ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities
Figures
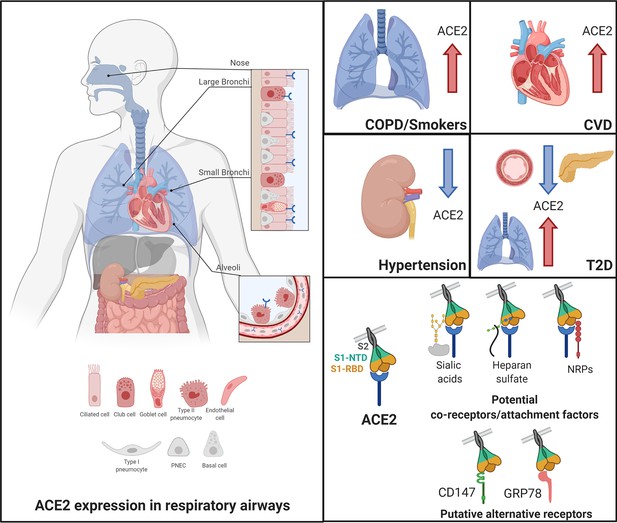
Angiotensin-converting enzyme 2 (ACE2), the proposed receptor of SARS-CoV-2, is expressed in the respiratory airways at low levels (blue) compared to the intestine, kidney, heart, and pancreas.
Low levels are also observed in the liver. In nasal and bronchial tissues, ACE2 is mainly expressed by ciliated, club, and goblet cells. It is also found in type-2 pneumocytes of alveoli and in endothelial cells of pulmonary capillaries. In comorbidities associated with a severity and poor prognosis of COVID-19, ACE2 levels are increased in the lungs of COPD and smokers and in the heart of patients with cardiovascular diseases (CVD). In contrast, patients with hypertension exhibit decreased levels of ACE2 in the kidney. In T2D patients, ACE2 is decreased in the pancreas and the vascular system but increased in the lungs. Current evidence supports a possible role of co-receptors or attachment factors, such as neuropilins, heparan sulfate, and sialic acids. The low detection of ACE2 in respiratory tissues also led to the speculation of a role of alternative receptors, such as CD147 and GRP78.
Tables
Measure of the dissociation constant (Kd) of ACE2 bound to immobilized SARS-CoV or SARS-CoV-2 S proteins by surface plasmon resonance (SPR) or biolayer interferometry binding (BLI) approaches.
| Reference | ACE2 protein PD domain | SARS-CoV S | SARS-CoV2 S | Method | Measured kd |
|---|---|---|---|---|---|
| Wrapp et al., 2020 | 1–615 aa | 306–577 aa | SPR | 325.8 nM | |
| 1–1208 aa | 14.7 nM | ||||
| Wang et al., 2020a | 19–615 aa | 306–527 aa | SPR | 408.7 nM | |
| 319–541 aa | 133.3 nM | ||||
| Lan et al., 2020 | 19–615 aa | 306–527 aa | SPR | 31.6 nM | |
| 319–541 aa | 4.7 nM | ||||
| Walls et al., 2020 | 1–614 aa | 306–575 aa | BLI | 1.2 nM | |
| 328–533 aa | 5 nM | ||||
| Wrapp et al., 2020 | 1–615 aa | 306–577 aa | BLI | 13.7 nM | |
| 319–591 aa | 34.6 nM |
mRNA levels of ACE2 found in the lungs, small intestine, kidney, and heart muscle reported in the Human Protein Atlas (HPA) consortium (Uhlén et al., 2015), the genome‐based tissue expression (GTEx) consortium (Keen and Moore, 2015).
Activity levels of the promoter of ACE2 assembled in the Fantom (FANTOM5) consortium (Yu et al., 2015). Protein-transcripts per million (pTPM). Scaled Tags Per Million (sTPM).
| Lung | Small Intestine | Kidney | Heart Muscle | |
|---|---|---|---|---|
| HPA (pTPM) | 1.7 | 31.1 | 107.2 | 31.1 |
| GTEx (pTPM) | 1.1 | 5.4 | 6.8 | 5.4 |
| FANTOM5 (sTPM) | 2.8 | 21.7 | 31.5 | 21.7 |
Available validation data for the antibodies used in the studies described in this review in accordance to the pillars defined by the Antibodypedia validation initiative (Uhlen et al., 2016).
Provider refers to the information found in the website of the company. IB: Immunoblot. IHC: Immunohistochemistry. IF: Immunofluorescence. 
 Enhanced validation,
Enhanced validation,  Supportive validation,
Supportive validation,  No data available, + Positive detection, +/- Weak detection, - Absence of detection.
No data available, + Positive detection, +/- Weak detection, - Absence of detection.
| Method | Antibodypedia | Provider | Additional information | |
|---|---|---|---|---|
| Ab15348 (or GTX15348) Immunogen: 788-805aa | IB |  | + Testis, intestines, lung, Calu-3 - Breast |  |
| IHC |  | + Testis, kidney, aorta and lung | + Intestine, heart, stomach, spleen (Independent antibody validation) (Lee et al., 2020) +, +/-, - Lung. Increased in COPD and smokers (correlation with mRNA) (Leung et al., 2020; Muus et al., 2020; Lee et al., 2020) | |
| IF |  |  |  | |
| MAB933 Immunogen: 18-74aa | IB |  | + Kidney - NSO cells | + Vero E6 cells - CHO cells (Jia et al., 2005) |
| IHC |  | + Kidney | + Orthogonal (RNA) and independent antibody validation +/-, - Lung (Hikmet et al., 2020; Aguiar et al., 2020; Muus et al., 2020; Lee et al., 2020) | |
| IF |  |  | + ALI-cultured ciliated airway epithelial cells (Martinez-Anton et al., 2013) | |
| AF933 Immunogen: 18-74aa | IB |  | + Ovary, testis and kidney | + Airway and distal lung, ALI-cultured airway epithelial cells (correlation with mRNA levels), Calu-3 and Caco-2 cells (Liao et al., 2013; Smith et al., 2020) - A549 (expected), Huh-7 cells (Liao et al., 2013; Smith et al., 2020) |
| IHC |  | + Kidney | + Testis, stomach, intestine (Independent antibody validation) +/-, - Lung (Ren et al., 2006; Muus et al., 2020; Lee et al., 2020) | |
| IF |  |  |  | |
| HPA000288 Immunogen: 1-111aa | IB |  | + Kidney, but several bands |  |
| IHC |  | + Intestine and kidney. - Tonsil | + Orthogonal (RNA) and independent antibody validation +/-, - Lung (Uhlén et al., 2015; Hikmet et al., 2020; Lee et al., 2020) | |
| IF |  |  |  | |
| Ab108252 Immunogen: 200-300aa | IB |  | + Testis, kidney, lung, HepG2, Caco-2 - A549 cells |  |
| IHC |  | + Kidney |  | |
| IF |  |  |  | |
| Ab239924 Immunogen: 200-300aa | IB |  | + Testis, kidney | + ACE2 transfected A459 - Non transfected A549 cells (Blanco-Melo et al., 2020) |
| IHC |  | + Testis, kidney | + Intestine, heart, stomach, spleen (Independent antibody validation), +/- Lung (Lee et al., 2020) | |
| IF |  |  |  | |
| NBP2-67692 Immunogen: 200-300aa | IB |  | + Kidney |  |
| IHC |  | + Kidney | + Testis and intestine (Independent antibody validation) - Lung (Lee et al., 2020) | |
| IF |  | + HepG2, MCF-7, 293 cells |  | |
| Anti-ACE2489 Immunogen: 489-508aa | IB |  | +ACE2 transfected CHO cells - Non transfected CHO cells |  |
| IHC |  |  | + Heart (No staining with secondary antibody alone) (Qian et al., 2013) | |
| IF |  |  |  | |
| Sc-20998 Immunogen: 631-805aa | IB |  | Antibody discontinued | Detection in rat tissue only (Sims et al., 2005) |
| IHC |  | Antibody discontinued |  | |
| IF |  | Antibody discontinued |  | |
| Homemade antibody Immunogen: 206-225aa | IB |  |  | Detection in rat tissue only (Mossel et al., 2008) |
| IHC |  |  |  | |
| IF |  |  |  |





