Mating Behaviour: To mate or not to mate
The theory of natural selection is often summarised with the phrase ‘the survival of the fittest’. But simply surviving is not enough; organisms must also reproduce, otherwise there would be no evolution. In the animal kingdom, for example, colourful feathers, sophisticated songs and elaborate courtship rituals are all employed to increase the chances of a successful copulation. Naturalists have marvelled at these mating rituals for hundreds of years and filled countless notebooks with descriptions and drawings of them. However, a more detailed understanding of mating behaviour has had to wait for arrival of new techniques in genetics and neuroscience, and the rise to fame of the vinegar fly, Drosophila melanogaster, as a model organism.
Vinegar flies (or common fruit flies) perform an elaborate courting ritual: first, a male orients towards and follows a female; then he touches her abdomen with his foreleg and, if she responds, he extends one of his wings and performs a song-like vibration with it. Finally he licks the female’s genitalia and attempts to copulate with her. Now, in eLife, Benjamin Kallman, Heesoo Kim and Kristin Scott of the University of California at Berkeley identify further pathways that guide mating decisions in male flies (Kallman et al., 2015).
With the discovery of the transcription factor Fruitless (which is expressed only in males, and called FruM for short), we now know that around 1,500 neurons orchestrate courtship behaviour in these flies (Manoli et al., 2005; Stockinger et al., 2005). However, little is known about how these neurons are connected to achieve this behaviour. Previous studies have shown that chemicals present on the cuticle can affect courtship behaviour in flies (Yamamoto and Koganezawa, 2013). The cuticular pheromones produced by a female fly promote mating and allow the courting male to identify her as a female of the same species; the pheromones produced by males have the opposite effect and inhibit courtship. Flies detect these compounds via a pair of sensory neurons housed in their leg bristles. The neurons that respond to male pheromones are called M cells, and those that respond to female pheromones are called F cells (Figure 1; Pikielny, 2012; Thistle et al., 2012).
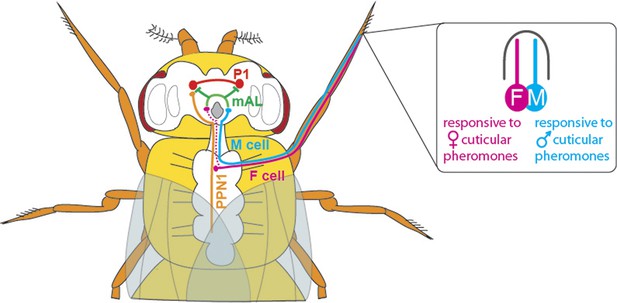
The neuronal circuitry underlying male-specific courtship behaviour in flies.
The leg bristles of flies contain neurons called F cells (shown in pink) that respond to female pheromones, and M cells (blue) that respond to male pheromones. Kallman et al. show that the F cells connect with PPN1 neurons (orange) in the ventral nerve cord; the PPN1 neurons then activate P1 neurons (red) in a part of the higher brain called the protocerebrum. The M cells project to the mAL neurons (green), which inhibit the P1 neurons. The F cells also indirectly activate the mAL neurons (represented by the dotted line).
Kallman et al. used genetic and behavioural tools to show that activating the F and M cells can modify male courtship behaviour. In a second step, they were able to identify the connection between the F and M cells in the leg and a cluster of neurons (called P1 neurons) that is found only in the brain of male flies. Neurobiologists have become increasingly interested in P1 neurons over the last decade, and have shown that activating them correlates with the start of courtship behaviour (Clowney et al., 2015; Kohatsu et al., 2011). However, until recently, the roads that lead to P1 were hardly known.
Kallman et al. then monitored activity in the P1 cluster when they activated either F cells or M cells in the forelegs of male flies. F cell activation was seen to excite the P1 cluster and resulted in enhanced courtship, whereas M cell activation did not excite the P1 cluster and may even have suppressed it. However, given that F cells terminate in the fly’s thorax, Kallman et al. found that another class of neurons, called PPN1, serves as the link between F cells and the P1 cluster in the higher brain (Figure 1).
To uncover the targets of the M cells, Kallman et al. looked at the neurons that express the FruM transcription factor and observed how their activity changed when M cells were stimulated. They found that the M cells activated a cluster of neurons called mAL (Figure 1); this cluster had previously been shown to send out inhibitory signals (Kimura et al., 2005). Inactivating the mAL neurons led to males courting each other. But how does mAL normally inhibit male-male courtship? In a final set of experiments, Kallman et al. showed that mAL neurons form connections with P1 neurons and inhibit their activity. Thus, while activating F cells led to an overall activation of P1, activating M cells resulted in an overall inhibition of P1. This suggests that the balance between excitation and inhibition determines this male-specific behaviour.
The work of Kallman, Kim and Scott represents a beautiful example of how sensory information can be processed by a very restricted number of neurons to generate highly complex forms of behaviour. The future will tell whether this is a general feature of sensory networks.
References
-
Genes and circuits of courtship behaviour in Drosophila malesNature Reviews Neuroscience 14:681–692.https://doi.org/10.1038/nrn3567
Article and author information
Author details
Publication history
- Version of Record published: December 23, 2015 (version 1)
Copyright
© 2015, Campetella et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,569
- views
-
- 177
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
Cholecystokinin (CCK) is an essential modulator for neuroplasticity in sensory and emotional domains. Here, we investigated the role of CCK in motor learning using a single pellet reaching task in mice. Mice with a knockout of Cck gene (Cck−/−) or blockade of CCK-B receptor (CCKBR) showed defective motor learning ability; the success rate of retrieving reward remained at the baseline level compared to the wildtype mice with significantly increased success rate. We observed no long-term potentiation upon high-frequency stimulation in the motor cortex of Cck−/− mice, indicating a possible association between motor learning deficiency and neuroplasticity in the motor cortex. In vivo calcium imaging demonstrated that the deficiency of CCK signaling disrupted the refinement of population neuronal activity in the motor cortex during motor skill training. Anatomical tracing revealed direct projections from CCK-expressing neurons in the rhinal cortex to the motor cortex. Inactivation of the CCK neurons in the rhinal cortex that project to the motor cortex bilaterally using chemogenetic methods significantly suppressed motor learning, and intraperitoneal application of CCK4, a tetrapeptide CCK agonist, rescued the motor learning deficits of Cck−/− mice. In summary, our results suggest that CCK, which could be provided from the rhinal cortex, may surpport motor skill learning by modulating neuroplasticity in the motor cortex.
-
- Neuroscience
Probing memory of a complex visual image within a few hundred milliseconds after its disappearance reveals significantly greater fidelity of recall than if the probe is delayed by as little as a second. Classically interpreted, the former taps into a detailed but rapidly decaying visual sensory or ‘iconic’ memory (IM), while the latter relies on capacity-limited but comparatively stable visual working memory (VWM). While iconic decay and VWM capacity have been extensively studied independently, currently no single framework quantitatively accounts for the dynamics of memory fidelity over these time scales. Here, we extend a stationary neural population model of VWM with a temporal dimension, incorporating rapid sensory-driven accumulation of activity encoding each visual feature in memory, and a slower accumulation of internal error that causes memorized features to randomly drift over time. Instead of facilitating read-out from an independent sensory store, an early cue benefits recall by lifting the effective limit on VWM signal strength imposed when multiple items compete for representation, allowing memory for the cued item to be supplemented with information from the decaying sensory trace. Empirical measurements of human recall dynamics validate these predictions while excluding alternative model architectures. A key conclusion is that differences in capacity classically thought to distinguish IM and VWM are in fact contingent upon a single resource-limited WM store.






