Circularization restores signal recognition particle RNA functionality in Thermoproteus
Figures
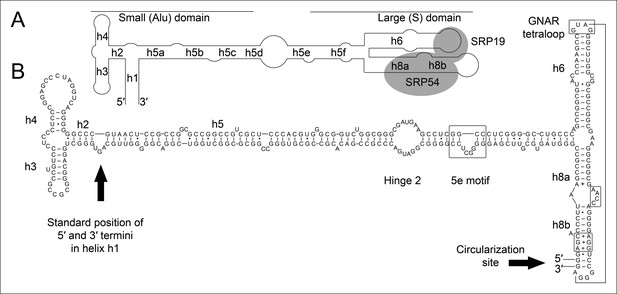
Secondary structure and sequence of the T. tenax SRP RNA.
(A) Schematic representation of the canonical archaeal SRP RNA structure. The approximate location of small and large domains, helices (h1 h8) and binding sites of SRP19 and SRP54 proteins are indicated. SRP RNA termini are located in helix h1 in the small domain. (B) Sequence and structure of the permuted SRP RNA in T. tenax. Conserved features and tertiary interactions are indicated. SRP RNA termini are located at the tip of helix h8 in the large domain.
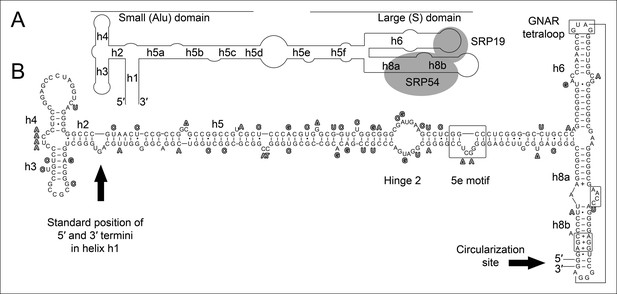
Conservation of permuted SRP RNAs.
Nucleotides that differ between the permuted SRP RNAs of T. uzoniensis and T. tenax are highlighted.
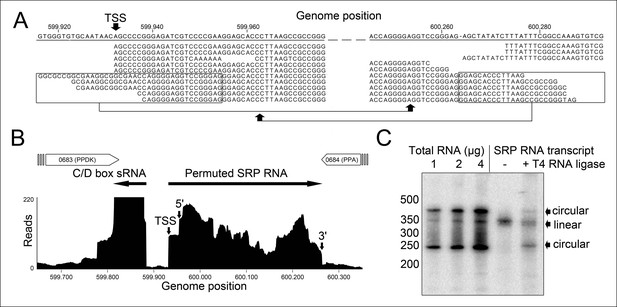
Identification of a permuted circular SRP RNA.
(A) Illumina Hiseq2000 example reads are mapped to the T. tenax reference genome (top sequence) and indicate the transcription start site (TSS) of SRP RNA precursors or contain the circularization junction. Parts of these reads (boxed) map to the distant SRP RNA terminus and highlight ligated 5′ and 3′ ends in helix h8b. (B) Sequence coverage at the intergenic region between the genes TTX_0683 and TTX_0684. Reads were obtained for the permuted SRP RNA and an adjacent C/D box sRNA (>10000 reads). (C) Northern Blot analyses verify the presence of the permuted RNA. Three signals were identified that might represent different stable structures of SRP RNA molecule. 15 ng of linear or circularized SRP RNA transcripts serve as size-markers and verify the altered running behavior of different SRP RNA forms.
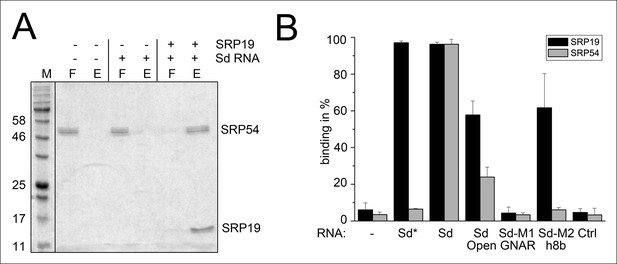
Binding of SRP19 and SRP54 to the S-domain of the permuted SRP RNA.
(A) Proteins of flow-through (F) and elution (E) fractions of DEAE-columns are separated on 15% SDS polyacrylamide gels next to a protein marker (M). SRP54 has weak affinity to the S-domain (Sd) RNA. The addition of SRP19 triggers interaction of SRP54 with Sd RNA. (B) Analysis of three independent binding assays of SRP19 and SRP54 to the different S-domain constructs (Sd: S-domain, Open: non-circularized version of the S-domain, GNAR: point-mutation in the GNAR motif, h8b: triple mutation in the helix 8b, Ctrl: control RNA of similar length). Sequences are listed in Tab. S2. Binding of the individual proteins is indicated as Sd*.
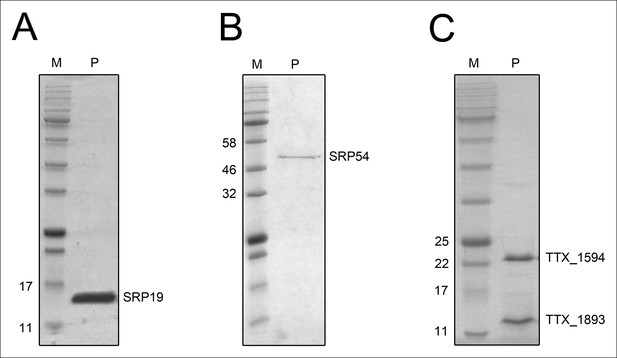
Purified recombinant T. tenax SRP proteins and splicing endonuclease.
The purified proteins (P) were separated on 15% SDS-polyacrylamide gels next to protein marker (M). (A) SRP19 (theoretical MW: 11.4 kDa) was purified via heat-precipitation (70°C) followed by a Ni-NTA chromatography. (B) SRP54 (theoretical MW: 48.8 kDa) was purified via Ni-NTA chromatography, followed by heat-precipitation (70°C) and heparin cation-exchange chromatography. (C) The two subunits of the splicing endonuclease (TTX_1594: catalytic subunit, theoretical MW: 20.8 kDa, TTX_1893: structural subunit, theoretical MW: 11 kDa) were produced together and purified via heat-precipitation (80°C) and Ni-NTA chromatography.
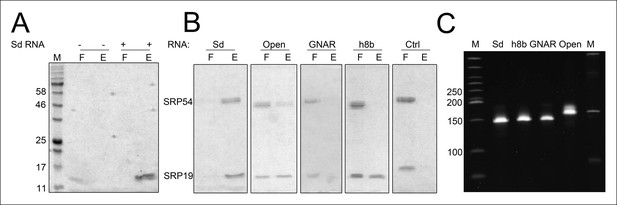
Binding of SRP19 and SRP54 to SRP RNA variants.
Proteins of flow-through (F) and elution (E) fractions of DEAE-columns are separated on 15% SDS polyacrylamide gels next to a protein marker (M). (A) SRP19 shows binding to the S-domain of the SRP RNA (Sd RNA). (B) SRP19, SRP54 and different S-domain constructs were loaded (Sd: S-domain, Open: non-circularized version of the S-domain, GNAR: point-mutation in the GNAR motif, h8b: triple mutation in the helix 8b, Ctrl: control RNA with similar length). (C) Loading control of the SRP S-domain RNA constructs. To verify the integrity and running behavior of the four constructed S-domain RNA variants, 100 ng of the transcripts were loaded onto a 8% denaturing polyacrylamide gel next to a DNA ladder (M1, bands marked in bp) as well as a RNA ladder (M) and SYBR Gold stained.
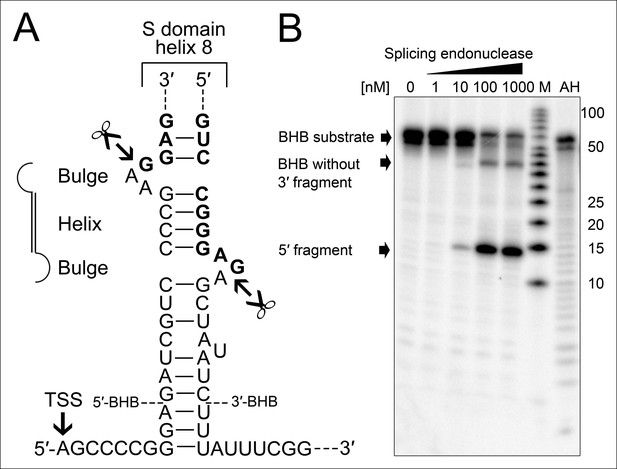
Processing of the SRP RNA circularization site by the T. tenax tRNA splicing machinery.
(A) Structure of the proposed BHB motif at the junctions of the leader and trailer sequences (normal letters) and the SRP RNA (bold letters). Cleavage sites are indicated. (B) Increasing concentrations of recombinant T. tenax splicing endonuclease reveal a distinct processing pattern of 5′-radioactively labeled BHB substrates of the SRP RNA precursor. A label size marker (M) and alkaline hydrolysis (AH) ladder are included. The BHB substrate termini are indicated in Figure 4A and the internal sequence is closed by a 3 bp stem and GNAR loop (Supplementary file 1B).
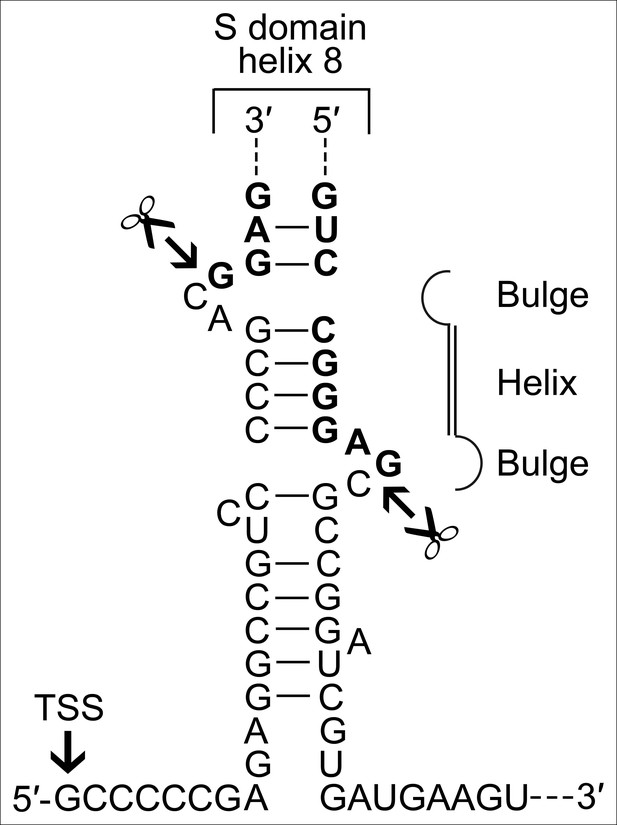
A BHB motif is present at the termini of the permuted T. uzoniensis SRP RNA gene.
Structure of the proposed BHB motif at the junctions of the leader and trailer sequences (normal letters) and the SRP RNA (bold letters). Cleavage sites are indicated.
Additional files
-
Supplementary file 1
Signal peptide analysis and oligonucleotide sequences.
This text-file contains two extended tables. Supplementary file 1A contains a list of predicted signal peptides for T. tenax. Supplementary file 1B lists all oligonucleotides and RNA sequences that were used in this study for cloning and RNA substrate generation.
- https://doi.org/10.7554/eLife.11623.011





