Activities of visual cortical and hippocampal neurons co-fluctuate in freely moving rats during spatial behavior
Figures
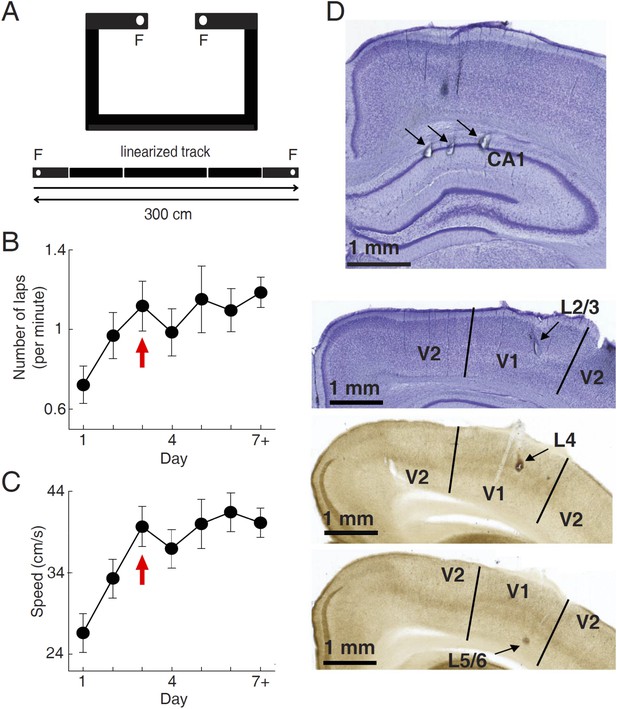
Behavioral task and recording sites.
(A) A C-shaped track where rats ran back and forth for food rewards. Bottom: two linearized trajectories, each 300 cm long. Vertical white lines: corners of the track. F: food wells. (B, C) The mean number of laps per minute (B) and mean running speed (C) on each day of track running, averaged over all trajectories and all animals. The number for Day 7 + included those days ≥ Day 7. Arrows: the day when the mean number of lap and speed were stabilized. Number of trajectories: N = 30, 26, 22, 22, 18, 16, 26 for Day 1 to Day 7+, respectively. (D) Nissl- (top 2 sections) and AChE-stained (bottom 2 sections) coronal brain sections to show recording sites (arrows) in the hippocampal CA1, and those in different layers of V1 (L2/3, L4 and L5/6). V2: the secondary visual cortex.
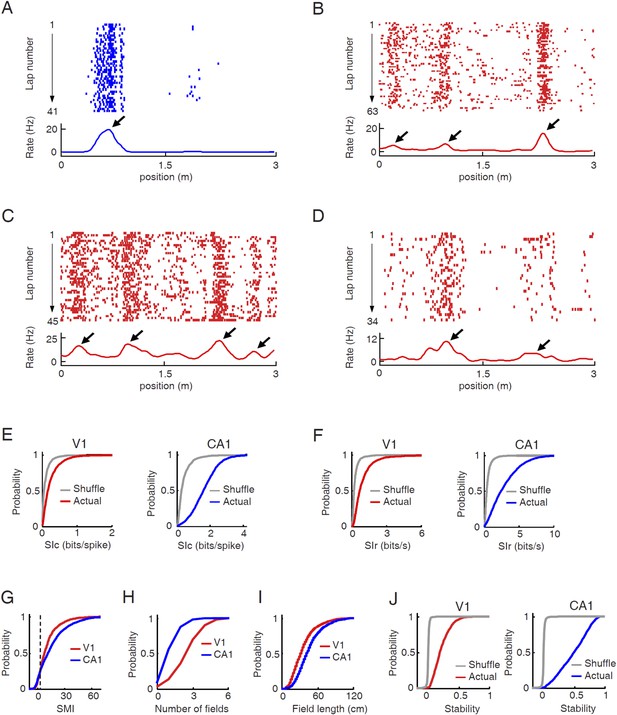
V1 cells fired predominantly at specific locations during track running.
(A–D) Firing activities of a CA1 place cell (A) and 3 V1 cells (B , C, D in layer L2/3, L4, L5/6 respectively). For each panel, the top displays the spike raster of a cell within every lap of running on a trajectory, which is linearized and plotted as the x-axis. Each tick represents a spike. The bottom is the firing rate curve averaged across all the laps. Arrows: firing fields. (E) Cumulative distributions of spatial information content (SIc) values of V1 and CA1 cells with actual and shuffled spiking activities. (F) Same as E, but for spatial information rate (SIr). (G–I) Cumulative distribution of spatial modulation index (SMI, G), number of fields per trajectory (H), and field length (I) for V1 and CA1 cells. (J) Same as E, but for spatial stability.
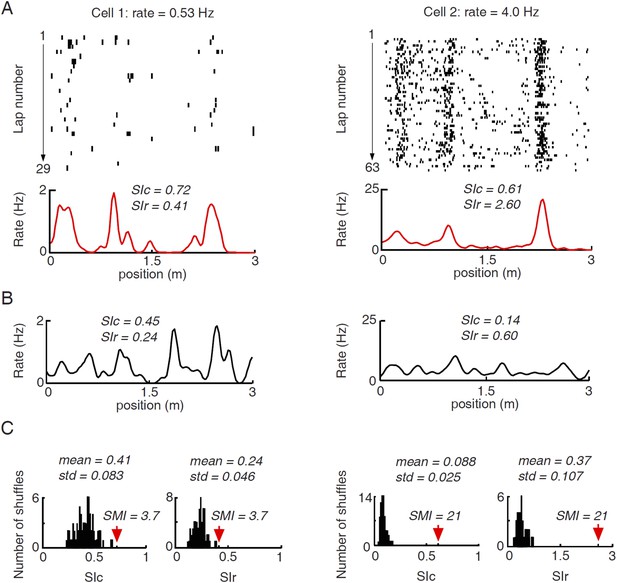
Illustration of computing spatial modulation indices (SMIs) for two example cells with different firing rates.
(A) Lap by lap spike raster and spike rate curves of the two cells averaged across all laps. The plots are arranged the same way as in Figure 2A–D. Spatial information content (SIc) and spatial information rate (SIr) computed from the rate curves are shown. Note that the higher-rate Cell 2 appeared more spatially modulated than Cell 1, but had a lower SIc. (B) An example of shuffled firing rate curve for each of the two cells, generated via circularly shifting the spike trains in A by a random time interval for every lap. Note the comparable peaks between the actual and the shuffled rate curves in the low-rate Cell 1, but not in Cell 2. The SIc and SIr computed from the shuffled rate curves were shown. (C) Histograms of SIc and SIr values computed from 100 randomly shuffled firing rate curves for the two cells. The mean and standard deviation (std) of each histogram are shown. Red arrows mark the actual values. SMI is computed as (actual value−mean)/std. It can be seen that the shuffle-generated values differed between SIc and SIr for the same cell and between the two cells, even though the shuffled spike trains by definition contained no spatial information. The shuffled spikes of the low-rate Cell 1 yielded a higher SIc, but a lower SIr, than the shuffled spikes of the high-rate Cell 2. The examples illustrate the rate–dependence of SIc and SIr. Second, by normalizing the actual SIc/SIr values relative to their shuffle-generated values, SMIs computed from SIc and SIr become equivalent.
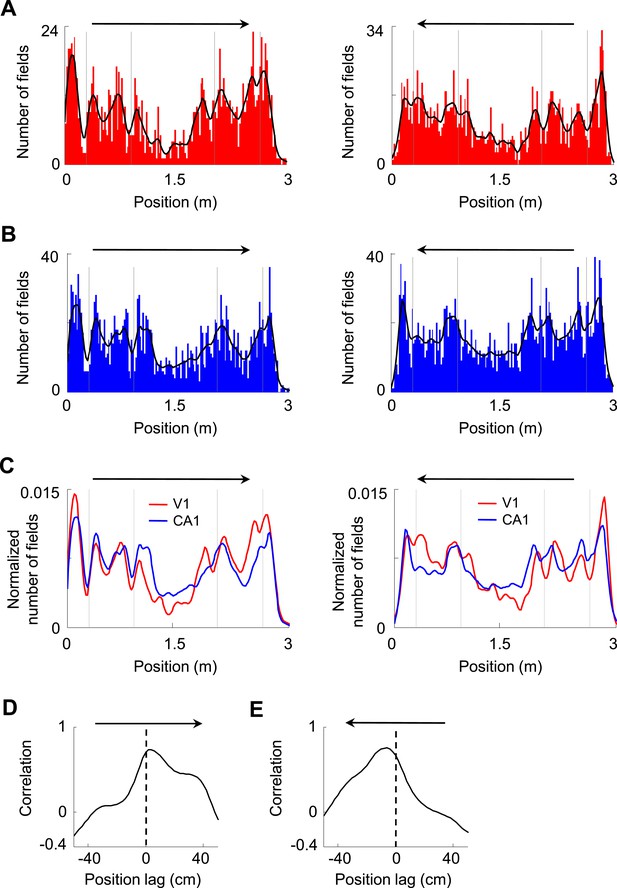
Firing fields of V1 and CA1 cells appeared to accumulate around the landmarks of the track.
(A, B) Histograms of the number of firing fields for V1 (A) and CA1 (B) cells on the two trajectories of the track with opposite running directions. Vertical gray lines: landmark positions (corners) of the track. Arrows: running directions. Black lines: smoothed curves of the histograms. Note that the number of fields tended to peak before and after the landmarks for both running directions and for both V1 and CA1 cells. (C) The smoothed curves for V1 and CA1 cells in A and B are normalized (by total number of firing fields) and re-plotted together for each of the running direction (arrow). Note that the V1 curve (red) tended to rise and fall slightly earlier than the CA1 (blue) curve on both running directions. (D, E) Cross-correlogram between the V1 and CA1 smoothed field curves in C on each of the running directions (arrow).
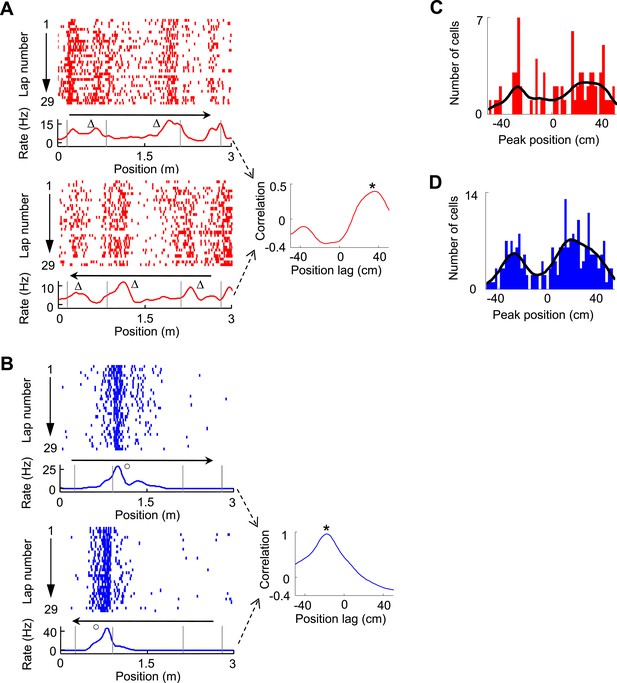
Bi-directional firing of V1 and CA1 cells on the C-shaped track.
(A) Firing activity of a V1 cell (lap-by-lap spike raster and average firing rate curve; see Figure 2 legend for details) on two trajectories with opposite running directions (left) and the cross-correlogram of the cell's two firing rate curves (right). Vertical Gray lines: land mark positions (corners) of the track. Arrows: running directions. Note that the peaks appeared before the animal passed the same landmarks (∆, prospective firing) on both running directions, resulted in a primary peak (*) at a positive position lag in the cross-correlogram. (B) Same as A, but for an example of CA1 cell showing consistent firing after a landmark (o, retrospective firing) on both directions. (C, D) Histograms of the cross-correlogram peak positions of all V1 (C) and CA1 (D) cells with significant bi-directional firing. Black lines: smoothed curves of the histograms. Note that both the V1 and CA1 distributions appeared to be bi-modal, suggesting prospective or retrospective firing for V1 and CA1 bi-directional cells.
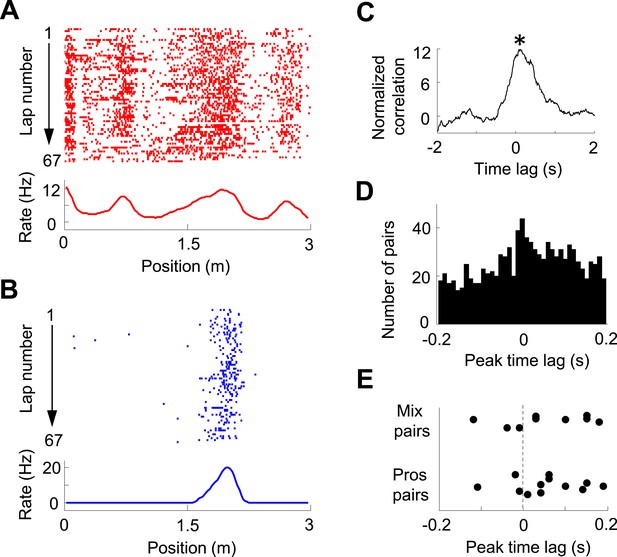
Pair-wise cross-correlation between V1 and CA1 cells.
(A, B) Firing activity (lap-by-lap spike raster and average firing rate curve; see Figure 2 legend for details) of a pair of V1 (A) and CA1 (B) cells on a trajectory of the C-shaped track. (C) Normalized cross-correlogram of the two cells in A and B. *: peak time of the cross-correlogram. (D) Histogram of the peak times for all highly significantly correlated pairs of V1 and CA1 cells (see ‘Materials and methods’). Note the bias of peak times toward positive time lags. (E) Peak times of those highly correlated V1-CA1 pairs with both displaying prospetive firing (Pros pairs) and of those pairs with one displaying prospective while the other displaying retrospective firing (Mix pairs). Each dot is a pair.
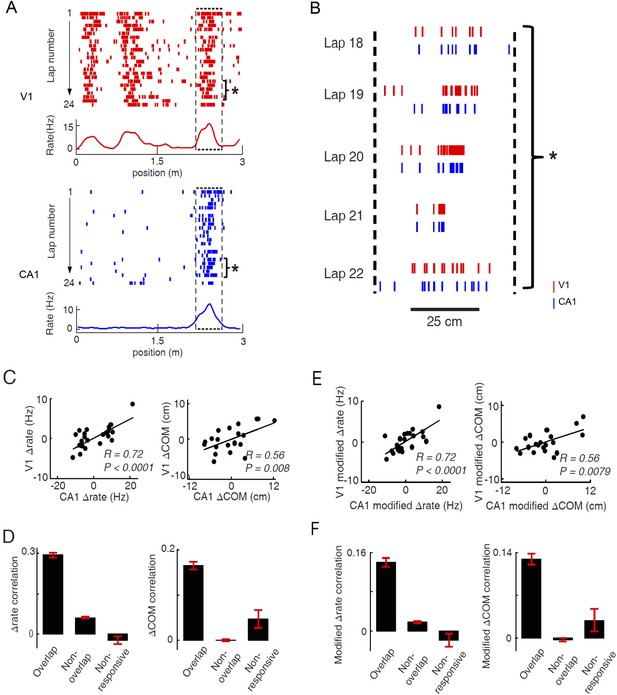
Pairs of V1 and CA1 cells with overlapping firing fields displayed correlated lap-by-lap fluctuations in firing rate and firing location.
(A) Lap-by-lap spike raster and the average rate curves (see Figure 2 legend for details) of a pair of V1 and CA1 cells on the same trajectory. Boxes: the overlapping firing fields of the two cells. (B) The spikes within the marked laps (*) of the two cells in A are expanded and plotted together. Note the correlated lap-by-lap shifting of the V1 and CA1 cells in their spikes. (C) The lap-by-lap fluctuations in firing rate (ΔRate) and COM (ΔCOM) within the firing fields of the two CA1 and V1 cells in A. Each dot is a lap. Solid line: linear regression. R, P: Pearson's correlation between the CA1 and V1 fluctuations and the associated p-value. (D) Average correlation in ∆rate and ∆COM for overlapping, non-overlapping, and non-responsive pairs of CA1 and V1 cells (see text for definitions). (E, F) Same as C and D, but for modified ∆rate and modified ∆COM after removing the modulations of firing rate and COM by speed and head direction.
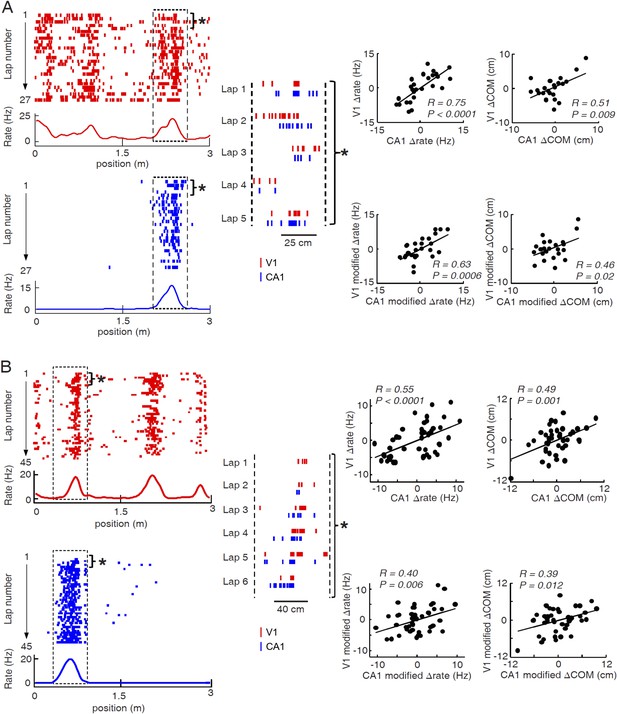
Two more examples of overlapping V1-CA1 cell pairs with correlated lap-by-lap fluctuations in, each from a different animal.
For each example (A or B), plotted on the left are the lap-by-lap spike raster and firing rate curves of the V1 (red) and CA1 (blue) cells, while plotted on the right are the lap-by-lap fluctuations in rate (∆rate) and COM (∆COM) for the two cells on the left, and in rate (modified ∆rate) and COM (modified ∆COM) after the modulation by speed and head direction was removed. See the main figure (Figure 6) legend for details.
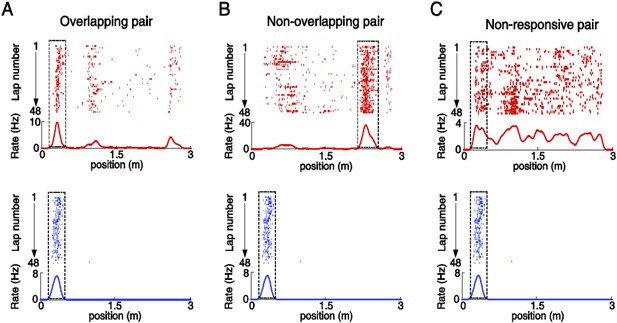
Illustration of overlapping, non-overlapping, and non-responsive V1-CA1 cell pairs.
(A) An overlapping pair: a location-responsive V1 cell (top) and a CA1 place cell (bottom) with spatially overlapping firing fields (with 50–100% overlap). (B) A non-overlapping pair: a location-responsive V1 cell (top) and a CA1 place cell (bottom) with spatially non-overlapping firing fields. (C) A non-responsive pair: a non-location-responsive V1 cell (top) and a CA1 place cell (bottom). Boxed areas: the spatial intervals for probing the co-fluctuation of a given pair. For overlapping pair (A) and non-overlapping pairs (B), the spatial intervals were their firing fields. For Non-responsive pairs (C), the spatial interval for the location-responsive cell was its firing field, whereas for the non-location-responsive cell it was the location-responsive cell's firing field shifted with a small random distance that yielded a random overlap of 50–100% with the firing field of the other cell. The same V1 cell and CA1 place cell can be involved in multiple pairs. In this example the same CA1 place cell appeared in 3 pairs.
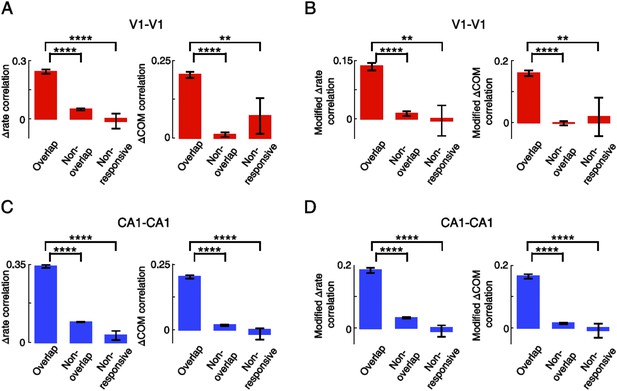
Overlapping V1-V1 and CA1-CA1 cell pairs displayed correlated lap-by-lap fluctuation in firing rate and COM within their firing fields.
(A) Average correlation in ∆rate and ∆COM for pairs of V1 location-responsive cells with overlapping firing fields (Overlapping), pairs of V1 location-responsive cells with non-overlapping firing fields (Non-overlapping), and pairs made of one location-responsive V1 cell and one non-location-responsive V1 cell (Non-responsive). (B) Same as (A), but after the modulation by speed and head direction was removed. (C, D) Same as A and B, but for CA1-CA1 cell pairs. Number of V1-V1 pairs: N = 803 overlapping pairs; 953 non-overlapping pairs; 30 non-responsive pairs. Number of CA1-CA1 pairs: N = 1621 overlapping pairs; 11,273 non-overlapping pairs; 121 non-responsive pairs. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001; t-test.
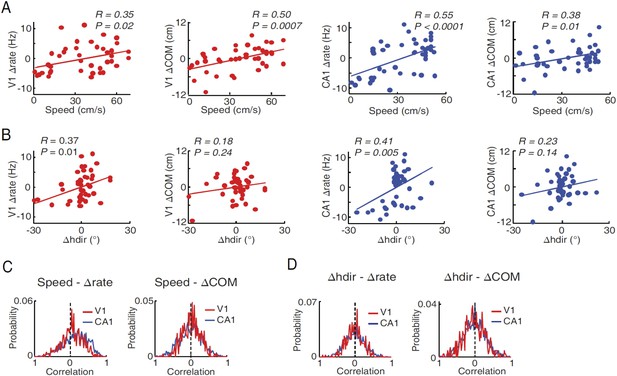
Modulation of V1 and CA1 firing activities by speed and head direction.
(A) Correlations between lap-by-lap speed and Δrate and between lap-by-lap speed and ΔCOM for example V1 and CA1 cells (the same as in Figure 6—figure supplement 1, panel B). Solid line: linear regression. R, P: Pearson's correlation and the associated p-value. Overall, speed was significantly correlated (p < 0.05) with Δrate in 25% (N = 107 out of 428) of V1 location-responsive cells and 41% (N = 619 out of 1510) of CA1 place cells, and with ΔCOM in 14% (N = 60) of V1 location-responsive cells and 25% (N = 378) of CA1 place cells. (B) Same as A, but between head direction fluctuation (Δhdir) and Δrate and between Δhdir and ΔCOM for the same V1 and CA1 cells in A. Δhdir was significantly correlated with Δrate in 12% (N = 51) of location-responsive V1 cells and in 21% (N = 317) of CA1 place cells, and with ΔCOM in 23% (N = 98) of location-responsive V1 cells and in 23% (N = 347) of CA1 place cells. (C) Distributions of speed-Δrate and speed-ΔCOM correlation values for all CA1 place cells and location-responsive V1 cells. Dashed lines: 0 correlation. The speed-Δrate distribution was skewed to the positive side for both V1 (0.064 ± 0.014; p < 0.0001, t-test compared with 0) and CA1 cells (0.16 ± 0.009, p < 0.0001), indicating that at the population level both V1 and CA1 cells increased their firing rates as speed increased. The speed-ΔCOM distribution was slightly but significantly skewed toward a positive mean for V1 (0.029 ± 0.011, p = 0.014), but not for CA1 cells (0.010 ± 0.008, p = 0.18), suggesting that, as speed increased, V1 cells' firing locations tended to move slightly forward along the animal's movement direction. (D) Same as (C), but for Δhdir-Δrate and Δhdir-ΔCOM distributions. The Δhdir–Δrate distribution was centered near 0 for both V1 (−0.0052 ± 0.011, p = 0.44) and CA1 (0.0057 ± 0.0073, p = 0.64) cells, indicating no systematic relationship between firing rate and head direction at the population level, even though each individual cell could increase or decrease firing rate as head direction was changed from left to right or vice versa. This result is expected if we assume that the V1 or CA1 cells as a group should not show any preferred head direction, even through individual V1 cells are tuned to particular directions. The Δhdir - ΔCOM distribution was similarly centered near 0 for both V1 (0.009 ± 0.015, p = 0.53) and CA1 (0.003 ± 0.008, p = 0.68) (right).
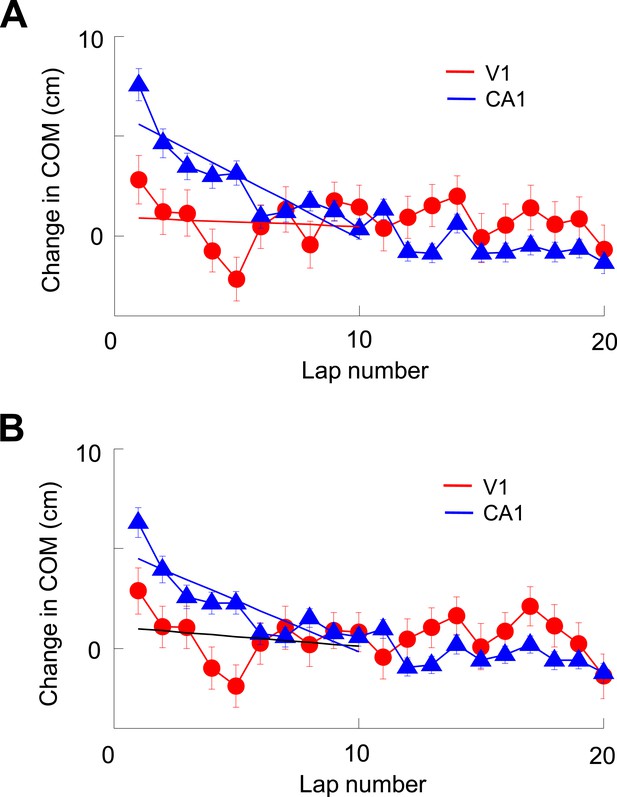
V1 location-responsive cells showed much less lap-by-lap backward shift in their firing locations than CA1 place cells.
(A) Average lap-by-lap changes in COM for V1 location-responsive cells (red, N = 670) and CA1 place cells (blue, N = 1743). The COM change of a firing field at each lap was computed relative to its stabilized value, which was the average of those values at laps #21–25. Solid lines: linear regressions between the COM change and lap numbers for the first 10 laps. It can be seen that the COMs of CA1 place fields significantly and systematically shifted backward (COM decreased with lap number) along the animal's moving direction (p < 0.0001, one-way ANOVA; Pearson's R = −0.89, p = 0.0006). The COMs of V1 firing fields appeared to shift backward during the first 5 laps or so, but fluctuated forward/backward in later laps. As a result, there was no significant change in COM (p = 0.09, one-way ANOVA) within the first 10 laps and no significant correlation between average COM change and lap number (Pearson's R = 0.10, p = 0.79). In addition, the average change in COM of V1 firing fields was significantly less than that of CA1 place fields within the first 10 laps (p < 0.0001, two-way ANOVA). (B) Same as A, but for lap-by-lap COM change of V1 and CA1 cells after removing the modulation by speed and head direction. The results are similar. There was a systematic backward shifting of the modified COM for CA1 place fields (p < 0.0001, one-way ANOVA; Pearson's R = 0.86, p = 0.0014), but not so for V1 firing fields (p = 0.13; R = 0.23, p = 0.53; comparison between CA1 and V1: p < 0.0001, two-way ANOVA). Therefore, the analysis indicates that V1 firing fields showed much less dynamics at the short-term lap-by-lap time scale than CA1 place fields.
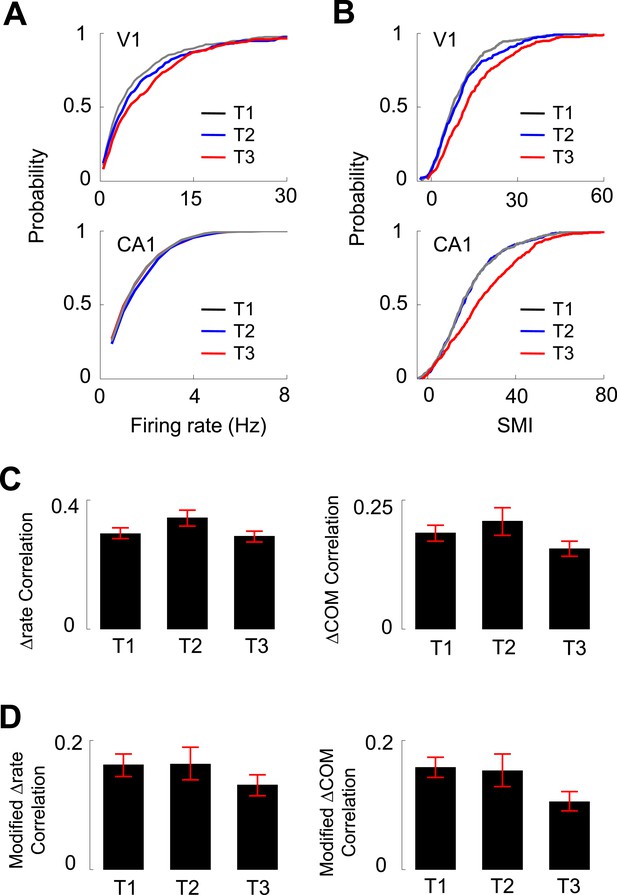
Experience dependence of V1 and CA1 firing activities on the C-shaped track.
(A, B) Cumulative distributions of overall firing rate (A) and SMI (B) of active V1 and CA1 cells on different days (T1 – T3, see texts for definition). (C) The average (mean ± S.E.) correlation in the lap-by-lap Δrate (left) and ΔCOM (right) fluctuations for overlapping V1-CA1 cell pairs on different days. (D) Same as C, but for the correlation in modified Δrate and ΔCOM after removing the modulation by speed and head direction.
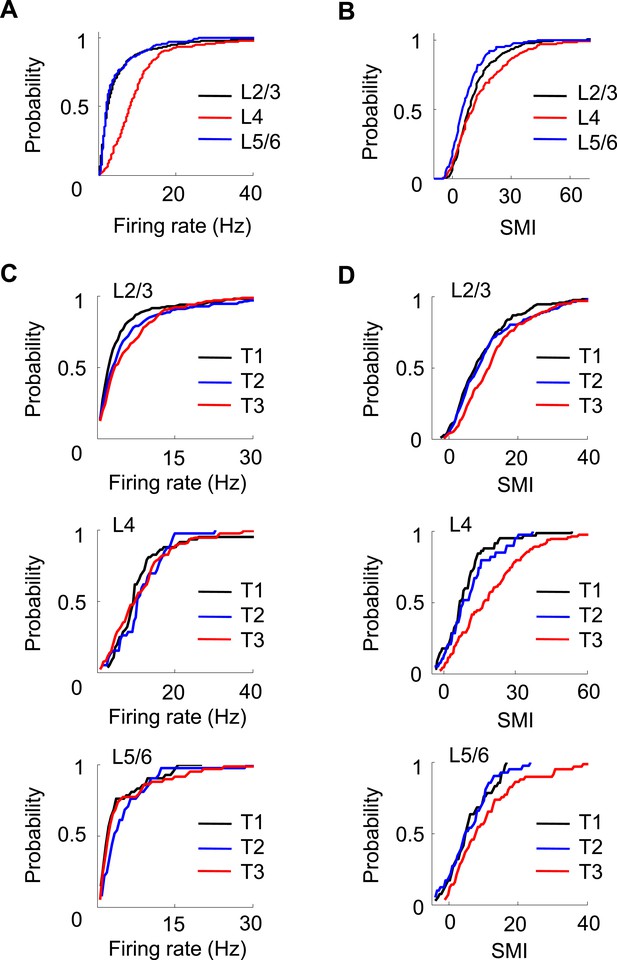
Layer differences in V1.
(A, B) Cumulative distributions of overall firing rate (A) and SMI (B) for active cells in L2/3, L4 and L5/6. (C, D) Cumulative distributions of firing rate and SMI for L2/3, L4, and L5/6 cells on different days (T1–T3).





