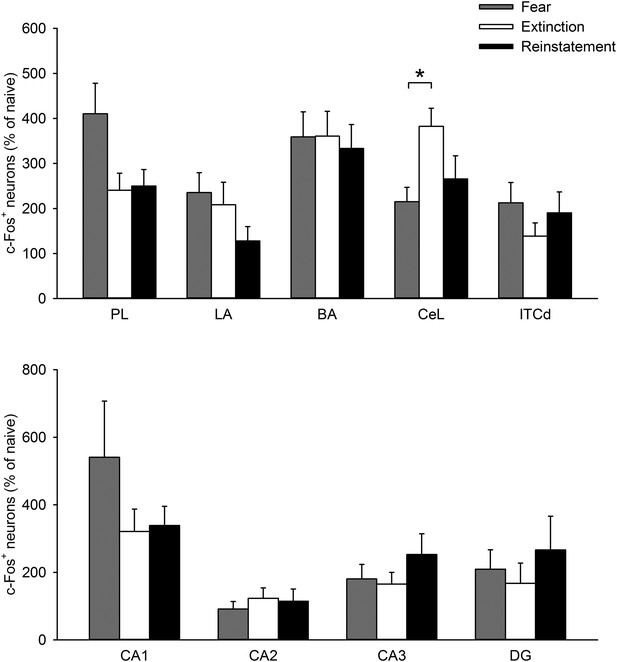
The density of c-Fos+ cells in the PL, LA, BA, CeL, ITCd, CA1, CA2, CA3, and DG was comparable between the Extinction and Reinstatement groups (PL, F(2,28) = 3.6, p = 0.041; LA, F(2,26) = 1.4, p = 0.27; BA, F(2,26) = 0.068, p = 0.93; CeL, F(2,26) = 4.8, p = 0.017; ITCd, F(2,27) = 0.97, p = 0.39; CA1, F(2,22) = 1.3, p = 0.29; CA2, F(2,22) = 0.29, p = 0.75; CA3, F(2,22) = 1.0, p = 0.38; DG, F(2,22) = 0.46, p = 0.64; Tukey's test, CeL: PFear vs Extinction = 0.015).
PL: prelimbic cortex, LA: lateral amygdala, BA: basal amygdala, CeL: lateral subdivision of central nucleus of the amygdala, ITCd: dorsal intercalated amygdala neurons. *p < 0.05. Data represent mean ± standard error.
Histological verification of cannula placements in the experiment with muscimol infusions into the IL.
https://doi.org/10.7554/eLife.08274.007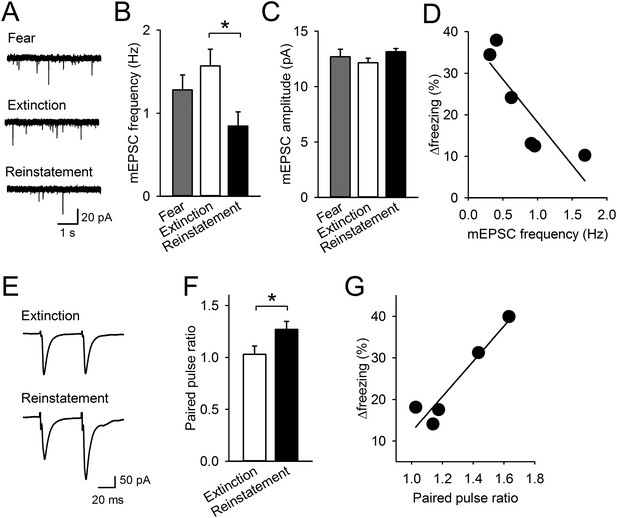
Reinstatement is associated with presynaptic depression in the IL.
(A) Representative miniature excitatory postsynaptic current (mEPSC) traces. (B) IL neurons had lower mEPSC frequency in the Reinstatement group (n = 8 neurons from 6 mice) than the Extinction group (n = 8 neurons from 4 mice) (F(2,21) = 3.9, p = 0.037; PExtinction vs Reinstatement = 0.030). (C) mEPSC amplitude did not differ across groups (F(2,21) = 1.9, p = 0.38). (D) mEPSC frequency negatively correlated with Δfreezing (different degrees of freezing time between tests 1 and 2) in the Reinstatement group (r = −0.83, p = 0.040). (E) Representative traces of EPSCs evoked by paired-pulse stimulation. (F) IL neurons had a higher paired-pulse ratio (PPR) in the Reinstatement group (n = 8 neurons from 5 mice) than the Extinction group (n = 8 neurons from 4 mice) (t(14) = 2.2, p = 0.049). (G) PPR positively correlated with Δfreezing in the Reinstatement group (r = 0.95, p = 0.012). *p < 0.05. Data represent mean ± standard error.
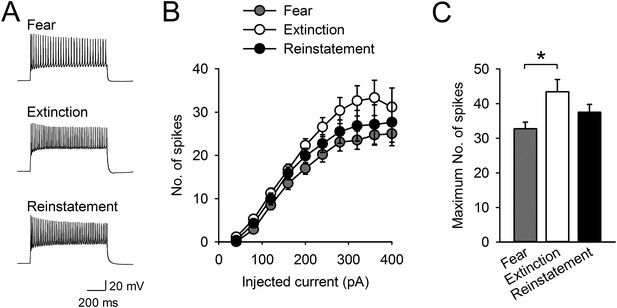
Intrinsic excitability of infralimbic neurons did not change with fear reinstatement.
(A) Representative traces showing spikes in response to a 400-pA current pulse. (B) The number of action potentials in response to depolarizing steps at different current intensities (n = 21, 20, 20 neurons). (C) The maximum number of evoked spikes at any current step increased with fear extinction (F(2,58) = 4.1, p = 0.022; Tukey's test, p = 0.016) and did not change with fear reinstatement. *p < 0.05. Data represent mean ± standard error.
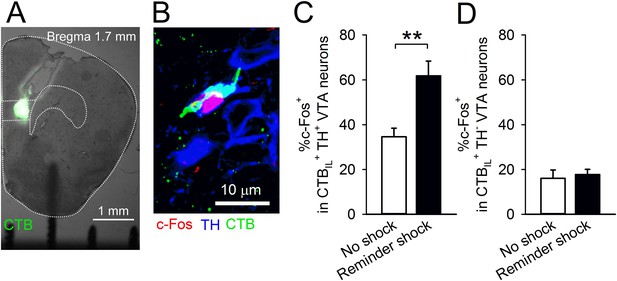
A reminder shock activates dopaminergic VTA neurons projecting to the IL.
(A) Coronal brain section of a mouse with Alexa 488-conjugated cholera toxin subunit B (CTB) infusion into the IL. (B) A representative immunofluorescence image of the ventral tegmental area (VTA) neurons with c-Fos, tyrosine hydroxylase (TH), and CTB. (C) A reminder shock increased the proportion of c-Fos+ neurons in IL-projecting TH+ VTA neurons (no shock: n = 7, reminder shock: n = 6 mice; t(11) = 4.3, p = 0.0012). (D) A reminder shock did not increase the proportion of c-Fos+ neurons in IL-projecting TH− VTA neurons (no shock: n = 7, reminder shock: n = 6 mice). **p < 0.01, Data represent mean ± standard error.
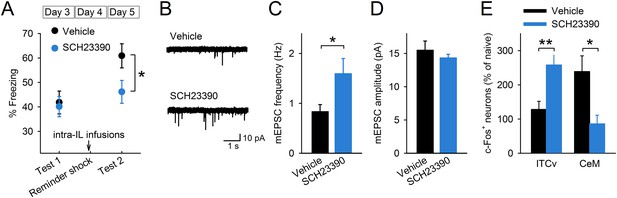
D1Rs in the IL mediate reinstatement.
(A) SCH23390 infusions into the IL before a reminder shock suppressed reinstatement (phosphate-buffered saline [PBS]: n = 15, SCH23390: n = 14 mice; t(27) = 2.2, p = 0.039). (B) Representative mEPSC traces. (C) SCH23390-infused mice demonstrated higher mEPSC frequency (n = 9, 10 neurons; t(17) = 2.2, p = 0.044). (D) SCH23390 infusions had no effects on mEPSC amplitude. (E) SCH23390-infused mice demonstrated higher and lower c-Fos+ cell density in the ventral intercalated amygdala neurons (ITCv) and the central nucleus of the amygdala (CeM), respectively (n = 7–8 mice; ITCv: t(13) = 3.0, p = 0.0093; CeM: t(14) = 2.9, p = 0.011). **p < 0.01, *p < 0.05. Data represent mean ± standard error.
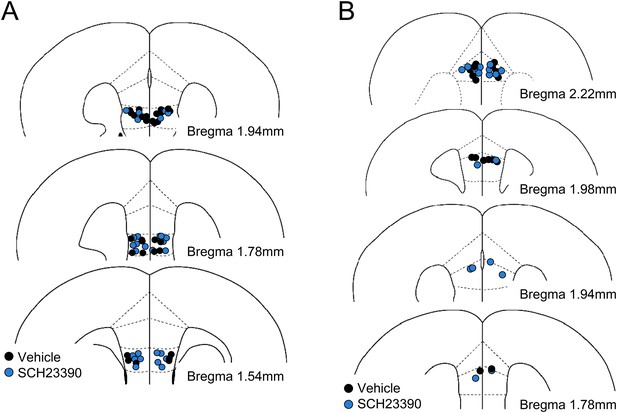
Histological verification of cannula placements in the experiment with SCH23390 infusions into the infralimbic (A) and the prelimbic (B) cortices.
https://doi.org/10.7554/eLife.08274.013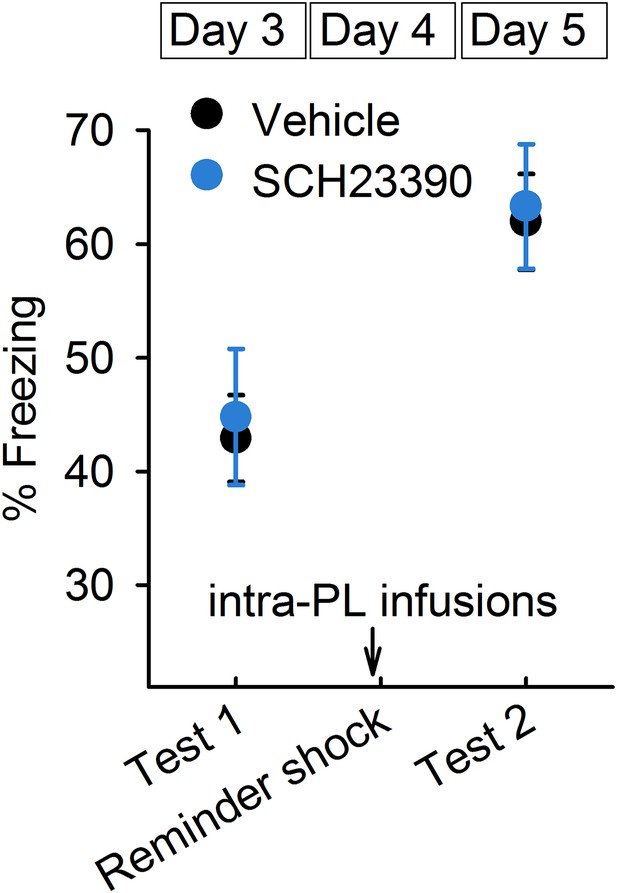
SCH23390 infusions into the prelimbic cortex had no effects on reinstatement (n = 9 mice).
Data represent mean ± standard error.
Tables
Electrophysiological properties of IL neurons
| Fear | Extinction | Reinstatement | |
|---|---|---|---|
| Resting potential (mV) | −70.7 ± 1.1 | −72.0 ± 1.0 | −70.6 ± 0.7 |
| Input resistance (MΩ) | 276.5 ± 24.6 | 391.9 ± 32.4* | 362.6 ± 30.9 |
| Spike amplitude (mV) | 75.5 ± 1.5 | 72.9 ± 2.0 | 76.3 ± 1.2 |
| First interspike interval (ms) | 7.9 ± 0.5 | 8.8 ± 0.6 | 8.9 ± 0.4 |
| Rheobase (pA) | 78.1 ± 5.7 | 65.5 ± 4.9 | 70.0 ± 6.1 |
| Spike threshold (mV) | −37.3 ± 0.7 | −37.2 ± 1.0 | −35.1 ± 0.6 |
| Voltage sag (mV) | −3.0 ± 0.3 | −3.1 ± 0.2 | −3.8 ± 0.4 |
| Half width of spike (ms) | 1.01 ± 0.03 | 0.98 ± 0.02 | 1.04 ± 0.02 |
| fAHP (mV) | −17.3 ± 0.7 | −16.7 ± 0.7 | −16.7 ± 0.7 |
| mAHP (mV) | −1.6 ± 0.5 | −0.9 ± 0.4 | −1.0 ± 0.5 |
-
*
p < 0.05 vs Fear, Tukey's test.
-
fAHP, fast afterhyperpolarization; mAHP, medium afterhyperpolarization.





