Some mechanistic underpinnings of molecular adaptations of SARS-COV-2 spike protein by integrating candidate adaptive polymorphisms with protein dynamics
Abstract
We integrate evolutionary predictions based on the neutral theory of molecular evolution with protein dynamics to generate mechanistic insight into the molecular adaptations of the SARS-COV-2 spike (S) protein. With this approach, we first identified candidate adaptive polymorphisms (CAPs) of the SARS-CoV-2 S protein and assessed the impact of these CAPs through dynamics analysis. Not only have we found that CAPs frequently overlap with well-known functional sites, but also, using several different dynamics-based metrics, we reveal the critical allosteric interplay between SARS-CoV-2 CAPs and the S protein binding sites with the human ACE2 (hACE2) protein. CAPs interact far differently with the hACE2 binding site residues in the open conformation of the S protein compared to the closed form. In particular, the CAP sites control the dynamics of binding residues in the open state, suggesting an allosteric control of hACE2 binding. We also explored the characteristic mutations of different SARS-CoV-2 strains to find dynamic hallmarks and potential effects of future mutations. Our analyses reveal that Delta strain-specific variants have non-additive (i.e., epistatic) interactions with CAP sites, whereas the less pathogenic Omicron strains have mostly additive mutations. Finally, our dynamics-based analysis suggests that the novel mutations observed in the Omicron strain epistatically interact with the CAP sites to help escape antibody binding.
eLife assessment
This important study investigates various variants of the SARS-COV-2 spike protein using established computational methods, complemented by experimental validation efforts. The evidence, bolstered by an evolutionary approach and protein dynamics, is solid. Placing this research in the broader context of the field could further enrich the article. It will interest biophysicists focused on allostery and protein evolution.
https://doi.org/10.7554/eLife.92063.3.sa0Introduction
Since 2019, the evolution of SARS-CoV-2 in humans has been characterized by the spread of mutations, many notably found within the spike (S) glycoprotein. The S protein is directly related to the human immune response to COVID-19 and, as such, has been one of the most studied and targeted proteins in SARS-CoV-2 research (Shang et al., 2020; Harvey et al., 2021; Jackson et al., 2022; Carabelli et al., 2023; Markov et al., 2023). Subsequently, research into the biophysical properties and mutational patterns associated with S protein evolution not only remains critical to understanding the pandemic but also emerges as a useful system to understand the mechanics of molecular adaptation within viruses.
For successful infection of a human host, the S glycoprotein of SARS-CoV-2 binds to the human ACE2 (hACE2) receptor through its receptor-binding domain (RBD). Evidence indicates that fine-tuning S protein interactions with hACE2 significantly affects viral reproduction (Rehman et al., 2020; Saputri et al., 2020; Rochman et al., 2021). Previous evolutionary studies show a complex network of interactions among mutated residues (Changeux and Edelstein, 2005; Doshi et al., 2016; O’Rourke et al., 2016; Mishra and Jernigan, 2018). Therefore, there has been a vast effort to uncover which mutations are important steps of adaptation for the S protein (Cagliani et al., 2020; Damas et al., 2020; Singh and Yi, 2021; Kistler et al., 2022; Maher et al., 2022; Neher, 2022). In particular, a significant aspect of many such studies was a focus on understanding adaptive mutations of SARS-CoV-2 that contributed to the leap to human hosts (Cagliani et al., 2020; Damas et al., 2020; Singh and Yi, 2021; Starr et al., 2022c). This is because SARS-CoV-2 has continuously mutated since its early detection (Kistler et al., 2022), causing the emergence of CDC-designated ‘variants of concern’ (VOCs) that may be driven by an accelerated substitution rate (Tay et al., 2022).
Predicting how new mutations impact the biophysical properties of the S protein remains a challenge, let alone explaining their complex interactions with one another and how they might affect hACE2 binding; many factors affect hACE2 interactions. Binding affinity with hACE2 can be enhanced directly through stronger receptor interactions or mediated through changes in RBD opening (Teruel et al., 2021b; Zhang et al., 2021; Díaz-Salinas et al., 2022). The RBD exhibits both ‘closed’ and ‘open’ conformational states. In the closed state, the RBD is shielded from receptor binding. In the open state, the RBD is accessible for hACE2 binding (Kirchdoerfer et al., 2016; Gur et al., 2020; Henderson et al., 2020; Hoffmann et al., 2020). While some mutations may affect the transition between these states (Henderson et al., 2020; Yurkovetskiy et al., 2020; Gobeil et al., 2021a; Sztain et al., 2021; Zhang et al., 2021; Shoemark et al., 2022), other mutations may allosterically regulate RBD openings through Furin cleavage site (residue ID range: 681–695) interactions to regulate hACE2 binding (Deng et al., 2021; Gobeil et al., 2021b; Khan et al., 2021; Laiton-Donato et al., 2021).
Moreover, as new mutations accumulate, culminating in the emergence of a new VOC, these mutations must occur on varied sequence backgrounds containing neutral, nearly-neutral, and adaptive mutations. While many studies have explored the impacts of individual mutations, VOCs result in a substantial difference in protein function compared to their individual effects (Moulana et al., 2022; Starr et al., 2022c; Moulana et al., 2023; Witte et al., 2023). Here we integrate an evolutionary approach with protein dynamics analysis to address the fundamental mechanisms of mutations dictating VOCs and the impact of their epistatic interaction on the function of the S protein. Many earlier studies have combined phylogeny and evolutionary theory to identify adaptive mutations and analyze how the viral sequence has changed over time (Frost et al., 2018; Boni et al., 2020; Cagliani et al., 2020; Damas et al., 2020; Tang et al., 2020). Similarly, we first use a well-established evolutionary probability (EP) approach (Liu et al., 2016) that utilizes phylogenetic trees in combination with the neutral theory of molecular evolution to determine candidate adaptive polymorphisms (CAPs) using the early Wuhan sequence as a variant. CAPs are substitutions in SARS-CoV-2 that are rarely observed in other closely related sequences (Figure 1A), which implies a degree of functional importance and makes them candidates for adaptation (Liu et al., 2016). Adaptation in this case means a virus which can successfully infect human hosts. As CAPs are unexpected polymorphisms under neutral theory, their existence implies a non-neutral effect. This can come in the form of functional changes (Liu et al., 2016) or compensation for functional changes (Ose et al., 2022b). Therefore, we suspect that these CAPs may be partially responsible for the functional change allowing the infection of human hosts. In support of this method, we find an overlap between our list of sites containing CAPs and putative adaptive sites identified by others (Cagliani et al., 2020; Singh and Yi, 2021; Kistler et al., 2022; Starr et al., 2022b). Second, we use a suite of computational tools to analyze how CAPs that arose in the early and late phases of the COVID-19 pandemic modulate the dynamics of the S protein. We also explore the complex interactions between these sets of CAPs to gain mechanistic insight into the behavior of molecular adaptation involving the S protein. In particular, we focused on how mutations modulate protein dynamics as we and others have previously found that rather than changing a protein’s structure solely, mutations modulate conformational dynamics, leading to changes in biophysical properties such as stability, flexibility, and allosteric dynamic coupling, any of which may affect protein binding (Swint-Kruse et al., 1998; Keskin et al., 2000; Bhabha et al., 2013; Nussinov and Tsai, 2013; Campbell et al., 2016; Ma and Nussinov, 2016; Saavedra et al., 2018; Kuzmanic et al., 2020).
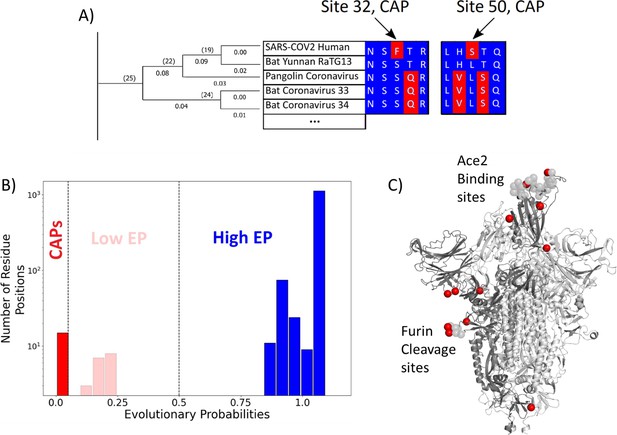
Evolutionary probability (EP) in SARS-CoV-2.
(A) The EPs of each amino acid in the S protein sequence are calculated by taking the multiple sequence alignment of the S proteins through their evolutionary tree and using Bayesian inferences to determine the likelihood of finding a particular residue at a particular location within a given sequence. Simply, if the residue is found at a location ‘x’ in closely related sequences, it will have a higher EP at location ‘x’ in the target sequence. Residues with an EP < 0.05 in the target sequence are candidate adaptive polymorphisms (CAPs) (red). CAPs are found at sites 32, 50, 218, 346, 372, 478, 486, 498, 519, 604, 681, 682, 683, 684, and 1125. (B) The distribution of EP scores of the wild-type residues in the S protein. Here, lower EP scores are shown in red, and higher EP scores in blue. While the vast majority of the wild-type (reference) protein consists of high EP residues, a few residues have low EP. (C) The CAPs are also highlighted as red spheres in the open configuration of the S protein, with the open chain in a darker shade. We observe that a majority of the CAP positions reside at the receptor-binding domain (RBD) and the Furin cleavage site (676-689; Wrobel et al., 2020) shown as transparent light gray spheres.
With this evolutionary-dynamics unified approach, we aim to answer the following questions about VOCs: Are all the mutations in VOCs adaptive in nature? Are they coupled to one another or provide some measure of biophysical, dynamical, or mechanical epistasis? While many of these mutations are found within the RBD, numerous others are located distal to this region; hence, we aim to investigate the functional roles of distal mutations, particularly from a protein dynamics perspective. Our integrated analysis revealed that protein dynamics play a significant role in the evolution of the S protein. The flexibility of sites within the S protein shows a strong, direct correlation with substitution rate, and newly evolving CAPS are mostly additive mutations that modulate the dynamics of the hACE2 binding site. Yet other CAPs, 346R, 486F, and 498Q, show highly epistatic (i.e., non-additive) modulation of the hACE2 binding site and provide immune escape benefits.
Results and discussion
Candidate adaptive mutations in the S protein are recognized via EPs
SARS-CoV-2 is part of a family of coronaviruses, many of which infect mainly animals and are less capable of infecting humans (Dicken et al., 2021). Therefore, to identify the most likely mutations responsible for the infection of human hosts (i.e., putative adaptive mutations for humans), we estimated the (neutral) EP scores of mutations found within the S protein (Liu et al., 2016). EP scores of the amino acid variants of the S protein were obtained using a maximum likelihood phylogeny (Kumar et al., 2018) built from 19 orthologous coronavirus sequences. Sequences were selected by examining available non-human sequences with a sequence identity of 70% or above to the human SARS CoV-2’s S protein sequence. This cutoff allows for divergence over evolutionary history such that each amino acid position had ample time to experience purifying selection, whilst limiting ourselves to closely related coronaviruses. (Figure 1A). The likelihood of finding a particular amino acid in the sequence is then determined using a Bayesian framework, with calculations carried out by MEGA X software (Kumar et al., 2018). As apparent in the name, EP scores obtained for the amino acids in the sequence provide information regarding the likelihood of finding them at their position, given the history of the sequence. Amino acid residues receiving low EP scores (<0.05) at a position are less likely to be found in a given position within the sequence because they are non-neutral. Generally, positions with low EP amino acids are far less common than those containing mutations with high EP, a trend also realized in the CoV-2 S protein (Figure 1B).
Of particular interest is an observed evolutionary change where an amino acid with high EP is replaced by an amino acid residue with low EP. While amino acids with low EP should be harmful or deleterious to viral fitness due to functional disruption or change, fixation of a low EP amino acid at a position suggests an underlying mechanism for natural selection to operate. These fixed, low EP mutations are called CAPs as they are predicted to alter protein function, and adaptive pressures may drive their prevalence (Kumar and Patel, 2018). Indeed, there is an overlap between these CAPs and the mutations suggested by other methods to be adaptive for the S protein (Cagliani et al., 2020; Singh and Yi, 2021; Kistler et al., 2022; Starr et al., 2022b). Within these studies, sites 478, 486, 498, and 681 have been implicated in SARS-CoV-2 evolution, leaving the remaining 11 CAPs as undiscovered candidate sites for adaptation.
Interestingly, most of the CAP residues are at functionally critical sites, including the RBD and the Furin cleavage site (Figure 1C). As mentioned earlier, the RBD plays a key role in initiating the infection of a healthy cell by binding with the host organism’s ACE2 protein. Before ACE2 binding, one chain of the homotrimer comprising the S protein must open to expose the RBD (Kirchdoerfer et al., 2016; Henderson et al., 2020; Hoffmann et al., 2020; Sztain et al., 2021). The Furin cleavage site plays a key role in the opening process as the binding of host cell protease Furin aids in the cleavage of the S protein into two domains: S1 and S2 (Wrobel et al., 2020: 13). Another host cell protease, TMPRSS2, facilitates viral attachment to the surface of target cells upon binding either to sites Arg815/Ser816, or Arg685/Ser686 which overlap with the Furin cleavage site 676–689, further emphasizing the importance of this area (; Fraser et al., 2022). Similar cleavage sites have been found in related coronaviruses, including HKU1 and Middle East respiratory syndrome coronavirus (MERS-CoV), which infects humans (Chan et al., 2008: 1; Millet and Whittaker, 2014; Millet and Whittaker, 2015), and the acquisition of similar cleavage sites is associated with increased pathogenicity in other viruses such as the influenza virus (Steinhauer, 1999). Interestingly, however, CAPs do not display such an overwhelming tendency to occur at well-known critical sites within human proteins studied with similar methods (Ose et al., 2022b), yet mutations at those sites are associated with disease, indicating their critical role in inducing functional change. Therefore, the identified CAPs in the S protein, which are signs of recent evolution, can provide mechanistic insights regarding the molecular adaptation of the virus. In particular, we aimed to analyze how these CAP positions in the S protein modulate the interaction with hACE2 using our protein dynamics-based analysis (Gerek and Ozkan, 2011; Nevin Gerek et al., 2013; Larrimore et al., 2017; Kumar et al., 2015).
Asymmetry in communications among the network of interactions in spike describes how CAPs regulate the dynamics of the S protein
A mutation at a given amino acid position inevitably not only alters local interactions, but this change cascades through the residue–residue interaction network, which gives rise to a variation in native ensemble dynamics to modulate function (Dror et al., 2012; Labbadia and Morimoto, 2015; Sekhar and Kay, 2019; Campitelli et al., 2020.). Many groups have already examined the conformational dynamics of the S protein using normal mode analysis to explore mutation sites and interactions with different receptors (Zhou et al., 2020; Majumder et al., 2021; Teruel et al., 2021a; Verkhivker, 2022). However, our study will focus mainly on the role of CAPs, of which F486 and Q498 have already been identified through perturbation response scanning (PRS) as potential allosteric sites (Verkhivker, 2022). Thus, we analyze the internal dynamics of the system to understand the functional role of CAPs in S proteins. This analysis allows us to gain a mechanistic understanding of the relationship between CAP mutations and biophysical outcomes (Teruel et al., 2021b). First, we implement the dynamic coupling index (DCI) approach to study long-distance coupling between the CAPs and the hACE2 binding sites emerging from the 3D network of interactions across the S protein system. DCI calculation combines PRS and linear response theory (LRT) to capture the strength of a displacement response for position i upon perturbation of position j, relative to the average fluctuation response of position i to all other positions in the protein. It represents the strength of dynamic coupling between positions i and j upon perturbation to j (Larrimore et al., 2017; Kumar et al., 2015).
Further, asymmetry can be captured in the DCI values as dynamic coupling is not necessarily symmetric due to an anisotropic interaction network. That is, each amino acid has a set of positions to which it is highly coupled, and this anisotropy in connections gives rise to unique differences in coupling between a given i, j pair of amino acids which do not have direct interactions. By calculating the coupling of the hACE2 binding interface in the RBD with respect to the CAP residue positions and vice versa, we can generate DCIasym (Figure 2A) as the difference between the normalized displacement response of position j upon a perturbation to position i (DCIij) and the normalized displacement response of position i upon a perturbation to position j (DCIji) (see ‘Methods’). If the DCIasym values significantly differ from zero, it shows asymmetry in coupling and presents a cause–effect relationship between the i, j pair in terms of force/signal propagation. This metric has been used previously in a variety of systems to analyze the unique behavior of positions within a protein and a given position’s propensity to effect biophysical changes upon mutation, particularly at long distances (Modi and Ozkan, 2018; Campitelli and Ozkan, 2020; Kolbaba-Kartchner et al., 2021; Ose et al., 2022a; Kazan et al., 2023; Campitelli et al., 2021).

Candidate adaptive polymorphisms (CAPs) control binding sites across chains.
(A) Schematic representation of DCIasym. (B) DCIasym of CAP residue positions with the binding interface of receptor-binding domain (RBD) in the open chain. Residues in the closed chains with a low evolutionary probability (EP) amino acid in the reference sequence dominate the binding site interface of RBD in the open chain. There is a significant difference between the asymmetry profiles of the closed (M = –0.06, SD = 0.33) and open (M = –1.68, SD = 0.89) conformations (p<0.001).
Recent work from our group has shown an enhancement in cross-chain communication within the main protease of SARS COV-2 compared to SARS COV-1 (Campitelli et al., 2022). Furthermore, previous studies have shown that allosteric inter-chain communication is important to S protein function (Zhou et al., 2020; Spinello et al., 2021; Tan et al., 2022; Xue et al., 2022). In support of these findings, we observe through DCIasym that when the S protein is in its pre-fusion conformation with one chain open, the CAPs in the closed chains have negative coupling asymmetry with respect to the hACE2 binding site interface in the RBD-open chain. This indicates an allosteric control where the hACE2 binding site is dominated by the dynamics of the CAPs in closed chains (Figure 2B, yellow bars). As this open-state RBD is critical for the viral infection of host cells (Kirchdoerfer et al., 2016), our results suggest that this type of closed-to-open cross-chain interaction is important for viral proliferation. Our prior studies on DCIasym show a similar trend in lactose inhibitor (LacI), a protein with a functional role in gene expression through binding DNA. The allosteric mutations (i.e., mutations on the sites that are far from the DNA binding sites) that alter DNA binding affinity not only exhibited unique asymmetry profiles with the DNA binding sites of LacI, but also regulated the dynamics of these binding sites (Campitelli et al., 2021).
Similarly, it is possible that mutations to such residue positions within the S protein can be used to regulate the dynamics of the hACE2 bindings sites of the open RBD state. We, therefore, propose that the residue positions with CAP substitutions hold the potential for mutations in the spike sequence which can alter the opening and closing dynamics of the RBD. This hypothesis is further supported by many mutations already observed at these residue positions which alter the infection rate (Brister et al., 2015). Interestingly, residues responsible for extremely low asymmetry values (<–4) lie overwhelmingly in the region 476–486. These same residues were suggested to stabilize S protein dynamics and prime it for host Furin proteolysis (Raghuvamsi et al., 2021).
Moreover, as a control, we performed the same analysis on the S protein with the RBDs of all chains in the closed configuration. In this case, we observed that the DCIasym of the CAPs residue positions with respect to the hACE2 interface in the other chains yields a largely symmetric distribution about 0 (Figure 2B, green bars). This verifies that the asymmetry in the coupling of CAPs with the exposed binding site interface in pre-fusion configuration results from one of the RBDs opening up and further suggests the allosteric role played by CAPs in locking the S protein in the RBD open state.
Dynamics analysis shows that rigid sites tend to be more highly conserved than flexible sites
CAPs represent important S protein amino acid changes between related coronaviruses across multiple species and the Wuhan-Hu-1 reference sequence (MN908947). Since SARS-CoV-2 first spread to humans, it has continued to mutate and evolve rapidly, particularly regarding the S protein (Amicone et al., 2022; Liu et al., 2022; Tay et al., 2022). Just as mutations leading to the Wuhan strain caused an increase in binding affinity to hACE2, continued evolution in human hosts has resulted in further altered binding affinities as well as different phenotypic outcomes for those infected (Ali et al., 2021; Barton et al., 2021; Ozono et al., 2021).
We explore whether protein dynamics has played a role in the selection of mutational sites during the evolution of the S protein since 2019. Our previous work has indicated that the rate of evolution per positional site exhibits a positive correlation with positional flexibility; generally, positions that exhibit higher flexibility are also sites that experience a higher number of amino acid substitutions (Liu and Bahar, 2012; Maguid et al., 2008; Maguid et al., 2006; Mikulska-Ruminska et al., 2019; Nevin Gerek et al., 2013). To confirm these findings for the evolution of the S protein using the sequenced variants of infected humans, we examine the flexibility of the S protein, an analysis conducted in other studies which resulted in several important findings (Nguyen et al., 2020; Socher et al., 2021; Teruel et al., 2021b; Pipitò et al., 2022; Verkhivker, 2022; Abduljalil et al., 2023). For example, Teruel et al., 2021a used their Elastic Network Contact Model to find how certain highly observed mutations make the open state more rigid and the closed state more flexible. For our own flexibility analysis, we measure the site-specific amino acid flexibility using the dynamic flexibility index (DFI). Using the same mathematical foundation as DCI, DFI evaluates each position’s displacement response to random force perturbations at other locations in the protein (Gerek and Ozkan, 2011; Nevin Gerek et al., 2013), and it can be considered a measure of a given position’s ability to explore its local conformational space. We found that the COVID-19 S protein shows the expected high correlation between the occurrence of mutations and site flexibility (Figure 3) when we compare %DFI (DFI ranked by percentile) to the average number of variants per position found within a given %DFI bin. Previous studies have indicated that rigid residues are critical for functional dynamics, thus more likely to impact function if mutated and, generally, can lead to a loss of function and thus more conserved (Kim et al., 2015; Butler et al., 2018; Modi et al., 2021b; Modi et al., 2021a; Kazan et al., 2022; Ose et al., 2022a; Stevens et al., 2022; Kumar et al., 2015). This analysis also agrees with these previous studies and highlights the power of negative selection, in line with the neutral theory of molecular evolution, stating that deleterious mutations (i.e., those on the rigid positions) should be eliminated and therefore not observed (Kimura, 1983).
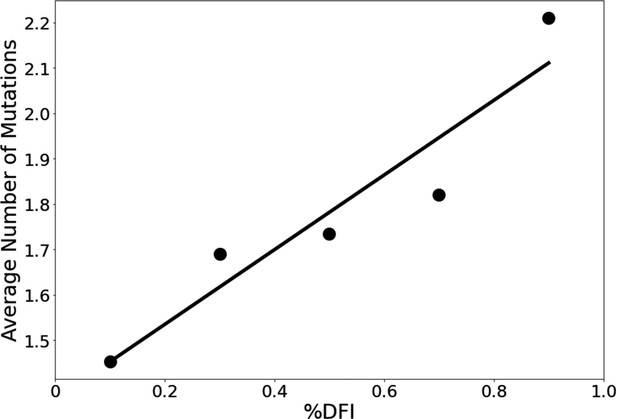
The average number of variants observed among residues of different flexibility.
Residues were sorted into one of five bins based on flexibility. Then, the average number of variants for residues within that bin was calculated. Here, the number of variants is defined as the number of different amino acid varieties found at that site. Mutational data was calculated across approximately 24,000 SARS-CoV-2 S protein sequences from the NCBI Datasets Project (Brister et al., 2015). Residue flexibility, as reported here via %DFI, was computed using PDB id 6vsb from the Protein DataBank (Berman et al., 2000). More rigid residues tend to have fewer variants (r = 0.94).
-
Figure 3—source data 1
A comparison of the amino acid sequences for different variant spike proteins used in this article.
Sequence MN90894.3 is used as the wild-type (reference) sequence. For each different variant, residue positions matching the reference sequence are shown in blue, while locations where the residue differs from the reference sequence are shown in yellow. For S protein locations not shown here, all variants presented have the same amino acid type as the reference sequence.
- https://cdn.elifesciences.org/articles/92063/elife-92063-fig3-data1-v1.xlsx
Continued mutations within human hosts have resulted in a multitude of variants. Indeed, by fitting various molecular clock models to genome sequence data, VOC emergence is punctuated by an episodic period of rapid evolution, with a substitution rate of up to fourfold greater than the background substitution rate (Kumar et al., 2021; Tay et al., 2022). With such an aggressive evolutionary rate, we are finding VOCs to consist of a number of different characteristic mutations, almost all of which are CAPs. Sequence differences between the various VOCs used in this article can be found in Figure 3—source data 1.
To explore the dynamic effects of the evolution of the spike in humans, we examine asymmetry with these new potentially adaptive sites, namely the low EP (CAP) characteristic mutation sites observed in the Delta variant, the widely dominant variant from December 2021 to January 2022 (Thye et al., 2021), and the Omicron variant, a highly transmissible variant whose lineages have remained dominant since January 2022 (Kim et al., 2021; Figure 4). This analysis revealed a mechanism similar to that for the CAPs in the reference protein (Figure 2) as the open-chain binding interface is also allosterically controlled by these potentially new adaptive sites. Regarding this, we see that the asymmetry is much more pronounced in observed mutations of Omicron variants, suggesting that these new mutations have a stronger power in controlling the dynamics of open-chain hACE2 binding interface compared to those observed in Delta variants. We surmise that the difference in virulence and infection rates between Omicron and Delta (Earnest et al., 2022; Bager et al., 2021; Sheikh et al., 2021; Twohig et al., 2022; Houhamdi et al., 2022; Menni et al., 2022) might be due to these specific CAPs within each variant and their differences in allosterically controlling the dynamics of open RBD binding sites as also observed in the DCIasym analysis of the Wuhan variant in Figure 2.
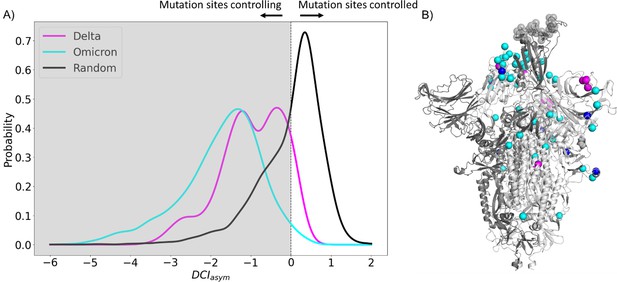
Mutations in variants of concern (VOCs) present different asymmetry profiles.
(A) DCIasym with low evolutionary probability (EP) characteristic mutation sites of Delta or Omicron strains in the closed chains and the binding interface of receptor-binding domain (RBD) in the open chain. Delta displays a second peak closer to zero, suggesting that Delta mutation sites (M = –0.98, SD = 0.80) have less allosteric control over the hACE2 binding sites than Omicron mutation sites (M = –1.74, SD = 1.00) (p<0.001). However, both sets of sites have far more control over hACE2 binding sites than expected, based on a random control group (M = 0.03, SD = 0.85) (p<0.001). (B) S protein structure showing binding interface sites (transparent gray), Delta mutation sites (magenta), Omicron mutation sites (cyan), and sites mutated in both Omicron and Delta (blue).
Experimental results motivate the use of EpiScore within the SARS-CoV-2 S protein
The fact that the identified CAPs in the reference protein and the more recently evolved CAPs of Delta and Omicron variants both show a high degree of control over the functional sites begs the question: what is the complex interaction between these previous and new CAP sites? Motivated by this concept, we explore the interplay of mutational pairs to understand the effects of the specific amino acid backgrounds associated with these two predominant variants. Some CAP sites in Delta and Omicron have already been considered adaptive (Kemp et al., 2021; Kistler et al., 2022; Maher et al., 2022; Neher, 2022).
It is well understood that the impact of even a single mutation to a protein sequence can sometimes dramatically alter the biophysical behavior of the system. However, the mechanistic impact of point mutations can only be fully understood when the sequence background upon which it is made is accounted for. This means that, in the case of strains with multiple mutations, the interplay between mutated positions will ultimately impact a protein as an aggregate behavior, where the presence of previous mutations may strongly (or weakly) influence some mutations. This concept of non-additivity is known as epistasis. In fact, studies of evolutionary pathways of mutations have suggested that a majority of the mutations have a second or a higher order epistasis among them (Bershtein et al., 2006). Nature exploits this higher order complex relationship between the mutations to evolve their function.
To computationally capture and interpret the pairwise effects of mutations, we have developed an in-house computational tool called EpiScore (Figure 5A). Here, we evaluate how a given position pair i j may affect other critical positions k of the protein. EpiScore is the relative coupling strength to a position k when positions i and j are perturbed simultaneously compared to the average dynamic coupling strength of i to k and j to k. EpiScore has previously been used successfully to capture overarching trends in GB1 deep mutational scan data as well as specific instances of the development of antibiotic resistance in various enzymatic systems (Campitelli and Ozkan, 2020). An EpiScore of 1 indicates perfect coupling additivity, and deviations from this value represent non-additive behavior between position pairs and functionally important sites. Prior EpiScore work has shown a difference in EpiScore between the sites of compensatory and non-compensatory mutations, where both yield distributions with peaks around 1, but non-compensatory mutations show higher deviation in their EpiScore distribution (Ose et al., 2022b).

EpiScore provides a measurement of epistasis.
(A) Schematic representation of cross-chain EpiScore, describing i, j, in chains B and C, respectively, and its impact in receptor-binding domain (RBD) binding position k in the open RBD conformer chain A. (B) Colors indicate EpiScore values for given mutation pairs, averaged over hACE2 binding sites. Cross-chain residue pairs in the upper right tend to be highly epistatic, similar to pairwise second-order interaction coefficients from Moulana et al., 2022.
Many studies have confirmed epistasis between residues within the S protein (Moulana et al., 2022; Starr et al., 2022a; Moulana et al., 2023; Witte et al., 2023). These epistatic residues can have various effects on hACE2 or antibody binding. Within SARS-CoV-2, here we calculate the EpiScore (Figure 5B) of a set of mutation pairs used by Moulana et al., 2022 and compare our results to quantified epistatic effects determined by the experimental hACE2 binding affinity of ‘first-order’ single mutation variants compared to ‘second-order’ mutation pairs. Our EpiScore results and the experimentally determined epistasis both captured highly epistatic behavior among residues 493, 496, 498, 501, and 505, as well as a lack of epistatic behavior for residues 339, 371, 373, and 375; however, EpiScore generally showed higher epistasis values than experiment for residues 417, 440, 446, 477, 478, and 484.
EpiScore highlights the epistatic relationship between the recent adaptive mutations in VOCs and the CAPs of the Wuhan reference
Seeking further to understand the role of epistasis within S protein variants, we explored the possibility of epistatic relationships between the CAPs of the Wuhan variant and the new CAPs in VOCs. Thus, we computed the EpiScore of these CAP positions in the closed RBDs (i.e., chains B and C) with respect to functional hACE2 binding interface sites of the open RBD chain (chain A) (Figure 5B) and obtained EpiScore distributions.
To contrast these variants, Omicron (Figure 6, cyan) shows a high proportion of additive mutations compared to the Delta variant (Figure 6, magenta), with a peak centered on 1. The comparatively more pathogenic Delta variant exhibited many non-additive mutations with EpiScores below one. This again suggests that the cross-communication between the open and closed chain of the S protein is important for regulating the function. Four out of seven low EP Delta mutation sites used in this analysis often resulted in EpiScores below 1. Each of those is found in the N-terminal domain (NTD) on or near the N3 loop and is implicated in antibody escape in recent studies (Chi et al., 2020; Weisblum et al., 2020; Harvey et al., 2021; Klinakis et al., 2021; Cantoni et al., 2022). The low EpiScores of NTD mutations suggest that they dampen the control of Wuhan variant CAPs over the hACE2 binding sites in addition to their effects on antibody binding. It is possible that what the Delta variant gained in transmission rate also came with being more harmfully pathogenic due in part to negatively epistatic interactions. In contrast, the mutations leading to the development of the Omicron strain were additive with respect to Wuhan variant CAPs, possibly leading to a lower pathogenicity and higher effective immune escape, resulting in an overall higher transmission rate. Another possible explanation for the higher transmission rate of Omicron comes from a different normal mode analysis study, which found that despite reduced binding affinity with hACE2, Omicron showed increased occupancy of the open state compared to the closed state, which would increase chances of interaction with hACE2 (Teruel et al., 2021b). It is worth noting that other variants contain NTD mutations which result in low EpiScores; however, the proportion of these mutations within the set is considerably less than in Delta (Figure 6—figure supplement 2).
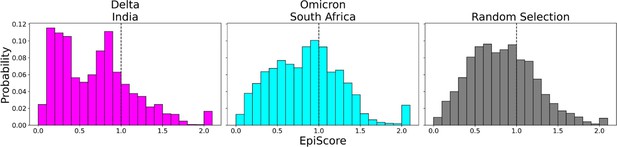
EpiScores of variants of concern (VOC) mutations.
Here, i = low evolutionary probability (EP) Delta mutation sites (magenta), low EP Omicron mutation sites (cyan), and a random selection of sites (gray), j = low EP sites in the Wuhan variant, and k = the binding interface of the open chain. EpiScores using sites of either variant (Delta: M = 0.70, SD = 0.50, Omicron M = 0.86, SD = 0.46) are significantly different (p<0.001) from a set of EpiScores using random sites (M = 0.84, SD = 0.41), but the distribution for Delta variants differs much more from the other two. EpiScores for other variants can be found in Figure 6—figure supplement 1.
One of the more notable features of generated EpiScore distributions is the presence of a tail of values upward of 2.0, indicating highly epistatic behavior. Interestingly, these tails are largely due to three different CAPs: 346R, 486F, and 498Q. Those residues are nearby one another within the protein structure and have been reported to play a role in antibody binding, either being known antibody binding sites (346R and 486F) or having received very high antibody accessibility scores (498Q) (Harvey et al., 2021; Raghuvamsi et al., 2021). These observed high EpiScore values also support other studies indicating the epistatic interactions between these CAPs and the mutations of the VOCs within the S protein are crucial for maintaining binding affinity of hACE2 whilst evading immunity (Hong et al., 2022; Starr et al., 2022b).
Inspection of EpiScores of Delta and Omicron potentially adaptive mutation sites with only CAP site 486F (a binding site for both hACE2 and antibodies) (Huang et al., 2020; Ali et al., 2021; Harvey et al., 2021; Raghuvamsi et al., 2021) shows highly epistatic interactions at other hACE2 binding sites (Figure 6). However, within a recent and rapidly spreading subvariant of Omicron, XBB 1.5, we see a mutation of S to P, a rare double nucleotide mutation, at site 486 (preceding Omicron variants included mutation F486S from the original CAP). This new variant has unprecedented immune escape capabilities, resisting neutralizing antibodies almost entirely (Qu et al., 2023). EpiScores of other XBB 1.5-specific mutation sites with 486P are almost entirely greater than 1, showing an even higher degree of epistasis with the binding sites of RBD (Figure 7). These results present a threefold importance for the S486P mutation: not only does this residue alter antibody (i.e., immune escape) and hACE2 binding by directly modifying a binding site, but it may also be responsible for modifying hACE2 binding via epistatic cooperation with other co-occurring mutations.

EpiScores with candidate adaptive polymorphism (CAP) site 486.
Here, i = low evolutionary probability (EP) Delta mutation sites (magenta), low EP Omicron mutation sites (cyan), and a random selection of sites (gray), j = site 486, and k = the binding interface of the open chain. CAP and hACE2 and antibody binding site 486 displays epistasis with almost all XBB 1.5 variant sites at almost every hACE2 binding site (M = 1.40, SD = 0.46) and presents a significantly different profile from other variant sites (p<0.01). EpiScores involving 486 for other variants can be found in Figure 7—figure supplement 1.
Change in flexibility of RBD binding site correlates with experimental binding affinities for Omicron and Omicron XBB variants
Experimental studies have tracked hACE2 binding for different variants since the virus first spread (Ali et al., 2021; Barton et al., 2021; Ozono et al., 2021; Wu et al., 2022). Within the Omicron variant, for example, characteristic mutations on the RBD are shown to increase the overall binding affinity of the virus to the ACE2 receptor, which is suspected to allow it to spread more easily (Kim et al., 2021). Furthermore, the new Omicron XBB and Omicron XBB 1.5 variants contain additional mutations in the RBD and antibody binding residues, which may further impact their dynamics and interactions with the host.
To gain deeper insights into the impact of dynamics on the binding affinity of hACE2 and antibodies with the recent Omicron XBB variants, we conducted molecular dynamics (MD) simulations. By analyzing the resulting trajectories, we investigated how these mutations influence the flexibility and rigidity of the RBD and antibody binding residues, consequently affecting their binding affinity and potential for immune evasion (Figure 8). To understand the overall flexibility changes, we measured the sum of DFI of the ACE2 binding residues, as well as the sum of DFI of the antibody binding residues, calculated from the MD trajectories and compared them with experimental viral binding (disassociation constants) and immunity evasion antibody IC50 values (Yue et al., 2023).
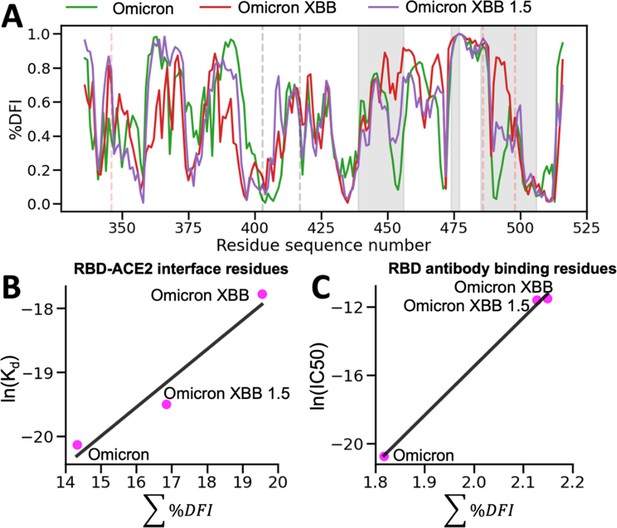
The %DFI calculations for variants Omicron, XBB, and XBB 1.5.
(A) %DFI profile of the variants are plotted in the same panel. The gray shaded areas and dashed lines indicate the ACE2 binding regions, whereas the red dashed lines show the antibody binding residues. (B) The sum of %DFI values of RBD-ACE2 interface residues. The trend of total %DFI with the log of Kd values overlaps with the one seen with the experiments (r = 0.97). (C) The receptor-binding domain (RBD) antibody binding residues are used to calculate the sum of %DFI. The ranking captured with the total %DFI agrees with the log of IC50 values from the experiments.
This investigation elucidated the impact of mutations in the RBD and antibody binding residues on the binding affinity of the S protein and immune evasion by modulating their flexibility and rigidity (Figure 8A). The Omicron XBB variant exhibits heightened flexibility in hACE2 and antibody binding residues, reducing infectivity and enhancing immune evasion. Conversely, the Omicron XBB 1.5 variant induces distinct dynamics in these regions, rendering the RBD-ACE2 interface more rigid while increasing flexibility in antibody binding residues. These effects indicate that Omicron XBB 1.5 retains its antibody escape capabilities while regaining ACE2 binding affinity comparable to previous Omicron variants, in accordance with experimental findings (Yue et al., 2023). These findings suggest that mutations in the RBD and antibody binding residues can have complex effects on the dynamics of the protein and, ultimately, on the virus’s ability to infect and evade the host immune system through an alteration of binding site dynamics. However, we do note that the effect of mutations will heavily depend on the genetic background in which they are mutated. The effects of a mutation on a Delta variant protein may differ entirely from the effects of a mutation on an Omicron variant protein (Figure 8—figure supplement 1).
Conclusion
We analyzed the evolutionary trajectory of the CoV-2 S protein in humans to understand the dynamic and epistatic interactions of the mutations defining specific VOCs. We first obtain the phylogenetic tree of the COV-2 S protein and identify the sites of certain recent mutations known as CAPs. CAPs are considered adaptive because mutations rarely tolerated in closely related sequences have suddenly become fixed, implying a degree of functional importance or evolution (Liu et al., 2016). In addition, our earlier work has shown that CAPS can also be compensatory; multiple CAPs may dynamically compensate for one another, changing the dynamic landscape and allowing for different mutations (Ose et al., 2022b). We then explored the mechanistic insights and epistatic relationship between the observed mutations in different VOCs and CAP sites, and, particularly, the relationship between CAP sites and the functionally critical RBD using our dynamic coupling analysis (Kumar et al., 2015).
We find a mechanistic pattern in the S protein evolution that is common amongst previously studied systems, where allosteric sites exert control over the dynamics of the binding sites, and mutations of these allosteric sites modulate function. Coupling our analysis with evolutionary theory showed that many of these allosteric sites regulating function of the S protein may have been subjected to adaptive evolution as observed in mutations in VOCs. Our dynamics analysis also provides a mechanistic insight where the Omicron-defining sites have greater control over the binding sites than the Delta variant and are dynamically additive with the functional advantage of CAPs, thus, the greatest infectivity may not be a coincidence.
Specifically, we find that the interactions between CAP sites and VOC-defining mutations show fingerprints of non-additive dynamics within the Delta variant. In contrast, mutations leading to the Omicron variant are largely additive, driving critical dynamical behavior closer to the patterns observed within the wild-type. These interactions may also drive observed behavior similar between the reference and Omicron strains yet differ in the delta strain, such as the severity of infection as evidenced by hospitalization rates (Houhamdi et al., 2022; Menni et al., 2022). It has also been shown that the Omicron variant has a lower binding affinity with hACE2 than previous variants (Wu et al., 2022), which may contribute to its low pathogenicity.
Long-ranged interactions between different sites within a given protein are critically important for protein function (Peters and Lively, 1999; Bershtein et al., 2006; Collins et al., 2006; Ekeberg et al., 2013; Levy et al., 2017; Harrigan et al., 2018; Otten et al., 2018; Rojas Echenique et al., 2019; Shimagaki and Weigt, 2019; de la Paz et al., 2020; Rizzato et al., 2020; Yang et al., 2020; Bisardi et al., 2022) and for the CoV-2 S protein in particular (Zeng et al., 2020; Castiglione et al., 2021; Dong et al., 2021; Garvin et al., 2021; Nielsen et al., 2022; Ramarao-Milne et al., 2022; Rochman et al., 2022; Rodriguez-Rivas et al., 2022). By showing dynamic differences between the interactions of CAPs, which have likely played a major role in allowing the virus to infect human hosts, the binding site, and the characteristic mutations of dominant Delta and Omicron strains, we see a ‘fine-tuning’ of protein behavior. As variants continue to evolve, Omicron subvariants are of growing concern due in large part to further increased immune evasion (Callaway, 2022; Wang et al., 2022a; Wang et al., 2022b), and we observe that the new mutations observed in antibody binding sites yield more epistatic interaction with the CAPs. In addition to supporting previous dynamic research on the S protein, this analysis provides the insight that CAP sites are of continued importance to protein function and should be given special attention when considering the impact of future mutations.
Methods
Dynamic flexibility and dynamic coupling
The DFI utilizes a PRS technique that combines the elastic network model (ENM) and LRT (Gerek and Ozkan, 2011; Nevin Gerek et al., 2013). In ENM, the protein is considered as a network of beads at Cα positions interacting with each other via a harmonic spring potential. Using LRT, ∆R is calculated as the fluctuation response vector of residue j due to unit force’s F perturbation on residue i, averaged over multiple unit force directions to simulate an isotropic perturbation.
where H is the Hessian, a 3N × 3N matrix that can be constructed from 3-D atomic coordinate information and is composed of the second derivatives of the harmonic potential with respect to the components of the position’s vectors of length 3N. The Hessian inverse in this equation may be replaced with the covariance matrix G obtained from MD simulations as follows:
MD simulations were used to obtain the DFI profiles of Omicron, Omicron XBB, and Omicron XBB 1.5. In order to obtain DFI, each position in the structure was perturbed sequentially to generate a Perturbation Response Matrix A
where is the magnitude of fluctuation response at position i due to perturbations at position j. The DFI value of position i is then treated as the displacement response of position i relative to the net displacement response of the entire protein, which is calculated by sequentially perturbing each position in the structure.
It is also often useful to quantify position flexibility relative to the flexibility ranges unique to individual structures. To that end, DFI can be presented as a percentile rank, %DFI. All %DFI calculations present in this work used the DFI value of every residue of the full spike structure for ranking. The DFI parameter can be considered a measure of a given amino acid position’s ability to explore its local conformational space.
Dynamic coupling index
Similar to DFI, the DCI (Larrimore et al., 2017; Kumar et al., 2015) also utilizes PRS with the ENM and LRT. DCI captures the strength of displacement response of a given position i upon perturbation to a single functionally important position (or subset of positions) j, relative to the average fluctuation response of position i when all of the positions within a structure are perturbed.
When only positional pairs are concerned, this expression reduces to
As such, this parameter represents a measure of the dynamic coupling between i and j upon a perturbation to j. As with DFI, DCIji can also be presented as a percentile-ranked %DCIji.
One of the most important aspects of DCI is that the entire network of interactions is explicitly included in subsequent calculations without the need for dimensionality reduction techniques. If one considers interactions such as communication directionality or dynamic coupling regulation between position pairs as inherent properties of an anisotropic interaction network, it is critical to include the interactions of the entire network to accurately model the effect one residue can have on another.
Here, we present two further extensions of DCI, which allow us to uniquely model coupling directionality and epistatic effects: DCIasym and EpiScore, respectively. Interestingly, we can capture asymmetry between different residues within a protein through DCI, as a coupling in and of itself is asymmetric within an anisotropic network. That is, each amino acid has a set of positions to which it is highly coupled, and this anisotropy in connections gives rise to unique differences in coupling between a given i j pair of amino acids which do not have direct interactions (Figure 2A). DCIasym, then, is simply DCIij (the normalized displacement response of position j upon a perturbation to position i) − DCIji (Equation (7)). Using DCIasym, we can determine a cause–effect relationship between the i j pair in terms of force/signal propagation between these two positions.
where a positive DCIasym value indicates communication from position i to position j.
EpiScore can identify or describe potential non-additivity in substitution behavior between residue pairs. This metric can capture the differences in a normalized perturbation response to a position k when a force is applied at two residues i and j simultaneously versus the average additive perturbation response when each residue i, j, is perturbed individually (Figure 5A, Equation 9).
EpiScore values <1 (>1) indicate that the additive perturbations of positions i and j generate a greater (lesser) response at position k than the effect of a simultaneous perturbation. This means that, when treated with a simultaneous perturbation at both sites i and j, the displacement response of k is lower (higher) than the average effect of individual perturbations to i and j, one at a time. As EpiScore is a linear scale, the further the value from 1, the greater the effect described above.
Molecular dynamics (MD)
The production simulations for the Omicron variants, including Omicron, Omicron XBB, and Omicron XBB 1.5, were performed with the AMBER software package. These variants, each characterized by specific mutations, were modeled based on the template PDB structure 6M0J. The initial protein configurations in the simulations were parameterized using the ff14SB force field (Maier et al., 2015). In order to create an appropriate solvation environment for the proteins, a solvation box was defined around them, maintaining a minimum separation distance of 16 Å from the protein to the box boundaries. This was accomplished by employing the explicit TIP3P water model (Sun and Kollman, 1995), with the addition of sodium and chloride ions to maintain overall charge neutrality.
The simulation procedure involves an initial energy minimization step, aimed at mitigating steric clashes and optimizing the system’s energy. The steepest descent algorithm was applied, encompassing 11,000 steps. Subsequently, the system underwent a gradual temperature increase (heat up), up to 300 K, and was subjected to production simulations under a constant number of particles, pressure, and temperature ensemble (NPT).
During these production simulations, temperature was maintained at 300 K, with pressure regulation set at 1 bar. Temperature regulation was achieved through the utilization of the Langevin thermostat (Hünenberger, 2005) and Berendsen barostat (Berendsen et al., 1984), featuring a collision frequency of 1.0 picoseconds⁻¹. Hydrogen atom bond lengths were constrained using the SHAKE algorithm (Pearlman et al., 1995). The production trajectories were simulated for 1 µs each.
To ensure the reliability of the simulations and assess their convergence, a convergence criterion was employed. The achieved convergence was determined by monitoring the root mean square deviation (RMSD) between the highest sampled conformation in consecutive time windows (Sawle and Ghosh, 2016). Specifically, convergence was defined as the point at which the RMSD between the highest sampled conformation in the last 300 ns window and the 300 ns window immediately preceding it dropped below 1 Å. Window sizes varying from 100 ns to 500 ns were employed to evaluate convergence, ensuring the robustness and stability of the obtained results.
To calculate DFI, covariance matrix data were computed over different time windows as discussed above. By default, utilizing the Hessian implies a restriction to a harmonic potential, assuming that the data are sampled from a Gaussian distribution. Ergodicity in both simulation time and initial structures sampled in each time interval ensures two key conditions: (i) consistency of potential energy across conformations sampled from the same distribution. (ii) The sampling of different initial conformations while computing covariance matrices at various time windows eliminates global motions and accurately captures equilibrium coordinates. Consequently, the final average DFI profiles are independent of time window size, resulting in consistent results across different time window sizes (e.g., 50 ns vs. 75 ns) and enabling the acquisition of statistically significant DFI values.
Statistical tests
Pearson correlation coefficients (r) were used to demonstrate linear relationships between continuous variables in Figures 3 and 8. Student’s independent t-tests were performed to demonstrate a significant difference between distributions in Figures 2, 4 and 6 (and Figure 3—source data 1, Figure 6—figure supplements 1 and 2), and Figure 7 (and Figure 7—figure supplement 1), as demonstrated by the p-value.
Data availability
The code to perform DFI and DCI analysis is available at https://github.com/SBOZKAN/DFI-DCI (copy archived at Ozkan, 2024). Molecular Dynamics data are available at https://github.com/SBOZKAN/COV2SPIKE_MD. The mutation sites and EP values are contained in Supplementary file 4. The alignment used to generate EP values is also contained within the supporting information files as "EP_alignment.fas". Protein Databank ID number 6VXX (Walls et al., 2020) was used for closed conformation DCI calculations. 6VSB (Wrapp et al., 2020) was used for DFI calculations, EpiScore calculations, and open conformation DCI calculations. 6M0J (Lan et al., 2020) was used in molecular dynamics simulations of the RBD.
-
RCSB Protein Data BankID 6VSB. Prefusion 2019-nCoV spike glycoprotein with a single receptor-binding domain up.
-
RCSB Protein Data BankID 6M0J. Crystal structure of SARS-CoV-2 spike receptor-binding domain bound with ACE2.
-
RCSB Protein Data BankID 6VXX. Structure of the SARS-CoV-2 spike glycoprotein (closed state).
References
-
How helpful were molecular dynamics simulations in shaping our understanding of SARS-CoV-2 spike protein dynamics?International Journal of Biological Macromolecules 242:125153.https://doi.org/10.1016/j.ijbiomac.2023.125153
-
The new SARS-CoV-2 strain shows a stronger binding affinity to ACE2 due to N501Y mutantMedicine in Drug Discovery 10:100086.https://doi.org/10.1016/j.medidd.2021.100086
-
Mutation rate of SARS-CoV-2 and emergence of mutators during experimental evolutionEvolution, Medicine, and Public Health 10:142–155.https://doi.org/10.1093/emph/eoac010
-
Hospitalisation associated with SARS-CoV-2 delta variant in DenmarkThe Lancet. Infectious Diseases 21:1351.https://doi.org/10.1016/S1473-3099(21)00580-6
-
Molecular dynamics with coupling to an external bathThe Journal of Chemical Physics 81:3684–3690.https://doi.org/10.1063/1.448118
-
Divergent evolution of protein conformational dynamics in dihydrofolate reductaseNature Structural & Molecular Biology 20:1243–1249.https://doi.org/10.1038/nsmb.2676
-
Modeling sequence-space exploration and emergence of epistatic signals in protein evolutionMolecular Biology and Evolution 39:msab321.https://doi.org/10.1093/molbev/msab321
-
NCBI viral genomes resourceNucleic Acids Research 43:D571–D577.https://doi.org/10.1093/nar/gku1207
-
Coevolving residues inform protein dynamics profiles and disease susceptibility of nSNVsPLOS Computational Biology 14:e1006626.https://doi.org/10.1371/journal.pcbi.1006626
-
The role of protein dynamics in the evolution of new enzyme functionNature Chemical Biology 12:944–950.https://doi.org/10.1038/nchembio.2175
-
The role of conformational dynamics and allostery in modulating protein evolutionAnnual Review of Biophysics 49:267–288.https://doi.org/10.1146/annurev-biophys-052118-115517
-
Substitutions at nonconserved rheostat positions modulate function by rewiring long-range, dynamic interactionsMolecular Biology and Evolution 38:201–214.https://doi.org/10.1093/molbev/msaa202
-
SARS-CoV-2 variant biology: immune escape, transmission and fitnessNature Reviews. Microbiology 21:162–177.https://doi.org/10.1038/s41579-022-00841-7
-
RETRACTED: spike protein, s, of human coronavirus HKU1: role in viral life cycle and application in antibody detectionExperimental Biology and Medicine 233:1527–1536.https://doi.org/10.3181/0806-RM-197
-
Allosteric mechanisms of signal transductionScience 308:1424–1428.https://doi.org/10.1126/science.1108595
-
Biomolecular simulation: A computational microscope for molecular biologyAnnual Review of Biophysics 41:429–452.https://doi.org/10.1146/annurev-biophys-042910-155245
-
Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activationNature Chemical Biology 18:963–971.https://doi.org/10.1038/s41589-022-01059-7
-
Neutral theory and rapidly evolving viral pathogensMolecular Biology and Evolution 35:1348–1354.https://doi.org/10.1093/molbev/msy088
-
Change in allosteric network affects binding affinities of PDZ domainsPLOS Computational Biology 7:e1002154.https://doi.org/10.1371/journal.pcbi.1002154
-
Conformational transition of SARS-CoV-2 spike glycoprotein between its closed and open statesThe Journal of Chemical Physics 153:075101.https://doi.org/10.1063/5.0011141
-
SARS-CoV-2 variants, spike mutations and immune escapeNature Reviews. Microbiology 19:409–424.https://doi.org/10.1038/s41579-021-00573-0
-
Controlling the SARS-CoV-2 spike glycoprotein conformationNature Structural & Molecular Biology 27:925–933.https://doi.org/10.1038/s41594-020-0479-4
-
Characteristics of the first 1119 SARS‐CoV‐2 Omicron variant casesJournal of Medical Virology 94:2290–2295.https://doi.org/10.1002/jmv.27613
-
Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19Acta Pharmacologica Sinica 41:1141–1149.https://doi.org/10.1038/s41401-020-0485-4
-
BookThermostat Algorithms for molecular Dynamics simulationsIn: Holm C, Kremer K, editors. Advanced Computer Simulation. Springer. pp. 105–149.https://doi.org/10.1007/b99427
-
Mechanisms of SARS-CoV-2 entry into cellsNature Reviews. Molecular Cell Biology 23:3–20.https://doi.org/10.1038/s41580-021-00418-x
-
Characterization of anticancer agents by their growth inhibitory activity and relationships to mechanism of action and structureAnti-Cancer Drug Design 15:79–98.
-
Higher infectivity of the SARS-CoV-2 new variants is associated with K417N/T, E484K, and N501Y mutants: An insight from structural dataJournal of Cellular Physiology 236:7045–7057.https://doi.org/10.1002/jcp.30367
-
BookThe neutral theory of molecular evolutionCambridge University Press.https://doi.org/10.1017/CBO9780511623486
-
N-terminal domain mutations of the spike protein are structurally implicated in epitope recognition in emerging SARS-CoV-2 strainsComputational and Structural Biotechnology Journal 19:5556–5567.https://doi.org/10.1016/j.csbj.2021.10.004
-
The role of rigid residues in modulating TEM-1 β-lactamase function and thermostabilityInternational Journal of Molecular Sciences 22:2895.https://doi.org/10.3390/ijms22062895
-
Neutral theory, disease mutations, and personal exomesMolecular Biology and Evolution 35:1297–1303.https://doi.org/10.1093/molbev/msy085
-
MEGA X: molecular evolutionary genetics analysis across computing platformsMolecular Biology and Evolution 35:1547–1549.https://doi.org/10.1093/molbev/msy096
-
An evolutionary portrait of the progenitor SARS-CoV-2 and Its dominant offshoots in COVID-19 pandemicMolecular Biology and Evolution 38:3046–3059.https://doi.org/10.1093/molbev/msab118
-
Investigating cryptic binding sites by molecular dynamics simulationsAccounts of Chemical Research 53:654–661.https://doi.org/10.1021/acs.accounts.9b00613
-
The biology of proteostasis in aging and diseaseAnnual Review of Biochemistry 84:435–464.https://doi.org/10.1146/annurev-biochem-060614-033955
-
Characterization of the emerging B.1.621 variant of interest of SARS-CoV-2Infection, Genetics and Evolution 95:105038.https://doi.org/10.1016/j.meegid.2021.105038
-
Potts Hamiltonian models of protein co-variation, free energy landscapes, and evolutionary fitnessCurrent Opinion in Structural Biology 43:55–62.https://doi.org/10.1016/j.sbi.2016.11.004
-
Sequence evolution correlates with structural dynamicsMolecular Biology and Evolution 29:2253–2263.https://doi.org/10.1093/molbev/mss097
-
A molecular evolutionary reference for the human variomeMolecular Biology and Evolution 33:245–254.https://doi.org/10.1093/molbev/msv198
-
Evolutionary conservation of protein backbone flexibilityJournal of Molecular Evolution 63:448–457.https://doi.org/10.1007/s00239-005-0209-x
-
Predicting the mutational drivers of future SARS-CoV-2 variants of concernScience Translational Medicine 14:eabk3445.https://doi.org/10.1126/scitranslmed.abk3445
-
ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SBJournal of Chemical Theory and Computation 11:3696–3713.https://doi.org/10.1021/acs.jctc.5b00255
-
Exploring the intrinsic dynamics of SARS-CoV-2, SARS-CoV and MERS-CoV spike glycoprotein through normal mode analysis using anisotropic network modelJournal of Molecular Graphics & Modelling 102:107778.https://doi.org/10.1016/j.jmgm.2020.107778
-
The evolution of SARS-CoV-2Nature Reviews Microbiology 21:361–379.https://doi.org/10.1038/s41579-023-00878-2
-
Characterization of differential dynamics, specificity, and allostery of lipoxygenase family membersJournal of Chemical Information and Modeling 59:2496–2508.https://doi.org/10.1021/acs.jcim.9b00006
-
Mutations utilize dynamic allostery to confer resistance in TEM-1 β-lactamaseInternational Journal of Molecular Sciences 19:3808.https://doi.org/10.3390/ijms19123808
-
Protein folding stability and binding interactions through the lens of evolution: a dynamical perspectiveCurrent Opinion in Structural Biology 66:207–215.https://doi.org/10.1016/j.sbi.2020.11.007
-
Compensatory epistasis maintains ACE2 affinity in SARS-CoV-2 Omicron BA.1Nature Communications 13:7011.https://doi.org/10.1038/s41467-022-34506-z
-
Structural dynamics flexibility informs function and evolution at a proteome scaleEvolutionary Applications 6:423–433.https://doi.org/10.1111/eva.12052
-
Does SARS-CoV-2 bind to human ACE2 More strongly than does SARS-CoV?The Journal of Physical Chemistry. B 124:7336–7347.https://doi.org/10.1021/acs.jpcb.0c04511
-
Biophysical and computational methods to analyze amino acid interaction networks in proteinsComputational and Structural Biotechnology Journal 14:245–251.https://doi.org/10.1016/j.csbj.2016.06.002
-
The red queen and fluctuating epistasis: a population genetic analysis of antagonistic coevolutionThe American Naturalist 154:393–405.https://doi.org/10.1086/303247
-
Data-driven platform for identifying variants of interest in COVID-19 virusComputational and Structural Biotechnology Journal 20:2942–2950.https://doi.org/10.1016/j.csbj.2022.06.005
-
Inference of compressed Potts graphical modelsPhysical Review. E 101:012309.https://doi.org/10.1103/PhysRevE.101.012309
-
Flexible, functional, and familiar: characteristics of SARS-CoV-2 Spike protein evolutionFrontiers in Microbiology 11:2112.https://doi.org/10.3389/fmicb.2020.02112
-
Convergence of molecular dynamics simulation of protein native states: feasibility vs self-consistency dilemmaJournal of Chemical Theory and Computation 12:861–869.https://doi.org/10.1021/acs.jctc.5b00999
-
An NMR view of protein dynamics in health and diseaseAnnual Review of Biophysics 48:297–319.https://doi.org/10.1146/annurev-biophys-052118-115647
-
On the origin and evolution of SARS-CoV-2Experimental & Molecular Medicine 53:537–547.https://doi.org/10.1038/s12276-021-00604-z
-
Allosteric cross-talk among spike’s receptor-binding domain mutations of the SARS-CoV-2 South African variant triggers an effective hijacking of human cell receptorThe Journal of Physical Chemistry Letters 12:5987–5993.https://doi.org/10.1021/acs.jpclett.1c01415
-
Hydrophobic solvation of methane and nonbond parameters of the TIP3P water modelJournal of Computational Chemistry 16:1164–1169.https://doi.org/10.1002/jcc.540160910
-
A glycan gate controls opening of the SARS-CoV-2 spike proteinNature Chemistry 13:963–968.https://doi.org/10.1038/s41557-021-00758-3
-
On the origin and continuing evolution of SARS-CoV-2National Science Review 7:1012–1023.https://doi.org/10.1093/nsr/nwaa036
-
The emergence of SARS-CoV-2 variants of concern is driven by acceleration of the substitution rateMolecular Biology and Evolution 39:msac013.https://doi.org/10.1093/molbev/msac013
-
Modelling conformational state dynamics and its role on infection for SARS-CoV-2 Spike protein variantsPLOS Computational Biology 17:e1009286.https://doi.org/10.1371/journal.pcbi.1009286
-
Allosteric determinants of the SARS-CoV-2 Spike protein binding with nanobodies: examining mechanisms of mutational escape and sensitivity of the omicron variantInternational Journal of Molecular Sciences 23:2172.https://doi.org/10.3390/ijms23042172
-
SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effectsNature Structural & Molecular Biology 27:763–767.https://doi.org/10.1038/s41594-020-0468-7
-
SARS-CoV-2 Omicron RBD shows weaker binding affinity than the currently dominant Delta variant to human ACE2Signal Transduction and Targeted Therapy 7:8.https://doi.org/10.1038/s41392-021-00863-2
Article and author information
Author details
Funding
Gordon and Betty Moore Foundation (AWD00034439)
- Nicholas James Ose
- Paul Campitelli
- Tushar Modi
- I Can Kazan
National Science Foundation (1715591)
- Sefika Banu Ozkan
National Science Foundation (1901709)
- Sefika Banu Ozkan
National Science Foundation (GCR 1934848)
- Sudhir Kumar
National Institutes of Health (R01GM147635-01)
- Sefika Banu Ozkan
National Institutes of Health (GM139540)
- Sudhir Kumar
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
Funding was provided to NJO, PC, TM, and ICK by the Gordon and Betty Moore Foundation (award number AWD00034439) and to SBO by the National Science Foundation (award numbers: 1715591 and 1901709) and the National Institutes of Health R01GM147635-01. SK acknowledges the National Science Foundation (GCR 1934848) and the National Institutes of Health (GM139540) grants.
Version history
- Sent for peer review: August 29, 2023
- Preprint posted: September 15, 2023 (view preprint)
- Preprint posted: November 6, 2023 (view preprint)
- Preprint posted: April 3, 2024 (view preprint)
- Version of Record published: May 7, 2024 (version 1)
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.92063. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2023, Ose et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 222
- views
-
- 16
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
- Structural Biology and Molecular Biophysics
The type II class of RAF inhibitors currently in clinical trials paradoxically activate BRAF at subsaturating concentrations. Activation is mediated by induction of BRAF dimers, but why activation rather than inhibition occurs remains unclear. Using biophysical methods tracking BRAF dimerization and conformation, we built an allosteric model of inhibitor-induced dimerization that resolves the allosteric contributions of inhibitor binding to the two active sites of the dimer, revealing key differences between type I and type II RAF inhibitors. For type II inhibitors the allosteric coupling between inhibitor binding and BRAF dimerization is distributed asymmetrically across the two dimer binding sites, with binding to the first site dominating the allostery. This asymmetry results in efficient and selective induction of dimers with one inhibited and one catalytically active subunit. Our allosteric models quantitatively account for paradoxical activation data measured for 11 RAF inhibitors. Unlike type II inhibitors, type I inhibitors lack allosteric asymmetry and do not activate BRAF homodimers. Finally, NMR data reveal that BRAF homodimers are dynamically asymmetric with only one of the subunits locked in the active αC-in state. This provides a structural mechanism for how binding of only a single αC-in inhibitor molecule can induce potent BRAF dimerization and activation.
-
- Structural Biology and Molecular Biophysics
Bacteria utilize various strategies to prevent internal dehydration during hypertonic stress. A common approach to countering the effects of the stress is to import compatible solutes such as glycine betaine, leading to simultaneous passive water fluxes following the osmotic gradient. OpuA from Lactococcus lactis is a type I ABC-importer that uses two substrate-binding domains (SBDs) to capture extracellular glycine betaine and deliver the substrate to the transmembrane domains for subsequent transport. OpuA senses osmotic stress via changes in the internal ionic strength and is furthermore regulated by the 2nd messenger cyclic-di-AMP. We now show, by means of solution-based single-molecule FRET and analysis with multi-parameter photon-by-photon hidden Markov modeling, that the SBDs transiently interact in an ionic strength-dependent manner. The smFRET data are in accordance with the apparent cooperativity in transport and supported by new cryo-EM data of OpuA. We propose that the physical interactions between SBDs and cooperativity in substrate delivery are part of the transport mechanism.






