Transcriptomics: Resting easy with a sleep regulator
We are able to sleep as a result of a delicate balance between competing signals from complex circuits of neurons. In the hypothalamus, neurons that produce a neuropeptide called hypocretin (Hcrt; also known as orexin) control the transition between being asleep and being awake (Adamantidis et al., 2007; de Lecea, 2015). These neurons also coordinate waking up (arousal) in response to various stimuli (Giardino and de Lecea, 2014; Bonnavion et al., 2015). Thus, finding out how the activity of Hcrt neurons is regulated will be vital for understanding how these neurons maintain a healthy balance between the sleep and the awake states, and how this equilibrium is disturbed in individuals with sleep disorders. Now, in eLife, Lior Appelbaum and colleagues – including Laura Yelin-Bekerman as first author – report a new role for a potassium ion channel in the regulation of arousal (Yelin-Bekerman et al., 2015).
Yelin-Bekerman et al. – who are based at Bar-Ilan University, Toldot Genetics, the Weizmann Institute of Science and the Massachusetts Institute of Technology – studied zebrafish (Danio rerio), which display decreased movement patterns during the night that are characteristic of sleep. Moreover, they only have 16–20 Hcrt neurons, compared to more than 5000 cells in mammals. Using transgenic zebrafish that express enhanced green fluorescent protein only in their Hcrt neurons (hcrt-eGFP) (Faraco et al., 2006), Yelin-Bekerman et al. used a technique called fluorescence-assisted cell sorting to isolate the hcrt-eGFP cells and extract messenger RNA (Figure 1A). They then amplified the RNA material before carrying out whole-transcriptome sequencing to measure gene expression. Applying their most conservative criteria, Yelin-Bekerman et al. generated a list of 20 gene transcripts that are found at higher levels in Hcrt neurons than in other brain cells. These genes included fam46a and other markers of Hcrt neurons that have previously been reported in higher vertebrates (Dalal et al., 2013). With a more relaxed threshold, 212 transcripts met the criteria for being enriched in Hcrt neurons.
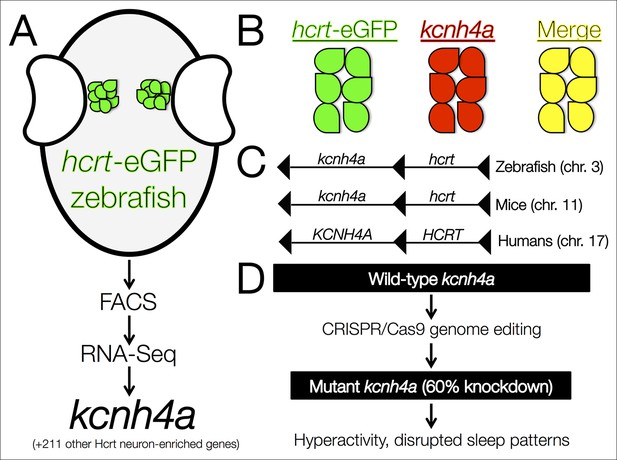
The kcnh4a gene is enriched in Hcrt neurons and promotes sleep in zebrafish.
(A) Fluorescent-assisted cell sorting (FACS) of neurons from hcrt-eGFP zebrafish followed by all-mRNA sequencing (RNA-seq) identified 212 genes that are significantly enriched in Hcrt neurons, including the voltage-gated potassium channel kcnh4a. (B) In situ hybridization for knch4a confirmed its expression in 100% of hcrt-eGFP neurons. (C) Genomic analyses revealed that kcnh4a lies directly upstream of the hcrt gene, a phenomenon that is conserved from zebrafish to humans. (D) CRISPR/Cas9 genome editing produced a mutant zebrafish with a 60% reduction in kcnh4a levels, resulting in hyperactivity and disrupted patterns of nighttime sleep.
Using in situ hybridization, Yelin-Bekerman et al. then tested the degree to which the hcrt-eGFP and the 20 gene transcripts co-localize: they were able to confirm that several of the candidates are highly expressed within Hcrt neurons, and that others were actually expressed in nearby non-Hcrt neurons instead. Further analysis revealed that many of the genes contained conserved motifs for the binding of transcription factors, such as pax4, which is predicted to regulate as many as 44 genes in Hcrt neurons.
Of the transcripts that were verified as being enriched in Hcrt neurons, Yelin-Bekerman et al. focused on one called kcnh4a, which was the only one that was expressed in all hcrt-eGFP neurons in both zebrafish larvae and adults (Figure 1B). It encodes a potassium channel and further genetic analyses revealed that kcnh4a is located directly upstream of the hcrt gene in the zebrafish genome. In fact, these two genes are directly adjacent to each other throughout the animal kingdom, including in mice and humans (Figure 1C). The expression patterns and tight genetic linkage of the two genes suggests that well-preserved mechanisms govern their interactions at the protein level.
Pursuing this rationale, Yelin-Bekerman et al. examined whether the Kcnh4a protein is important for behaviors that are regulated by Hcrt neurons (Figure 1D). The authors used CRISPR/Cas9 genome editing to produce mutant zebrafish that had reduced levels of kcnh4a expression. Compared to wild-type zebrafish, these mutants showed significantly increased movement in both the light and dark periods of a 24-hour cycle. In further analyses in which sleep was defined as a period of inactivity that is longer than six seconds (Yokogawa et al., 2007), Yelin-Bekerman et al. reported decreased overall sleep time and shorter average bouts of sleep in kcnh4a mutants relative to control zebrafish, specifically during the dark periods.
An obvious next step is to determine whether Kcnh4a is also co-expressed with the Hcrt neuropeptide in mammals, and whether it plays a similar role in the sleep-wake transition. In vivo imaging with genetically-encoded calcium or voltage sensors in kcnh4a mutants will likely be useful in understanding the role of this potassium channel in the intrinsic properties of Hcrt neurons. Also, the unique properties of the zebrafish model make it possible to image the activity of single neurons and entire networks during 24-hour periods, and to monitor changes induced by sleep or wake-promoting drugs. In fact, Kchn4a is an intriguing potential new drug target, and may be highly relevant for therapeutic approaches to treat conditions like insomnia and narcolepsy.
References
-
Regulation of hypocretin (orexin) expression in embryonic zebrafishJournal of Biological Chemistry 281:29753–29761.https://doi.org/10.1074/jbc.M605811200
-
Hypocretin (orexin) neuromodulation of stress and reward pathwaysCurrent Opinion in Neurobiology 29:103–108.https://doi.org/10.1016/j.conb.2014.07.006
-
Optogenetic control of hypocretin (orexin) neurons and arousal circuitsCurrent Topics in Behavioral Neurosciences 25:367–378.https://doi.org/10.1007/7854_2014_364
Article and author information
Author details
Publication history
- Version of Record published: December 10, 2015 (version 1)
Copyright
© 2015, Giardino et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,186
- views
-
- 155
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
Recent studies show that, even in constant environments, the tuning of single neurons changes over time in a variety of brain regions. This representational drift has been suggested to be a consequence of continuous learning under noise, but its properties are still not fully understood. To investigate the underlying mechanism, we trained an artificial network on a simplified navigational task. The network quickly reached a state of high performance, and many units exhibited spatial tuning. We then continued training the network and noticed that the activity became sparser with time. Initial learning was orders of magnitude faster than ensuing sparsification. This sparsification is consistent with recent results in machine learning, in which networks slowly move within their solution space until they reach a flat area of the loss function. We analyzed four datasets from different labs, all demonstrating that CA1 neurons become sparser and more spatially informative with exposure to the same environment. We conclude that learning is divided into three overlapping phases: (i) Fast familiarity with the environment; (ii) slow implicit regularization; and (iii) a steady state of null drift. The variability in drift dynamics opens the possibility of inferring learning algorithms from observations of drift statistics.
-
- Neuroscience
Nociceptive sensory neurons convey pain-related signals to the CNS using action potentials. Loss-of-function mutations in the voltage-gated sodium channel NaV1.7 cause insensitivity to pain (presumably by reducing nociceptor excitability) but clinical trials seeking to treat pain by inhibiting NaV1.7 pharmacologically have struggled. This may reflect the variable contribution of NaV1.7 to nociceptor excitability. Contrary to claims that NaV1.7 is necessary for nociceptors to initiate action potentials, we show that nociceptors can achieve similar excitability using different combinations of NaV1.3, NaV1.7, and NaV1.8. Selectively blocking one of those NaV subtypes reduces nociceptor excitability only if the other subtypes are weakly expressed. For example, excitability relies on NaV1.8 in acutely dissociated nociceptors but responsibility shifts to NaV1.7 and NaV1.3 by the fourth day in culture. A similar shift in NaV dependence occurs in vivo after inflammation, impacting ability of the NaV1.7-selective inhibitor PF-05089771 to reduce pain in behavioral tests. Flexible use of different NaV subtypes exemplifies degeneracy – achieving similar function using different components – and compromises reliable modulation of nociceptor excitability by subtype-selective inhibitors. Identifying the dominant NaV subtype to predict drug efficacy is not trivial. Degeneracy at the cellular level must be considered when choosing drug targets at the molecular level.





