Identification of a novel spinal nociceptive-motor gate control for Aδ pain stimuli in rats
Figures
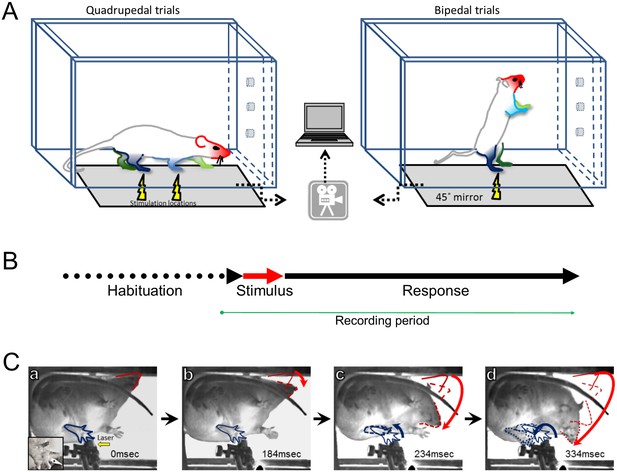
Experimental design and protocol.
(A) Adult unrestrained rats were placed in a Plexiglas chamber which allows them to move freely. The chamber was placed on an elevated glass or a wire mesh surface depending on the stimulus type (i.e. laser; sharp or blunt). Stimulus location is indicated by the yellow lightning symbol and the limbs and head are color coded. Selective thermal activation was achieved using a brief (100 ms), small-diameter (1.6 mm), and high-energy (5000 mA) infrared diode laser pulse. Pinprick stimuli were applied by pushing a sharp pin against the foot pad until the skin was dimpled but not penetrated and a rounded wooden stick was used for non-noxious blunt stimulus. A high-speed camera captured the animal’s postural changes via a mirror under the platform surface. The collected data were transferred and stored on a computer for off-line analysis. (B) The experimental protocol had three phases: habituation– allowing the rat to get used to the chamber and the required posture (on four or two limbs); stimulus – sensory stimulation was delivered to the target limb; response– a 500–800 ms window where any postural changes or movements were documented. (C) A typical response to a thermal Aδ afferent activation. A sequence of video frames (a–d) demonstrating the response to a laser pulse applied to the left hind limb of rat standing on four limbs (Video 1) . For simplicity, only the stimulated limb (blue) and the head (red) are marked. Immediately after the stimulus (a– 0 ms), all limbs are in place and no movement has occurred. Bottom left insert: zoom in to the stimulated hindpaw where the black dot represents the laser target. The head was the first body part to move (b), followed by the movement of the stimulated limb (c) while the head continued moving toward the stimulated area. Red and blue arrows demonstrate the overall trajectory of the head and left hindlimb movement, respectively.
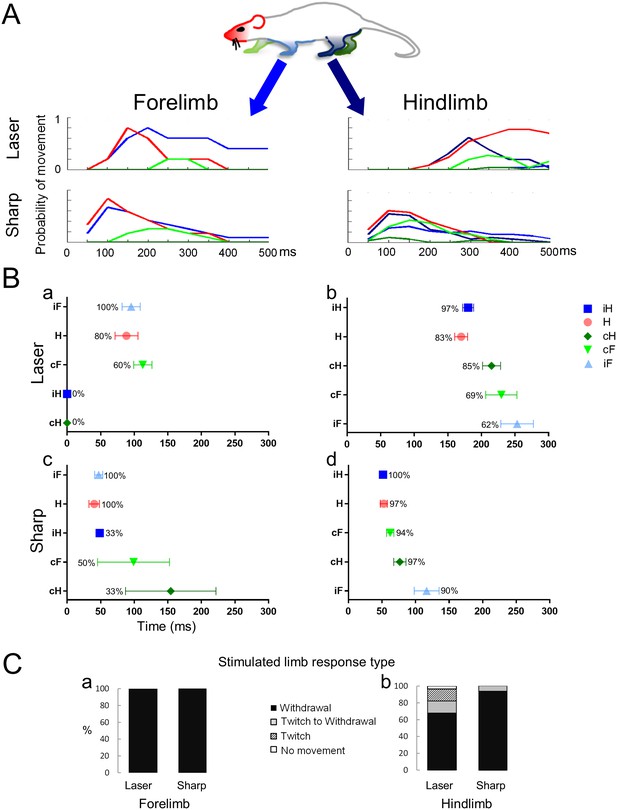
Noxious stimuli evoke a complex multi-segmental response.
The graphs show the temporal progression of the head and limb responses to a noxious stimulus applied to the forelimb or to the hindlimb. (A) Color codes for the head and the limbs and movement histograms showing the probability of a head or limb movement in 50 ms bins. (B) Scatter plots showing the mean first response (± S.E.M) of the movements and the probability of a movement (%) evoked by stimuli given to the forelimb (Ba and Bc) or the hindlimb (Bb and Bd). The averaged first response time for the stimulated limb is on top (ipsilateral forelimb for Ba and Bc; ipsilateral hindlimb for Bb and Bd). The other limbs and the head are presented according to their appearance after the stimulus. Notice that the head is the most likely to move regardless the stimulus type or location and that the hindlimbs do not move when the laser stimulus is applied to a forelimb paw. (C) Bars representing the frequency distribution of the response type (full withdrawal, twitch to withdrawal, twitch only, and no movement of the stimulated limb) of the stimulated limb. The stimulated forelimb responded with a full withdrawal 100% of the times to both sharp and laser stimuli. H– Head (●); iF- ipsilateral forelimb (▲); iH– ipsilateral hindlimb (■); cF– contralateral forelimb (▼); cH– contralateral hindlimb (♦).
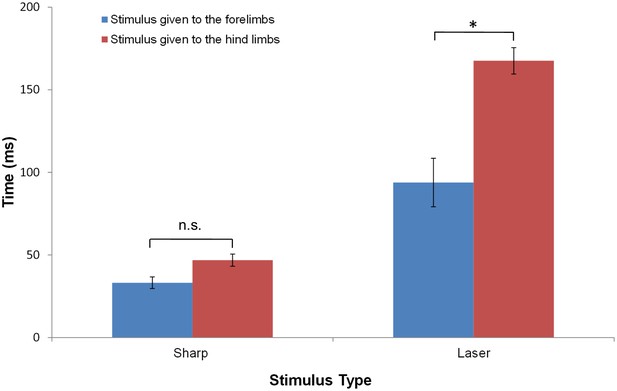
The average response latency to Aδ noxious stimulus is dependent on the stimulus modality and location.
The bar plots show the average latency and standard error of the mean for the first response of any limb following a sharp or a laser stimulus to the fore- (blue) or hind- (red) limb of a rat standing on all four limbs. Collectively, the data show that the earliest responses were to sharp stimuli and the slowest were to laser stimuli. The averaged first response latencies for forelimb stimulation were shorter than those for hindlimb stimulation. The shortest latency was found when the sharp stimulus was applied to the forelimb (33.2 ± 13.0 ms, n = 14) or to the hindlimbs (46.9 ± 21.0 ms, n = 31). These latencies were not significantly different. A significant difference in the latencies between forelimb and hindlimb stimulation was found for the responses to laser stimulation (Forelimb: 93.9 ± 33.0 ms, n = 5; Hindlimb: 167.5 ± 43.0 ms, n = 29; *p<0.0001). (Two-way ANOVA; Tukey’s multiple comparisons test).
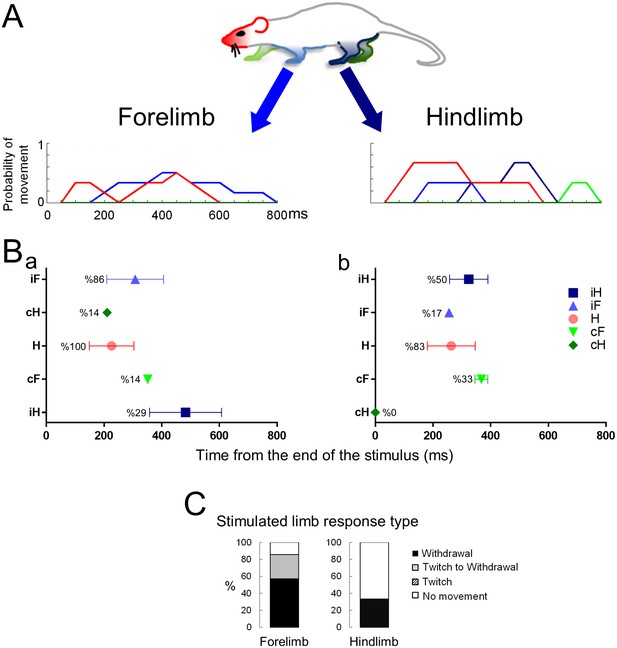
The response to a blunt stimulus when the animal is standing on four legs.
Movement histograms (A) and averaged first response scatter plots with the probability (%) of a movement (B) showing the movements evoked by stimuli to the forelimb (left panels) or hindlimb (right panels) paws. (C) Bar plots representing the proportion of withdrawals (■), twitches (■), or no movements (□) of the stimulated limb. Color codes for the head and the limbs as in Figure 2. H– Head; iF- ipsilateral forelimb; iH– ipsilateral hindlimb; cF– contralateral forelimb; cH– contralateral hindlimb.
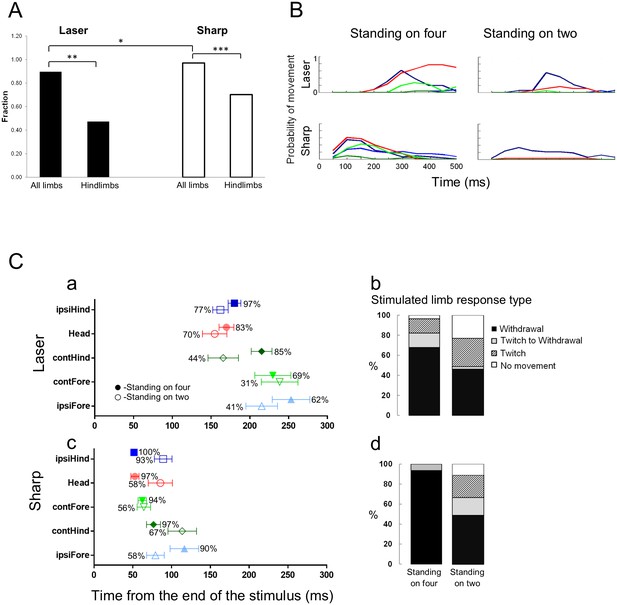
Standing on hindlimbs decreased the response frequency to noxious stimuli and resulted in fewer stimulus-evoked limb movements.
Comparison of the response rate to laser or sharp stimulus given to the hindlimbs under two different postural conditions. (A) A significant difference in the response rate was found when the rats were standing only on their hindlimbs compared to all four limbs. The proportion of rats responding to the laser or sharp stimulus was significantly lower when the rats were standing on their hindlimbs (laser on four vs. laser on hindlimbs, **p<0.01; sharp on four vs. sharp on hindlimbs, ***p<0.001; Fisher Exact test 2 × 2 contingency table, see also Table 1). A significant difference was also found between the response rate to laser and sharp stimuli when the rats were standing on all four limbs (*p<0.05). No significant difference was found between the fraction of rats responding to laser stimulus compared to the sharp stimulus (laser on hindlimbs vs. sharp on hindlimbs, p>0.1). (B) Movement histograms show a reduction in the number of moving limbs when the rat is standing on its hindlimbs (the probability histograms on the left are adapted from Figure 2a for comparison). (C) The effects of posture on the averaged first response (plots with probability of movement (%)) (Ca, Cc) and the stimulated limb response type distribution (Cb and Cd). Filled symbols represent the average first response when the rat is in quadrupedal stance (from Figure 2 for comparison). Unfilled symbols represent the average first response when the rat is rearing on its hindlimbs. (Data presented on the left part of B and C is adapted from Figure 2 for comparison).
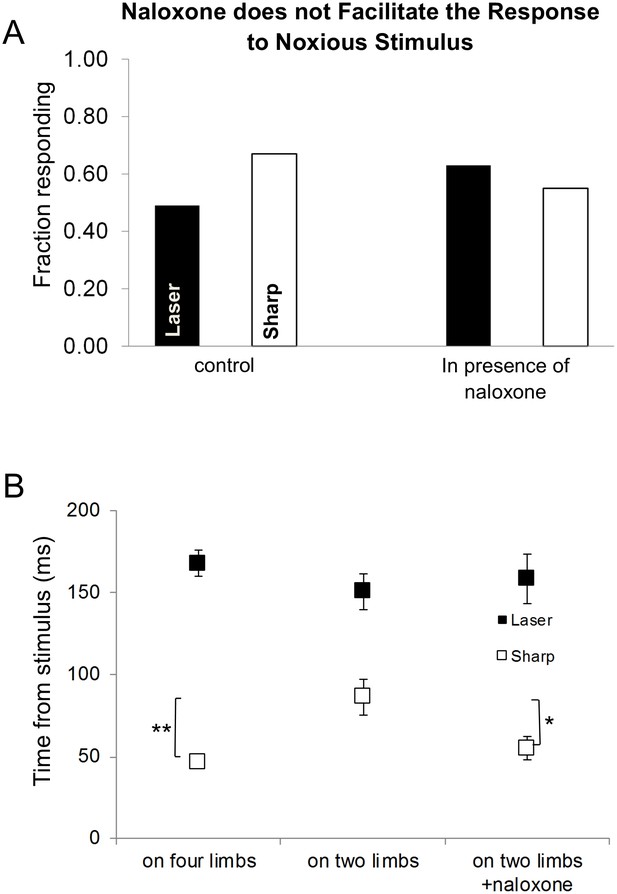
The effects of posture on the latency of the first response.
(A) Systemic naloxone (5 mg/kg) did not enhance the response rate to noxious stimulus and did not reverse the postural inhibition of paw withdrawal when the animal was standing on its hindlimbs. In the presence of naloxone, when the rat was standing on its hindlimbs, the response rate to noxious stimuli was not statistically different from that in control conditions (laser: control vs. naloxone, p>0.5; sharp: control vs. naloxone, p>0.7; laser naloxone vs. sharp naloxone, p=1) (Bars for control conditions are adapted from Figure 4). (B) In general, the first response latency to the laser stimulus was significantly longer than that to the sharp stimuli for all postural conditions. There was no significant difference in the response latencies of animals that were standing on four vs two limbs in the presence of naloxone for laser stimulus (Laser (black rectangle): 167.5 ± 8.0 ms vs. 150.3 ± 11.0 ms vs. 158.5 ± 15.0 ms; two-way ANOVA). First response latency to the sharp stimulus was significantly longer when animals stood on their hindlimbs compared to standing on four limbs (sharp (white rectangle), four limbs– 46.9 ± 4.0 ms vs. on two limbs– 86.3 ± 11.0 ms; *p<0.005; two-way ANOVA). The presence of naloxone reduced significantly the average response time to noxious sharp stimuli (on two limbs +naloxone– 55.1 ± 7.0 ms; vs. on two limbs *p<0.05) but not for animals standing on four limbs (p=0.6).
Videos
Forelimb laser stimulus when the rat is standing on all four limbs.
https://doi.org/10.7554/eLife.23584.005Forelimb sharp stimulus when the rat is standing on all four limbs.
https://doi.org/10.7554/eLife.23584.006Hindlimb laser stimulus when the rat is standing on all four limbs.
https://doi.org/10.7554/eLife.23584.007Hindlimb sharp stimulus when the rat is standing on all four limbs.
https://doi.org/10.7554/eLife.23584.008Forelimb blunt stimulus when the rat is standing on all four limbs.
https://doi.org/10.7554/eLife.23584.014Hindlimb blunt stimulus when the rat is standing on all four limbs.
https://doi.org/10.7554/eLife.23584.015Hindlimb laser stimulus when the rat is rearing up.
https://doi.org/10.7554/eLife.23584.018Hindlimb sharp stimulus when the rat is rearing up.
https://doi.org/10.7554/eLife.23584.019Hindlimb laser stimulus when the rat is rearing up in the presence of naloxone.
https://doi.org/10.7554/eLife.23584.020Hindlimb sharp stimulus when the rat is rearing up in the presence of naloxone.
https://doi.org/10.7554/eLife.23584.021Tables
Comparison between the response rate for different sensory stimuli applied to the fore– or hindlimb of a rat standing on four or two limbs. Results of Fisher Exact test, 2 × 2 contingency tables. Values in red are significant. Values in black are not significant. In parenthesis is the rate for full withdrawal for each condition.
| Laser | Sharp | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Standing on four | Standing on two | Standing on four | Standing on two | |||||||
| Forelimb (%100) | Hindlimb (%82.8) | Hindlimb (%48.7) | Hindlimb+NL (%63.2) | Forelimb (%100) | Hindlimb (%100) | Hindlimb (%66.7) | Hindlimb+NL (%55) | |||
| Laser | Standing on four | Forelimb (%100) | ||||||||
| Hindlimb (%82.8) | 1.0 | |||||||||
| Standing on two | Hindlimb (%48.7) | >0.05 | ||||||||
| Hindlimb+NL (%63.2) | >0.2 | >0.1 | >0.4 | |||||||
| Sharp | Standing on four | Forelimb (%100) | 1.0 | >0.1 | ||||||
| Hindlimb (%100) | 1.0 | <0.05 | <0.001 | <0.001 | 1.0 | |||||
| Standing on two | Hindlimb (%66.7) | >0.3 | >0.1 | >0.1 | >0.7 | <0.05 | <0.001 | |||
| Hindlimb+NL (%55) | >0.1 | >0.5 | >0.7 | >0.7 | <0.001 | <0.001 | >0.4 | |||
-
Table 1—source data 1
Proportion of withdrawals for different sensory stimuli applied to the fore- or hindlimb of a rat standing on four or two limbs.
Source data and Column histogram presenting the number of trials the rats responded with a full withdrawal to a stimulus. Each column presents the total number of trials. (Black: full withdrawal; Gray: no withdrawal). l-laser stimulus; s-sharp stimulus; 4-animal on all four limbs; 2-animal on two hind limbs; f -forelimb stimulation; h -hindlimb stimulation.
- https://doi.org/10.7554/eLife.23584.010
Comparison between the average first response latencies to different sensory stimuli applied to the fore- or hindlimb of a rat standing on four limbs. Results of two-way ANOVA analysis with Tukey’s multiple comparisons test. In parenthesis is the average latency and standard deviation per each condition.
| Sharp–Forelimb (33.2 ± 13.0 ms) | Laser—Hindlimb (167.5 ± 43.0 ms) | Laser—Forelimb (93.9 ± 33.0 ms) | |
|---|---|---|---|
| Sharp– Hindlimb (46.9 ± 21.0 ms) | ns | <0.0001 | <0.05 |
| Sharp–Forelimb (33.2 ± 13.0 ms) | <0.0001 | <0.01 | |
| Laser– Hindlimb (167.5 ± 43.0 ms) | <0.0001 |





