Crystal structure of dopamine receptor D4 bound to the subtype selective ligand, L745870
Figures
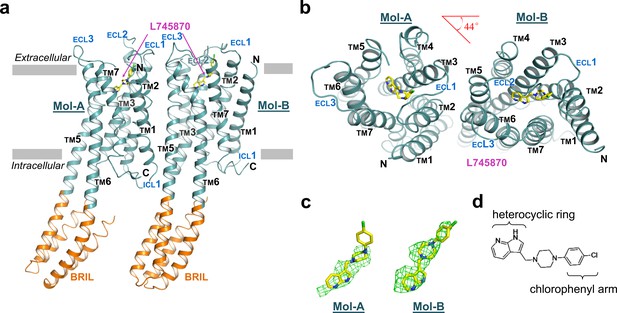
The overall structure of mouse DRD4 in complex with L745870.
(a) Ribbon representation of mouse DRD4 (TM domain presented in cyan, ligand in yellow, and BRIL fusion in orange), as viewed parallel to the membrane with approximate membrane boundaries indicated with gray lines. (b) Top view of the mouse DRD4 structure. Rotation angle between Mol-A and Mol-B calculated with PyMOL is shown as a red angle sign. (c) The L745870 antagonist is shown in stick representation with carbon, nitrogen and chloride atoms shown in yellow, blue, and green, respectively. Fo−Fc omit density map for L745870 is contoured at 2.5 σ. (Also see Figure 1—figure supplement 5) (d) Chemical structure of L745870.
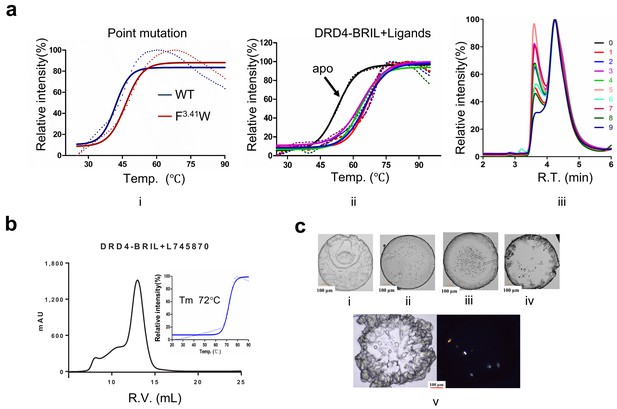
Profiles of DRD4 in different constructs or under different conditions.
(a) The ThermoFluor (Alexandrov et al., 2008) and monodispersity validation of different constructs of DRD4. The melting temperature (Tm) of WT is approx. 43°C (i, blue curve, solid line, Boltzmann sigmoid fitting, dashed line, experimental data), and the F3.41W mutation construct elevated Tm ~5°C (panel i, red curve). Tm of the BRIL-fusion protein reached above 50°C (panel ii, black curve), and when it was incubated with DRD4 ligands Tm was raised above 70°C (ii, colored curves). The size exclusion chromatography assay with nanofilm SEC-250 (Sepax Technologies) shows that the monodispersity of the fusion protein (panel iii) was improved differently with different ligands. 0: no ligands during purification; 1: thioridazine (Seeman and Lee, 1975) (Sigma-Aldrich); 2: ABT-724 (Brioni et al., 2004) (Tocris); 3: (-)quinpirole (Millan et al., 2002) (Tocris); 4: PD-168568 (Belliotti et al., 1998) (Tocris); 5: Mesoridazine (Choi et al., 2004) (Sigma-Aldrich); 6: Fananserin (Truffinet et al., 1999) (Sigma-Aldrich); 7: spiperone (Hidaka et al., 1995) (Sigma-Aldrich); 8: L745870; and 9: L750667 (Schetz et al., 2000) (Tocris). (b) The DRD4-L745870 complex shows a single peak in Superdex200 (R.V., retention volume). The Tm of the complex was determined as ~72°C. (c) Initial crystal images under different conditions (panels i–iv: visual light image) and optimized crystals (panel v: visual light image and polarized light image) used for data collection.

Protein sequence alignment of D2-like family members.
Red stars below the TM2 and TM3 sequences indicate the non-conserved residue position in the extended pocket. Black boxes show the mutations (P5.52I, P6.38A, and C45.51R) in TM5, TM6, and ECL2, which facilitated the crystallization. Triangles mark the binding residues of Nemonaride (blue) and L745870 (purple). Alignment was generated with http://gpcrdb.org/ website.
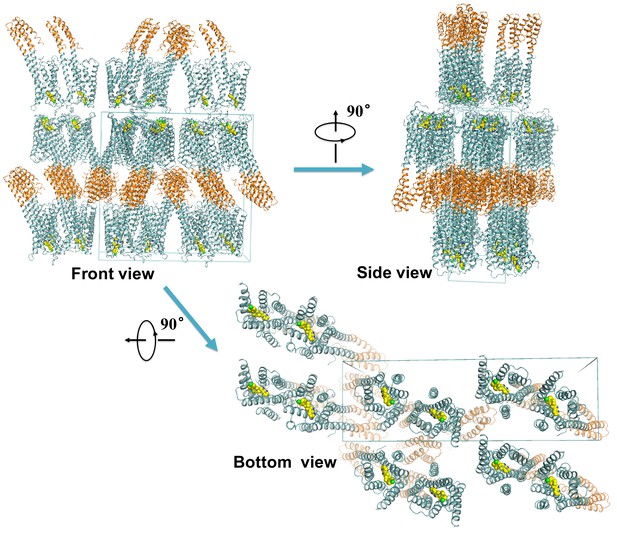
Crystal packing of the mouse DRD4.
The D4 dopamine receptor is shown in aquamarine, BRIL fusion protein is shown in brown, and L745870 is shown as yellow sphere models. The blue box depicts the crystallography unit cell.
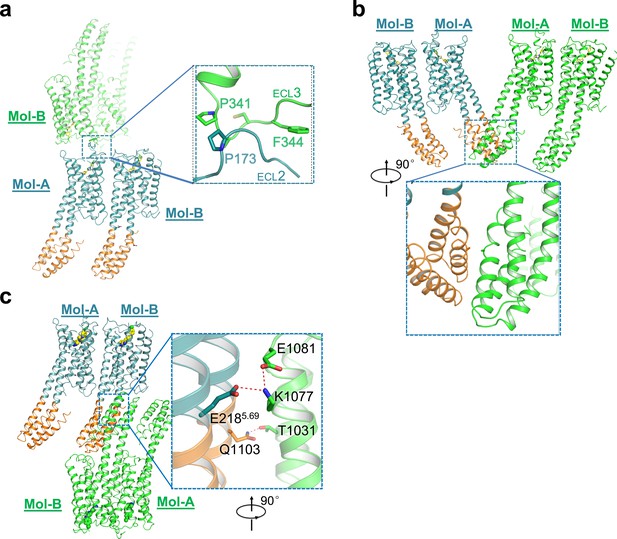
Interactions of different asymmetric units in mouse DRD4 crystal packing.
Head-to-head interactions through hydrophobic packing. P341 and F344 in ECL3 of Mol-B interacts with P173 in ECL2 of Mol-A from a neighboring asymmetric unit. No ordered interaction was detected between the BRILs of Mol-A from neighboring asymmetric units, in agreement with high B factors in this region. Salt bridges stabilize the interaction between BRILs in Mol-B from neighboring asymmetric units, which mediate the packing force between two membrane layers. Residue numbers 1001–1106 were assigned from the N-terminus to C-terminus of BRIL, independently of the receptor.

The B-factors distribution and representative electron density.
(a) B-factor putty representation of the mouse DRD4 structure. Blue-to-red rainbow spectrum is from the lowest (~30 Å2) to highest (>200 Å2). b, c, d. Representative |2Fo|–|Fc| electron density map (deep purple and magenta contoured at 1.0 σ) of TM5 and TM6 (b) and ligand-binding pocket (c). Panel d shows a side-by-side stereo view of the ligand pocket in Mol-B. The green density represents |Fo|–|Fc| omit map of the ligand, contoured at 2.5 σ.
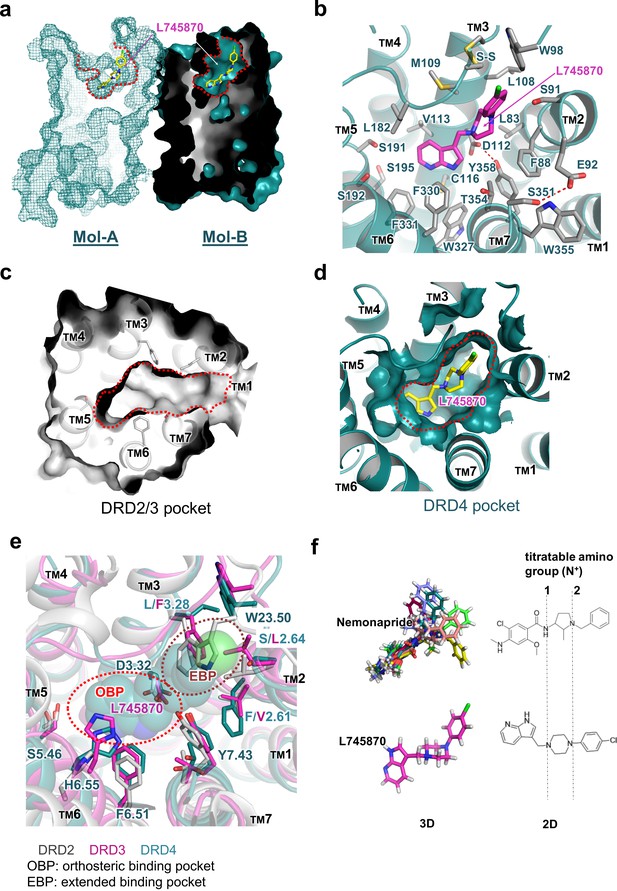
Molecular details of the L745870-binding site in DRD4.
(a) Mesh (Mol-A) and surface (Mol-B) representation of mouse DRD4 as viewed parallel to the membrane plane, clipped to reveal L745870 (colored as in Figure 1). (b) Interactions between the residues confining the binding pocket and L745870 (shown in magenta), with potential polar interactions depicted as dashed red lines. (c, d) Top view of surface representation of binding pocket in DRD2/3 and DRD4, respectively. L745870 is colored as in Figure 1. (e) Structural differences in the extended binding pockets (EBPs) of DRD4 (cyan), DRD2 (gray), and DRD3 (magenta). L745870 is shown as a space-filling model with carbon, nitrogen, and chloride atoms shown in cyan, blue, and green, respectively. (f) Comparison of nemonapride and L745870 in 3D models as well as 2D structures. The protonatable amino groups are marked with dashed lines in the 2D structures. For nemonapride, protonation occurs both at positions 1 and 2, whereas for L745870 it occurs mainly at position 1. The 3D steric conformations were generated with Maestro using the ligand preparation mode.
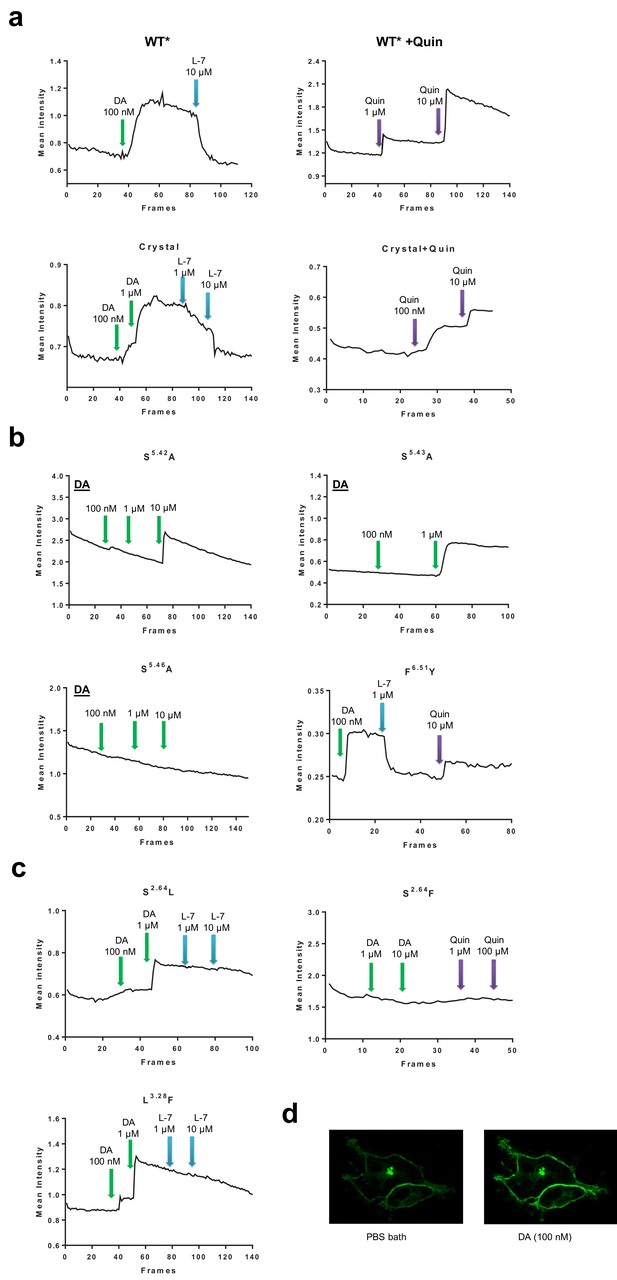
Representative results from Ligand binding-induced fluorescence intensity change with DRD4-cpGFP based dopamine assay.
(a) Fluorescence intensity of the WT* and ‘crystallization’ construct. The signals changed with different agonists or L745870 (labeled as L-7) at varied concentrations. Full length DRD4 with its ICL3 replaced with cpGFP is denoted here as WT*, and a similar cpGFP fusion but also containing mutations and truncations as the crystallization construct is labeled as ‘Crystal’. Events of addition of dopamine (DA) are depicted with green arrows; L-7, blue arrows; and quinpirole (Quin, an agonist of the D2-like subfamily), purple arrows. The reported fluorescence intensity (in arbitrary unit) is the mean value of all pixels within a selected area containing the cell(s) of interests. (b) Mutations in the orthosteric binding pocket. The mutation S5.42A lowered the dopamine activating ability, S5.46A eliminated the dopamine signal, and S5.43A showed no effect to dopamine response. In comparison, the mutation F6.51Y showed higher sensitivity to ligands used. (c) Mutations in the extending binding pocket. The mutations of S2.64L and L3.28F abolished the inhibition ability of L745870, yet S2.64F eliminated the dopamine signal all together. (d) Examples of confocal images of HEK cells either bathed in PBS or with additional dopamine.
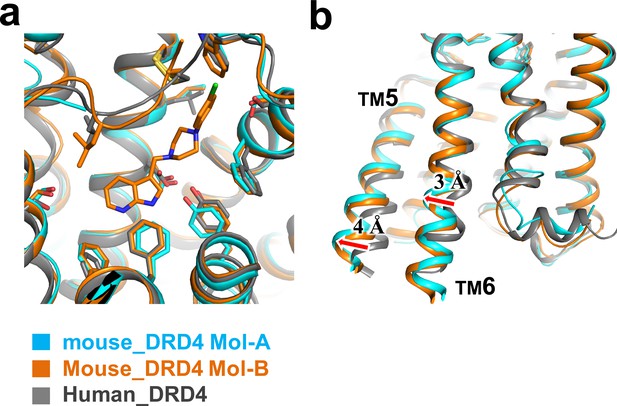
Structural comparison of mouse and human DRD4 receptors.
(a) Structural superposition of Mol-A and Mol-B of mouse DRD4 with human DRD4 (PDB ID: 5WIU). No significant conformational shift was observed around the binding pocket among the three structures. (b) Small outward shift of TM5 and TM6 in mouse DRD4 relative to human DRD4 may be explained by the BRIL conformation/crystal packing. Similar packing was observed in the A2A receptor structure (PDB ID: 4EIY).
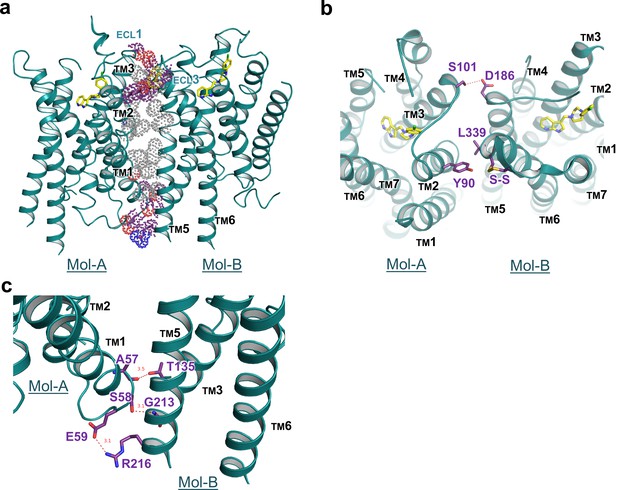
Interface details of mouse DRD4 in the asymmetric dimer.
(a) Overview of the interface of the mouse DRD4 dimer. Dot representations of the interacting residues are shown for the transmembrane domain (gray) and exo-membrane regions (colored). (b, c) Extracellular and intracellular faces of the DRD4 dimer. Red dashed lines depict potential polar interactions.
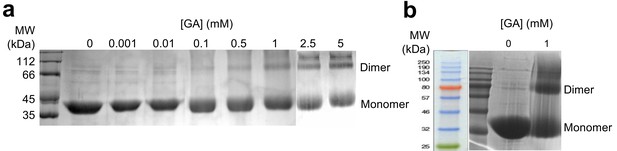
DRD4 cross-linked with glutaraldehyde.
(a) SDS-PAGE of DRD4 sample cross-linked with a concentration gradient of glutaraldehyde (GA). Bands of protein dimers are clearly visible at 0.5 mM GA. (b) Detailed marker shows that the molecular weight of cross-linked band is approx. 80 kDa, which is twice the size of a DRD4 monomer.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Mouse) | DRD4 | SUNGENE BIOTECH | GenBank: BC051421.1 | |
| Cell line (Spodoptera frugiperda) | Sf9 and High5 | Thermo Fisher Scientific | Sf9: B825-01 High5:B85502 | |
| Cell line (Homo sapiens) | HEK293t | ATCC | catalog numbers: CRL-3216 | |
| Transfected construct (Spodoptera frugiperda) | pFastBac1-DRD4-BRIL | This paper | Crystal construct | |
| Transfected construct (Homo sapiens) | pcDNA3.1-DRD4-cpGFP | This paper | Fluorescent sensor-based ligand-binding assay | |
| Recombinant DNA reagent | pFastBac1 | Thermo Fisher Scientific | catalog numbers: 10359016 | |
| Recombinant DNA reagent | pcDNA3.1 (plasmid) | Thermo Fisher Scientific | catalog numbers: V79020 | Mammalian expression vector |
| Commercial assay or kit | Bac-to-Bac Baculovirus Expression system | Thermo Fisher Scientific | Catalog number: 10359016 | |
| Chemical compound, drug | L745870 | Tocris | Catalog number: 158985-00-3 | |
| Software, algorithm | XDS program package | Kabsch, 2010 | ||
| Software, algorithm | PHENIX | Adams et al., 2010 | ||
| Software, algorithm | Maestro | SCHRÖDINGER | ||
| Software, algorithm | Fiji | Schindelin et al., 2012 |
Additional files
-
Supplementary file 1
Data collection and refinement statistics for the mouse DRD4 and L745870 complex.
a. Values in parentheses present the highest resolution shell. CC1/2** (Diederichs and Karplus, 2013). b. All outliers are located in loops not involved in ligand binding.
- https://cdn.elifesciences.org/articles/48822/elife-48822-supp1-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/48822/elife-48822-transrepform-v1.docx





