Cell Biology: A new regulator of caveolae signalling
More than sixty years ago, electron microscopy revealed that the plasma membrane of the cell contains specialized domains with distinct morphologies. These include small invaginations called caveolae that are found in the membranes of most cell types. For many years the functions of caveolae (‘little caves’ in Latin) remained elusive, but recent studies have implicated them in a range of processes, including lipid homeostasis, endocytosis and the protection of cells from mechanical stress (Parton and del Pozo, 2013). Now, in eLife, Peter Michaely at the University of Texas Southwestern Medical Centre and colleagues report that a protein called cavin-3, which is localized to caveolae, is involved in regulating the activities of two major intracellular signalling pathways and in controlling metabolism and cell proliferation (Hernandez et al., 2013).
Caveolae are formed through the actions of caveolins, a family of proteins that aggregate at the interface between the cytoplasm and the plasma membrane (Parton and del Pozo, 2013). Caveolins form complexes with additional proteins including members of the cavin family. Of these, cavin-1 is the best characterized and is known to be required for caveolae formation (Hansen and Nichols, 2010). Cavin-3 was originally identified as a binding partner and substrate of the enzyme PKC (protein kinase C), and was later shown to be associated with caveolae (Izumi et al., 1997; McMahon et al., 2009).
To address the role of cavin-3 in caveolae function and to determine its biological significance, Michaely and colleagues—including Victor Hernandez as first author—used biochemical and loss of function approaches in cultured fibroblasts, and also generated mice that lack cavin-3. They found that cavin-3 links caveolae with the actin cytoskeleton—the network of scaffolding that maintains a cell’s structure and shape. They provide evidence that this may be important for maintaining caveolae at the plasma membrane, and also for facilitating growth factor signalling through a cascade known as the ERK MAP kinase pathway.
Hernandez et al. showed that loss of cavin-3 expression triggers a reduction in ERK signalling, and that this change coincides with an increase in signalling by another protein kinase called Akt, which is a key regulator of cell growth and survival. The reciprocal changes in the activities of these two pathways may be linked. Thus, down-regulation of ERK activity leads to reduced expression of the transcription factor EGR1 (Early Growth Response protein 1) and, subsequently, to reduced expression of the gene that codes for a protein called PTEN. Since PTEN inhibits the action of Akt, the down-regulation of ERK ultimately leads to the up-regulation of Akt (Figure 1). Additional mechanisms for up-regulating Akt activity that are dependent on the ERK-EGR1 pathway clearly exist but have yet to be identified.
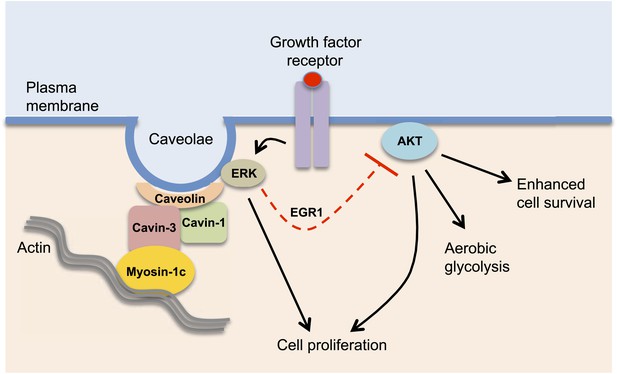
Cavin-3 regulates caveolae signalling.
Cavin-3 forms a complex with cavin-1 and caveolin and helps stabilize caveolae at the plasma membrane by linking them to the actin cytoskeleton via the protein myosin-1c. Caveolae at the plasma membrane can promote cellular growth by recruiting components of the ERK MAP kinase pathway and facilitating its activation by growth factors. ERK signalling can suppress (dashed red line) the activity of Akt, a protein kinase that is involved in many processes inside the cell. The loss of cavin-3 leads to enhanced Akt activity, which promotes cell survival and aerobic glycolysis, which may help explain why cavin-3 has tumour suppressor activity.
How does this signalling switch alter cellular function? It renders cells less susceptible to programmed cell death (apoptosis) and enhances aerobic glycolysis and cell proliferation. Aerobic glycolysis is the conversion of glucose to lactate despite oxygen being available—a phenomenon known as the ‘Warburg effect’—and is a hallmark of some rapidly dividing cancer cells (Cairns et al., 2011). Loss of cavin-3 protein has been reported in epithelial and glial-derived cancers, indicating that cavin-3 may be a tumour suppressor (Xu et al., 2001). The work of Hernandez et al. suggests that one way in which cavin-3 could suppress tumour formation is by limiting aerobic glycolysis. They demonstrate that introducing cavin-3 into a lung carcinoma cell line in which cavin-3 is absent, leads to enhanced ERK activation and a reduction in both Akt signalling and aerobic glycolysis. While cavin-3 knock-out mice do not generate tumours spontaneously, the loss of cavin-3 could contribute to metabolic changes that promote tumour growth. The mice develop normally but die prematurely following severe weight loss; this is likely due to reduced caveolae function as mutations in human genes encoding other caveolae-associated proteins are linked to muscle and fat degeneration (Parton and del Pozo, 2013). Precisely how the cellular and metabolic changes observed in cavin-3 depleted cells contribute to the mouse phenotype requires further research.
A major challenge for the future is to understand the cellular roles of distinct caveolae-associated protein complexes. The composition of the complexes can be altered in response to changes in the local environment or upon disease. In addition, some members of the caveolin and cavin families are only expressed in certain types of tissue, indicating that caveolae have cell-type specific functions (Bastiani et al., 2009; Hansen and Nichols, 2010). This is highlighted by recent work showing that, in contrast to the situation in fibroblasts, loss of cavin-3 does not affect the number of caveolae present in endothelial cell membranes (Hansen et al., 2013). It remains unknown whether there are changes in the balance of ERK and Akt signalling or in glucose metabolism in the endothelial cells. Cavin-3 is ubiquitously expressed; however, these findings suggest that it has cell-type specific functions that depend on its interaction with other caveolae proteins. This is supported by observations that cavin-3 is found in protein complexes of varying sizes in different tissues (Hansen et al., 2013).
The study by Michaely and colleagues contributes to the increasingly complex picture being drawn of how caveolae function and provides new insight into their regulation of intracellular signalling. The disruption of caveolae appears to contribute to many diseases including muscular dystrophies, cardiac disease and cancer. Therefore, a detailed understanding of the molecular mechanisms involved may provide potential avenues for therapeutic intervention.
References
-
Exploring the caves: cavins, caveolins and caveolaeTrends Cell Biol 20:177–186.https://doi.org/10.1016/j.tcb.2010.01.005
-
Caveolae as plasma membrane sensors, protectors and organizersNat Rev Mol Cell Biol 14:98–112.https://doi.org/10.1038/nrm3512
-
Inactivation of human SRBC, located within the 11p15.5-p15.4 tumor suppressor region, in breast and lung cancersCancer Res 61:7943–7949.
Article and author information
Author details
Publication history
Copyright
© 2013, Whitmarsh
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,609
- views
-
- 152
- downloads
-
- 7
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
The induction of adipose thermogenesis plays a critical role in maintaining body temperature and improving metabolic homeostasis to combat obesity. β3-adrenoceptor (β3-AR) is widely recognized as a canonical β-adrenergic G-protein-coupled receptor (GPCR) that plays a crucial role in mediating adipose thermogenesis in mice. Nonetheless, the limited expression of β3-AR in human adipocytes restricts its clinical application. The objective of this study was to identify a GPCR that is highly expressed in human adipocytes and to explore its potential involvement in adipose thermogenesis. Our research findings have demonstrated that the adhesion G-protein-coupled receptor A3 (ADGRA3), an orphan GPCR, plays a significant role in adipose thermogenesis through its constitutively active effects. ADGRA3 exhibited high expression levels in human adipocytes and mouse brown fat. Furthermore, the knockdown of Adgra3 resulted in an exacerbated obese phenotype and a reduction in the expression of thermogenic markers in mice. Conversely, Adgra3 overexpression activated the adipose thermogenic program and improved metabolic homeostasis in mice without exogenous ligand. We found that ADGRA3 facilitates the biogenesis of beige human or mouse adipocytes in vitro. Moreover, hesperetin was identified as a potential agonist of ADGRA3, capable of inducing adipocyte browning and ameliorating insulin resistance in mice. In conclusion, our study demonstrated that the overexpression of constitutively active ADGRA3 or the activation of ADGRA3 by hesperetin can induce adipocyte browning by Gs-PKA-CREB axis. These findings indicate that the utilization of hesperetin and the selective overexpression of ADGRA3 in adipose tissue could serve as promising therapeutic strategies in the fight against obesity.
-
- Cell Biology
- Chromosomes and Gene Expression
During oncogene-induced senescence there are striking changes in the organisation of heterochromatin in the nucleus. This is accompanied by activation of a pro-inflammatory gene expression programme – the senescence-associated secretory phenotype (SASP) – driven by transcription factors such as NF-κB. The relationship between heterochromatin re-organisation and the SASP has been unclear. Here, we show that TPR, a protein of the nuclear pore complex basket required for heterochromatin re-organisation during senescence, is also required for the very early activation of NF-κB signalling during the stress-response phase of oncogene-induced senescence. This is prior to activation of the SASP and occurs without affecting NF-κB nuclear import. We show that TPR is required for the activation of innate immune signalling at these early stages of senescence and we link this to the formation of heterochromatin-enriched cytoplasmic chromatin fragments thought to bleb off from the nuclear periphery. We show that HMGA1 is also required for cytoplasmic chromatin fragment formation. Together these data suggest that re-organisation of heterochromatin is involved in altered structural integrity of the nuclear periphery during senescence, and that this can lead to activation of cytoplasmic nucleic acid sensing, NF-κB signalling, and activation of the SASP.





