Precardiac deletion of Numb and Numblike reveals renewal of cardiac progenitors
Abstract
Cardiac progenitor cells (CPCs) must control their number and fate to sustain the rapid heart growth during development, yet the intrinsic factors and environment governing these processes remain unclear. Here, we show that deletion of the ancient cell-fate regulator Numb (Nb) and its homologue Numblike (Nbl) depletes CPCs in second pharyngeal arches (PA2s) and is associated with an atrophic heart. With histological, flow cytometric and functional analyses, we find that CPCs remain undifferentiated and expansive in the PA2, but differentiate into cardiac cells as they exit the arch. Tracing of Nb- and Nbl-deficient CPCs by lineage-specific mosaicism reveals that the CPCs normally populate in the PA2, but lose their expansion potential in the PA2. These findings demonstrate that Nb and Nbl are intrinsic factors crucial for the renewal of CPCs in the PA2 and that the PA2 serves as a microenvironment for their expansion.
https://doi.org/10.7554/eLife.02164.001eLife digest
Human embryos contain cells called ‘cardiac progenitor cells’ that serve as the building blocks to make the heart. Cardiac progenitor cells, or CPCs for short, initially move into areas of the embryo called the first and second heart fields, and then undergo a change to become specific types of heart cells: such as cardiac muscle cells. However, it is not known if CPCs are maintained during the development of the heart.
Now, Shenje, Andersen et al. have shown that Numb and Numblike—two proteins that are needed for the development of nerve cells—are also involved in the development of the heart. Mouse embryos without the genes for Numb and Numblike failed to develop hearts normally; and these mutants also had fewer CPCs in the ‘second pharyngeal arch’: a part of the embryo that becomes the sides and front of the neck. Experiments on wild-type mice showed that the CPCs multiplied within this arch, and then changed into specific heart cells as they left this structure. Furthermore, mixing CPCs in a petri dish with cells taken from this arch encouraged the CPCs to multiply without changing into specific cell types.
To investigate the importance of these two proteins further, Shenje, Andersen et al. engineered ‘chimeric’ mice in which some CPCs contained the Numb and Numblike genes and other CPCs did not. In most of these chimeric mice, the hearts developed normally, but the CPCs without the Numb or Numblike genes failed to multiply in the second pharyngeal arch. This shows that these genes must be present within an individual CPC to regulate the multiplication of that cell within this arch.
By uncovering how problems with the maintenance of CPCs can lead to heart defects—a very common birth defect in humans—this work may lead to new ways to prevent or treat congenital heart disease. Furthermore, identifying the other factors or mechanisms that can allow the long-term maintenance of CPCs in the laboratory will be crucial for research into heart regeneration, and for CPC-based treatments to repair the heart.
https://doi.org/10.7554/eLife.02164.002Introduction
Embryonic cardiac progenitor cells (CPCs), identified from early embryos or differentiating pluripotent stem cells, hold tremendous regenerative potential with their unique capability to expand and differentiate into nearly all cell types of the heart (Parmacek and Epstein, 2005; Kattman et al., 2006; Moretti et al., 2006; Kwon et al., 2007). Over the past decade, significant progress in developmental cardiology led to the identification of CPC markers and lineages (Cai et al., 2003; Kattman et al., 2006; Moretti et al., 2006; Kwon et al., 2009). However, CPCs are highly heterogeneous and it is unknown if they can undergo self-renewal without differentiation. Consequently, understanding the precise mechanisms of CPC self-renewal and maintenance remains a fundamental challenge.
Cardiogenesis initiates as the basic helix-loop-helix protein mesoderm posterior 1 (Mesp1) is transiently expressed in the nascent mesoderm during gastrulation (Saga et al., 1996). Mesp1+ cells migrate anteriorly and form the first heart field (FHF) and second heart field (SHF) (Saga et al., 2000). The FHF gives rise to the atria and left ventricle (LV), whereas the outflow tract (OT), right ventricle (RV) and some of atria are derived from the SHF (Buckingham et al., 2005). Before myocardialization, subsets of Mesp1 progeny express CPC markers including Islet1 (Isl1), fetal liver kinase 1 (Flk1), Nkx2.5, or myocyte-specific enhancer factor 2c (Mef2c) in precardiac mesoderm (Stanley et al., 2002; Cai et al., 2003; Verzi et al., 2005; Kattman et al., 2006). Isl1 and Flk1 expression is extinguished as CPCs adopt myocardial fates, but Nkx2.5 and Mef2c are continually expressed in cardiomyocytes (Edmondson et al., 1994; Tanaka et al., 1999). While CPCs expressing these markers have similar differentiation potential in vitro (Kattman et al., 2006; Moretti et al., 2006; Wu et al., 2006), it is unknown if a discrete population of stem cell-like CPCs exist to supply cells for cardiac growth and morphogenesis during development.
Numb and Numblike (Numbl)—mammalian Numb homologs sharing collinear topology and extensive sequence identity with functional redundancy—are evolutionarily conserved proteins that are required for the self-renewal of neural progenitors and mediate asymmetric cell divisions in various contexts of cell fate decisions (Zhong et al., 1997; Petersen et al., 2002, 2004; Roegiers and Jan, 2004), but their role in CPC development has not been explored. In the current study, we sought to identify and investigate CPCs affected by Numb and Numbl. By taking combinatorial approaches, we demonstrate that Mesp1+ progenitor-derived Isl1+ Nkx2.5− cells renew and expand without cardiac differentiation in the second pharyngeal arch (PA2) and that PA2 serves as their microenvironment during mammalian heart development.
Results
Numb and Numbl are required for heart development
Numb is expressed ubiquitously in developing mouse embryos (Zhong et al., 1997; Jory et al., 2009; Figure 1—figure supplement 1). To quantitatively examine the expression of Numb and Numbl in developing CPCs, we used the embryonic stem (ES) cell differentiation system that recapitulates early cardiogenesis (Kattman et al., 2011; Van Vliet et al., 2012; Figure 1A). Numb levels were relatively low at day 4, when Mesp1 was induced, but upregulated at day 6, when Isl1 appeared (Figure 1B). Numbl levels were also increased at day 6 (Figure 1B), implying that Numb and Numbl may have a role in CPC development after Mesp1 induction. To test this possibility, we deleted Numb in Mesp1+ progenitors, the earliest mesodermal progenitors giving rise to the entire heart and vasculature (Saga et al., 2000), by crossing Mesp1Cre mice with Numbflox mice (Zhong et al., 2000). The deletion did not affect LV formation, but resulted in a hypoplastic OT/RV and PA2 (Figure 1C,C′,D,D′). While the phenotype appeared to be confined to the OT, RV, and PA2, the penetrance was variable. Since Numbl-null mice are viable and fertile, but Numbl can compensate for loss of Numb function (Petersen et al., 2002), we extinguished Numb and Numbl expression in Mesp1+ progenitors by deleting Numb in the Numbl-null background (Petersen et al., 2002). The resulting Numb and Numbl double knockout (Numb/Numbl DKO) embryos showed a hypoplastic OT/RV and PA2 with complete penetrance (n = 12 embryos, Figure 1E,E′) and uniformly lethal by embryonic day (E) 10.0. These data suggest that Numb and Numbl are required for the formation of the PA2 and OT/RV.
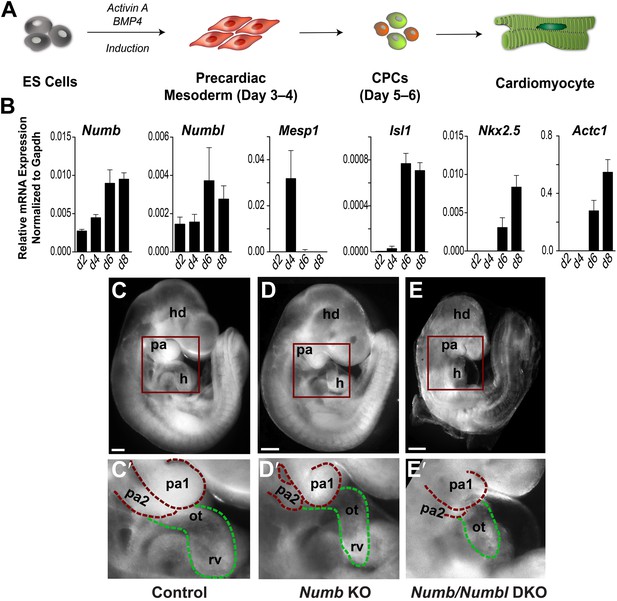
Numb and Numbl are required for PA2 and heart development.
(A) Schema of cardiac differentiation in ES cell system. (B) Expression profiles of genes indicated during cardiac differentiation of ES cells. Gene expression was analyzed by qPCR. Data are mean ± SD; n = 4; d, day. (C–E) Lateral views of control (C), Mesp1Cre; Numbflox/flox (Numb KO, D), Mesp1Cre; Numbflox/flox; Numbl–/– (Numb/Numbl DKO, E) embryos. (C′–E′) Enlargement of boxed areas in (C–E), showing normal, hypoplastic or atrophic PA2 and heart in control (C′), Numb KO (D′) or Numb/Numbl DKO (E′) embryos, respectively. Pharyngeal arches (red) and outflow tract/right ventricle (green) are outlined in dashes. Scale bars, 150 μm. hd, head; pa, pharyngeal arch; h, heart; ot, outflow tract; ra, right atrium; rv, right ventricle.
Deletion of Numb and Numbl depletes Mesp1+ cell progeny in PA2
Based on the Numb/Numbl DKO phenotype, we focused on investigating Mesp1 progeny contributing to the PA2 and OT/RV. To visualize Mesp1 progeny affected by Numb and Numbl, we introduced a Cre reporter (Ai9) into the DKO mouse embryos, in which red fluorescent protein (RFP) permanently marks Mesp1+ progenitors, and traced the RFP+ cells (Figure 2A). The resulting RFP+ cells showed a near complete deletion of Numb, confirmed by fluorescence-activated cell sorting (FACS) and quantitative PCR (qPCR) (Figure 2B). Interestingly, control and DKO littermates showed no noticeable morphological defects at E8.5 (8–12 somite stage) and were histologically indistinguishable (Figure 2—figure supplement 1). RFP+ cells were normally expressed the transient CPC marker Isl1 (Cai et al., 2003) from the primordial PA2 to the bulbus cordis (BC), and there was no significant difference in the number of RFP+ Isl1+ cells or the percentage of RFP+ Isl1+ cells positive for the mitosis marker phospho-histone H3 (PH3) (Figure 2—figure supplement 1B,B′,G,G′,K, Figure 2M). The RFP+ Isl1+ cells in PA2 primordia were continuous with the endo-, myo- and peri-cardial cell layers of the heart tube, and expressed the cardiac transcription factors Nkx2.5 and Mef2c from the distal BC and the sarcomeric protein α-Actinin from the proximal BC in both control and mutant embryos (Figure 2—figure supplement 1B–E′,G–J′). Thus, Numb/Numbl DKO does not appear to affect CPC and heart development at E8.5.
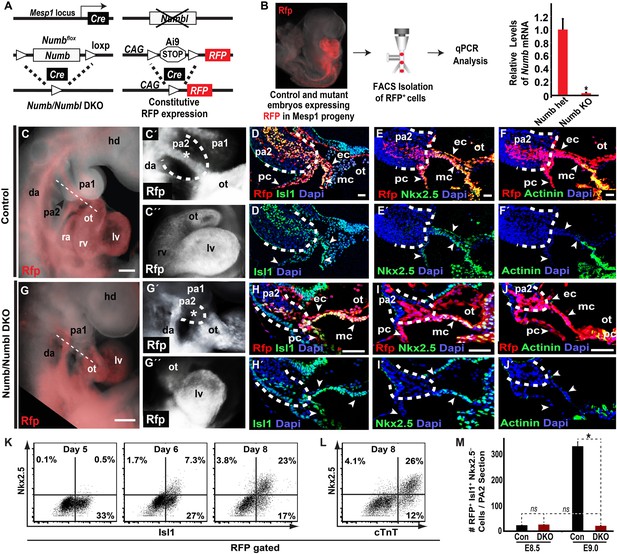
Deletion of Numb and Numbl depletes Mesp1+ progenitor-derived Isl1+ Nkx2.5– cells in PA2.
(A) Generation of Numb/Numbl DKO embryos expressing RFP in Mesp1 progeny (Mesp1Cre; Numbflox/flox; Numbl−/−; Ai9). (B) Relative mRNA levels of Numb in RFP+ cells isolated from control and Numb/Numbl DKO embryos. Data are mean ± SD; n = 9. (C, C′, G, G′) Lateral views of Mesp1+ cell-traced control (C and C′) or Numb/Numbl DKO (G and G′) embryos analyzed at E9.0. RFP marks Mesp1 progeny. Control embryos show continuous RFP expression from second pharyngeal arch (PA2, outlined) to heart (asterisk, C′), but the arch (outlined) is severely underdeveloped in Numb/Numbl DKO embryos without noticeable RFP expression (asterisk, G′). (C″ and G″) Frontal views of control (C″) or Numb/Numbl DKO (G″) heart. (D–F′), (H–J′), Representative confocal images of transverse sections through PA2 and outflow tract (OT) of control (D–F′) and Numb/Numbl DKO (H–J′) embryos. Cutting planes are shown in (C) and (G). Internal boundaries of PA2 are outlined in dashes. Dapi (blue) was used to counterstain the nuclei. (K and L) Representative plots of intracellular staining of RFP-gated cells. Isl1 and Nkx2.5 staining during day 5–8 (K) and cardiac troponin T (cTnT) and Nkx2.5 staining at day 8 (L). (M) Average number of RFP+ Isl1+ Mef2c−Nkx2.5− cells in PA2 section (12-micron) of indicated embryos and stages. Data are mean ± SD; n = 5; *p<0.05; ns, not significant. p values were determined using the paired Student t test. Scale bars, 25 μm (D–F and H–J), 150 μm (C and G). hd, head; pa, pharyngeal arch; ot, outflow tract; da, dorsal aorta; fe, foregut endoderm; ra, right atrium; rv, right ventricle; lv, left ventricle; ec, endocardial layer; mc, myocardial layer; pc, pericardial layer.
By E9.0 (18–22 somite stage) the RFP+ cells in the PA2 were continuous with the OT in control embryos, but were nearly absent in Numb/Numbl DKO embryos (Figure 2C–C″,G–G″). Histological analysis revealed that the PA2 contained a dense cluster of RFP+ Isl1+ cells in control embryos, but they were severely depleted in Numb/Numbl DKO embryos (Figure 2D,D′,H,H′,M). This suggests that Numb and Numbl are required for the formation of the RFP+ Isl1+ cell cluster in the PA2. Intriguingly, the RFP+ Isl1+ cells in the PA2 did not express the reported CPC genes Nkx2.5 and Mef2c (Dodou et al., 2004; Wu et al., 2006), but were continuous with the Nkx2.5+ Mef2c+ cells in the distal OT and thereafter the α-Actinin+ cells in the proximal OT and the RV (Figure 2E–F′,I–J′, Figure 2—figure supplement 2). This suggested that the transition of the Mesp1 lineage descendant to cardiomyocytes may occur through (1) Isl1+ Nkx2.5−/Mef2c−α-Actinin−cells, (2) Isl1+ Nkx2.5+/Mef2c+ α-Actinin−cells, (3) Isl1− Nkx2.5+/Mef2c+ α-Actinin+ cells. To test this possibility, we rederived ES cells expressing RFP in Mesp1+ cells from Mesp1Cre; Ai9 embryos, induced Mesp1+ precardiac mesoderm (Kattman et al., 2011), and analyzed CPC differentiation by FACS. RFP+ cells appeared on day 4 of differentiation and expressed Isl1 on day 5 (Figure 2—figure supplement 3, Figure 2K). The RFP+ Isl1+ cells started to express Nkx2.5 from day 6 and differentiated into cardiac troponin T+ (cTnT+) myocytes (Figure 2K,L). This indicates Mesp1+ progenitors are specified to Isl1+ Nkx2.5− CPCs and transition to Nkx2.5+/cTnT+ cells in an ES cell system as they differentiate into cardiac cells. Together, these data suggest that Numb and Numbl are required to form a dense population of Mesp1+ cell-derived Isl1+ Nkx2.5− cells in the PA2, which may give rise to Nkx2.5+ cardiac cells.
Mesp1 progeny expand in PA2 and migrate out to become heart cells
While PAs contain SHF progenitors (Kelly et al., 2001; Xu et al., 2004), it is unknown if Mesp1+ cell-derived Isl1+ Nkx2.5− cells in the PA2 give rise to cardiac cells. Based on the Numb/Numbl DKO phenotype, cardiac gene expression patterns, and ES cell data, we hypothesized that the Isl1+ Nkx2.5− cells expand without differentiation in the PA2 and migrate out of the arch to differentiate into cardiac cells. To test this, we examined proliferating cells in cardiac regions of E9.0 embryos by performing whole-mount staining with 5-ethynyl-2′-deoxyuridine (EdU), a nucleoside analog of thymidine. Remarkably, EdU+ cells were concentrated in the PA2 and rarely detected in other cardiac regions including the growing heart (Figure 3A,B). Consistently, about four percent of Mesp1+ cell-derived (RFP+) Isl1+ Nkx2.5− cells were PH3+ in the PA2, whereas PH3+ cells were nearly absent in RFP+ Isl1+/− Nkx2.5+ OT and α-Actinin+ RV cells (Figure 3C). Since DNA analogues are commonly used to show actively dividing stem cells and their lineages (Eisenhoffer et al., 2008; Snippert and Clevers, 2011), we labeled the proliferating cells by administering a single pulse of EdU, and monitored EdU+ cells in RFP+ cells (Figure 3D). EdU+ cells were first identified in the outer layer of the RFP+ cell cluster in PA2 after 2 hr, but not in the OT/RV (Figure 3D). By 4–8 hr, the RFP+ EdU+ cells were abundant in the PA2 and OT, and eventually found in the RV after 24–48 hr of chase (Figure 3D). RFP+ cells in the PA2 remained EdU+ (Figure 3D,E), implying their renewal in the PA2. The purdurance of the EdU pulse, determined by a sequential injection of EdU and 5-bromo-2′-deoxyuridine (BrdU, another thymidine analog), was shorter than 4 hr (Figure 3—figure supplement 1). These data suggest that Mesp1+ cell-derived Isl1+ Nkx2.5− cells in the PA2 migrate out of the arch and differentiate into cardiac cells.
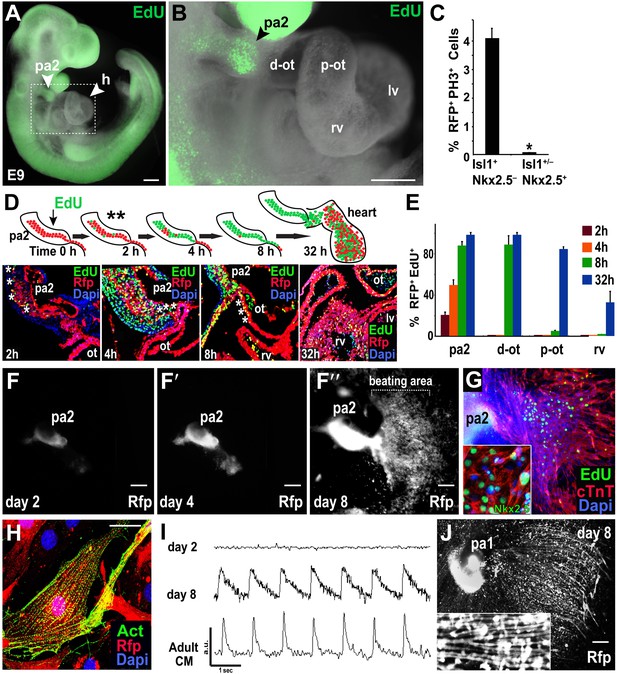
Mesp1+ progenitor-derived Isl1+ Nkx2.5– cells expand in PA2 and differentiate into Nkx2.5+ heart cells after leaving PA2.
(A) Whole-mount view of EdU (green)-treated embryo at E9.0. (B) Enlargement of the boxed area in (A), showing enrichment or lack of EdU+ cells in the PA2 or heart region, respectively. (C) Percentage of RFP+ PH3+ cells in Isl1+ Mef2c−Nkx2.5− cells and Isl1+/− Mef2c+ Nkx2.5+ cells. Data are mean ± SD; n = 4. (D) EdU pulse experiment. Top, EdU experiment schema. PA2s were also dissected out for ex vivo culture after 2 hr (**). Bottom, progeny of EdU+ cells at 2, 4, 8, and 32 hr after single pulse of EdU injection at E9.0 demonstrating that Mesp1 progeny in PA2 proliferate and migrate to form the OT/RV. (E) Quantification of RFP+ EdU+ cell progeny shown in (D). Data are mean ± SD; n = 3. (F–F″), Cultured PA2 explants in 2D culture form a sheet of beating cardiomyocytes (see Video 1). (G) EdU-pulsed PA2-derived sheet of beating cells stained with cTnT, EdU, or Nkx2.5 (inset). (H) Confocal image of RFP+ cells migrated from PA2 stained with cardiac α-actinin (Act). (I) Intracellular Ca2+ transients from day 2 and day 8 PA2 explants and adult cardiomyocyte (CM). a.u., arbitrary unit. (J) Cultured PA1 explants form myotube-like cells. Inset shows a magnified view. *p<0.05. D, day; Heart, E10.5 embryonic heart; ACTC1, actin, alpha, cardiac muscle 1. Dapi (blue) was used to counterstain the nuclei. p values were determined using the paired Student t-test. Scale bars, 10 μm (H), 150 μm (A and B), 250 μm (F–F″ and J). pa, pharyngeal arch; h, heart; d-ot, distal outflow tract; p-ot, proximal outflow tract; rv, right ventricle; lv, left ventricle.
To directly test the potential of RFP+ cells in the PA2, we labeled proliferating cells with EdU for 2 hr at E9.0, dissected out PA2s from embryos (asterisks, Figure 3D), and cultured the arches in 3D Matrigel culture. RFP+ cells were monitored in real-time with fluorescent time-lapse movies. After 2 days, the cluster of RFP+ cells expanded and exhibited multidirectional migration (Figure 3—figure supplement 2). The migrated RFP+ cells became contractile by day 4 (Figure 3—figure supplement 2; Videos 1 and 2). Although the range of their migration was somewhat limited, likely due to the extracellular environment of Matrigel, RFP+ cells continued to expand in the PA2 and differentiated into cardiomyocytes. When cultured in an uncoated dish, RFP+ cells expanded in the PA2 and progressively migrated out of the arch in a unidirectional fashion soon after being attached to the surface. However, no beating activity was observed on day 2 (Figure 3F). They migrated out of the arch progressively soon after being attached to the surface (Figure 3F). The migrated RFP+ cells started to spontaneously contract by day 4 (Figure 3F´). The expansion and migration of RFP+ cells appear to continue over 2 weeks and the beating area was correspondingly expanded (Figure 3F″; Video 3). The beating cells were positive for EdU, Nkx2.5, and cTnT/α-Actinin and exhibited a periodic Ca2+ oscillation pattern similar to that of adult cardiomyocytes (Figure 3G–I). To ascertain that the resulting cardiac cells were not derived from contamination from the adjacent OT, we isolated PAs from Nkx2.5GFP embryos (Biben et al., 2000) that express GFP in cardiac cells including the OT, but not in the PAs. Freshly dissected PA2s did not contain GFP+ cells, but cells expressed GFP 2–3 days after migration from the arch (Figure 3—figure supplement 3). We concluded that RFP+ Isl1+ cells expand in the PA2 and differentiate into cardiac cells as they migrate out of the arch. It is worth noting that PA1 cells also migrated out from the arch, but they remained GFP–and eventually formed myotube-like structures (Figure 3—figure supplement 3, Figure 3J). This is consistent with the previous finding that pharyngeal mesoderm is also the source of head skeletal muscles (Nathan et al., 2008).
3D-cultured PA2 in Matrigel.
This video shows expansion and migration of Mesp1 progeny (RFP+) in ex-vivo cultured PA2.
High magnification of inset in Video 1.
This video shows RFP+ beating cardiac myocytes at a migrating edge of ex-vivo cultured PA2.
2D-cultured PA2.
This video shows a beating monolayer of cardiomyocytes derived from RFP+ Mesp1 Progeny in ex-vivo cultured PA2.
PA2 cells promote the renewal and expansion of CPCs
To determine if the PA2 affects CPC expansion, we isolated Isl1+ Nkx2.5− CPCs from ES cells and cultured them with PA2 cells or heart cells derived from E9.0–9.5 embryos or without feeders. The CPCs were obtained by differentiating Isl1Cre; Ai9 ES cells (Uosaki et al., 2012) into Mesp1+ precardiac mesoderm (Kattman et al., 2011) and purifying RFP+ Isl1+ Nkx2.5− cells by FACS at day 5 (Figure 4A). The RFP+ cells spontaneously differentiate and form a sheet of beating cardiomyocytes (Uosaki et al., 2012). Strikingly, the co-cultured RFP+ cells formed distinct colonies (Figure 4A), which were never observed in control culture conditions, and their number was greatly increased over time (Figure 4B). The increase was inversely correlated with the appearance of Nkx2.5+/cTnT+ cells (Figure 4C, Figure 4—figure supplement 1), indicating PA2 cells promote expansion of Isl1+ Nkx2.5− CPCs and suppress their cardiac differentiation. This is unlikely due to the altered 3D environment as the effect was mimicked by PA2 cell-conditioned medium, but not by embryonic heart cells (Figure 4A,B, Figure 4—figure supplement 2).
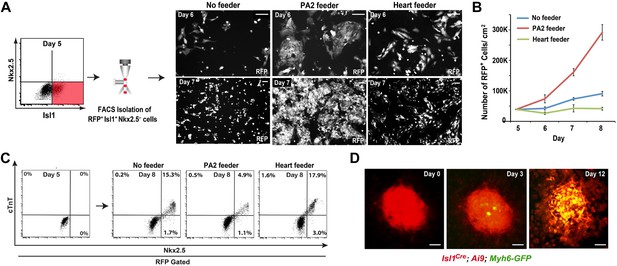
PA2 cells promote CPC expansion and suppress cardiac differentiation.
(A) FACS-purification of RFP+ Isl1+ Nkx2.5− CPCs induced from ES cell-derived precardiac mesoderm and their culture with no, embryonic PA2, or embryonic heart feeders. Images show RFP+ cells at day 6 and day 7. Isl1Cre; Ai9 ES cells were used to purify the CPCs at day 5 of cardiac differentiation, when Nkx2.5 is not expressed in Isl1+ CPCs. (B) Quantification of numbers of RFP+ cells cultured with no, embryonic PA2, or embryonic heart feeders. Data are mean ± SD; n = 3. (C) FACS plot of RFP+ Isl1+ Nkx2.5− CPCs differentiating into Nkx2.5+/cTnT+ cells with no, embryonic PA2, or embryonic heart feeders, determined at day 8. (D) Time-lapse images of Isl1+ Nkx2.5− CPC colony showing cardiac differentiation after removal of PA2 cells, indicated by GFP expression driven by Myh6 promoter. Scale bars, 50 μm.
To test if the colonies maintain differentiation potential, we established an Isl1Cre; Ai9; Myh6-GFP ES cell line, in which green fluorescent protein (GFP) is expressed when cells differentiate into cardiomyocytes (Ieda et al., 2010). RFP+ Isl1+ Nkx2.5− CPCs were FACS-purified from the line at day 5 of differentiation and co-cultured with PA2 cells to form colonies. In the presence of PA2 cells, the colonies continued to grow without GFP expression. However, when PA2 cells were removed after a week of co-culture, they began to express GFP from day 3 and differentiated into beating cardiomyocytes (Figure 4D). These data suggest that PA2 cells provide a cellular environment for the renewal and expansion of Isl1+ Nkx2.5− CPCs and suppress their cardiac differentiation.
Generation of Mesp1+ cell lineage-specific mosaicism
Although the Cre-mediated conditional deletion revealed a crucial role of Numb and Numbl for the development of Mesp1 progeny in the PA2, it was unclear if the Numb/Numbl DKO phenotype reflected the intrinsic role of Numb and Numbl. To address this question, we generated mosaic animals lacking Numb and Numbl in Mesp1 lineages (Figure 5A). We first established an Numb/Numbl DKO ES cell line, in which conditional deletion of Numb/Numbl occurs in Mesp1+ cells with resultant expression of RFP, by directly deriving ES cells from the DKO embryos. The ES cells were injected into host blastocysts of UBI-GFP/BL6 mice (Schaefer et al., 2001), which ubiquitously express GFP, to generate chimeras. In this system, Numb and Numbl deletion occurs in donor-derived Mesp1+ cells, and the DKO cells are traced by RFP expression. Donor-derived RFP+ cells were formed exclusively within Mesp1 lineages and distinguished from GFP+ host cells and RFP−GFP−donor progeny (Figure 5B–D). No cells were found double positive for RFP and GFP (Figure 5C,D), excluding the possibility of cell fusion, which potentially could rescue the phenotype of DKO cells. Therefore, we used wildtype blastocysts as hosts for further chimera generation. Consistent with the earlier mRNA analysis, Numb was absent in donor-derived RFP+ cells (Figure 5E).
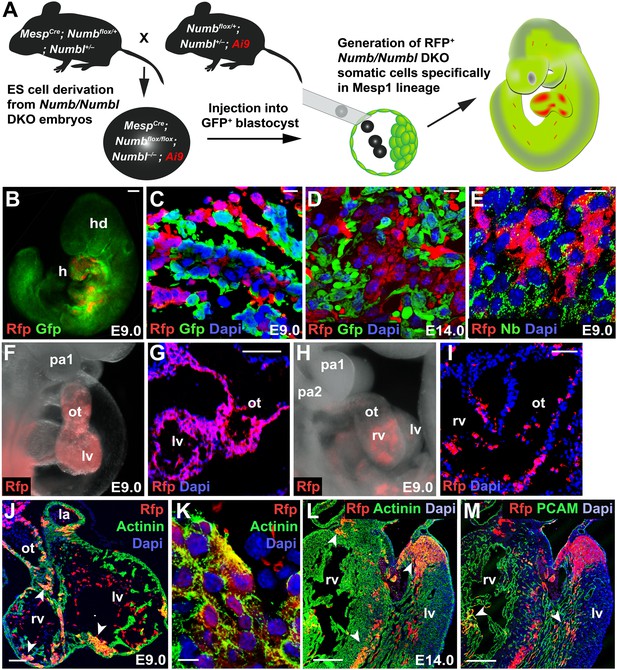
Generation of Mesp1 lineage-specific somatic cells lacking Numb and Numbl in vivo.
(A) Scheme for generation of RFP+ Numb/Numbl DKO cells in Mesp1 lineage. In this study, three independent sets of blastocyst injection were carried out and 16 chimeras were obtained from 132 embryos. (B–E) Chimeric embryos at E9.0 (B) generated with GFP+ host cells. Numb/Numbl DKO cells are shown in red. Sections were made transversely through cardiac region (C–E) and immunostained with RFP and GFP (C and D) or RFP and Numb (E) antibodies at corresponding stages. (F–I) Lateral views of chimera and sections, focused on PA and heart, showing contribution of RFP+ cells. Major contribution of RFP+ cells causes a phenotype similar to Numb/Numbl DKO embryos (F and G). (J–M) Confocal images of heart transverse sections at indicated stages. α-Actinin and PCAM are properly expressed in RFP+ cells (arrowheads). RFP+ area in (J) is enlarged in (K). Dapi (blue) was used to counterstain the nuclei. Scale bars, 10 μm (C, D, E, K), 100 μm (B, G, I, J), and 200 μm (L and M). a, anterior; p, posterior; hd, head; h, heart; pa, pharyngeal arch; ot, outflow tract; rv, right ventricle; lv, left ventricle; la, left atrium.
The phenotype of DKO chimeras depended on the contribution of the donor RFP+ cells. Major donor contribution caused a phenotype similar to DKO embryos (12.5% 2/16, Figure 5F,G). In most cases (87.5% 14/16), the chimeras were indistinguishable from wild-type embryos (Figure 5H,I). Donor RFP+ cells were normally populated in the PA2 and contributed to the OT, cardiomyocytes and endocardial cells (Figure 5J–M, Figure 5—figure supplement 1), indicating deletion of Numb and Numbl did not affect the migration or cardiac differentiation of CPCs.
NumbNumbl DKO CPCs fail to expand in PA2
In control embryos, the Isl1+ Nkx2.5– cells expanded dramatically after E8.5 in the PA2 (Figure 2C′,D,M). Proliferating cells, marked by PH3, were mostly found within the border of the cluster in control embryos, but were depleted in Numb/Numbl DKO embryos (Figure 6A–C‴). Likewise, the chimeras formed the Isl1+ cell cluster with PH3+ cells in the PA2 (Figure 6E). However, none of the RFP+ Isl1+ Nkx2.5− donor cells was found positive for PH3 in the PA2s of chimeric embryos analyzed at E9.0–9.5 (n = 10, Figure 6E–I), implying that Isl1+ Nkx2.5− cells in the PA2 lose their expansion potential in the absence of Numb and Numbl. There was no evidence of apoptosis in Numb/Numbl DKO cells in the PA2 (not shown). Consistent with the in vivo data, knockdown of Numb and Numbl in ES cell-derived Isl1+ Nkx2.5− CPCs, but not in Mesp1+ or Nkx2.5+ CPCs, resulted in a significant reduction of cell proliferation (Figure 6J, Figure 6—figure supplement 1). Conversely, increased levels of Numb in the Isl1+ Nkx2.5− CPCs promoted their proliferation (Figure 6K). These suggest that Numb and Numbl are cell-autonomous factors regulating the expansion of Isl1+ Nkx2.5− cells in the PA2.
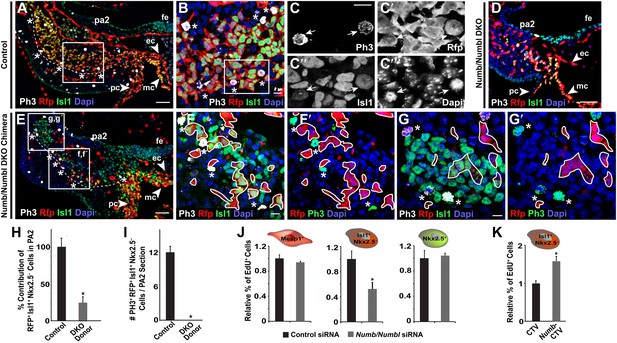
Numb and Numbl are required for proliferation of Mesp1+ progenitor-derived Isl1+ Nkx2.5– cells in PA2.
(A–G′) Confocal images of PA2 sections of control (A–C‴), Numb/Numbl DKO (D), and chimeric (E–G′) embryos, immunostained with PH3, RFP, Isl1 antibodies. Asterisks indicate PH3+ RFP+ Isl1+ (triple positive) cells located in outer layers of RFP+ Isl1+ cell population (outlined, A and E). Boxed areas in (A), (B), and (E) are shown in higher magnification in (B), (C–C‴), and (F–G′), respectively. No PH3+ cells are found in RFP+ cells (asterisks, F–G′). RFP+ cells were outlined in white (F–G′). (H and I) Percentage of donor-derived Isl1+ Nkx2.5− cells in PA2 is shown in (H) and number of PH3+ RFP+ Isl1+ cells per 12-micron PA2 section is shown (I). Data are mean ± SD; n = 10; *p<0.05. (J and K) Relative percentage of EdU+ cells in ES cell-derived Mesp1+ progenitor, Isl1+ Nkx2.5− CPCs or Nkx2.5+ CPCs transfected with control or Numb/Numbl DKO siRNA (J) or Isl1+ Nkx2.5− CPCs transfected with control (CTV) or Numb overexpression construct (CTV-Numb) (K). Data are mean ± SD; n = 3; *p<0.05. The Mesp1+, Isl1+ Nkx2.5−, or Nkx2.5+ cells were FACS-purified from day 4 Mesp1Cre; Ai9, day 5 Isl1Cre; Ai9, or day 6 Nkx2.5GFP ES cells, respectively. Dapi (blue) was used to counterstain the nuclei. p values were determined using the paired Student t test. Scale bars, 10 μm (B, C, F, G). 50 μm (A, D, E). fe, foregut endoderm; pa, pharyngeal arch ec, endocardial layer; mc, myocardial layer; pc, pericardial layer.
Discussion
Through the use of mouse genetics, lineage-specific mosaicism, embryonic stem cell systems, and ex-vivo organ culture, we have shown the existence of an undifferentiated and expansive population of CPCs and their microenvironment during development (Figure 7), which may provide a stem cell-niche paradigm in cardiovascular biology. Given that the heart grows rapidly in size and cell number at E8.5–10.5, the CPCs may serve as a renewable source to supply the number of cardiac cells needed to sustain the ensuing heart growth.
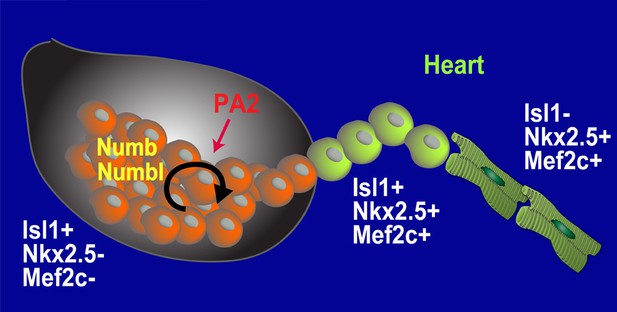
Model for Renewal and Niche of Mesp1+ progenitor-derived CPCs.
https://doi.org/10.7554/eLife.02164.023Although highly heterogeneous, CPCs are considered as multipotent cells that are destined to become heart cells. However, it remains unclear if they are capable to self-renew without differentiation. In mice, their precursors are identified as early as E5.75 by expression of the T-box transcription factor Eomesodermin in the epiblast (Russ et al., 2000) and specified to Mesp1+ cells at the onset of gastrulation at E6.5 (Costello et al., 2011). Mesp1+ cells are further specified to CPCs, vascular progenitors and some of the head mesenchyme that contribute to the entire heart, vasculature and subsets of head muscle cells, respectively (Saga et al., 2000). CPCs giving rise to the RV migrate through the OT from the SHF and express the transcription factors Isl1, Mef2c or Nkx2.5. These factors, however, are also expressed in neighboring cells including pharyngeal ectoderm and endoderm, foregut endoderm, neural progenitors and neural crest cells that are not originated from mesoderm (Lints et al., 1993; Edmondson et al., 1994; Cai et al., 2003), making it difficult to precisely discern CPCs. Moreover, it is unclear when and where CPCs are specified from Mesp1+ cells or their progeny. Thus, we traced Mesp1 lineages and examined the expression of CPC markers in Mesp1 progeny. Unexpectedly, heart cells rarely proliferated during early cardiogenesis, implying the existence of an alternate cell source for the ongoing growth of the heart. Indeed, we found a proliferative cluster of Mesp1+ cell-derived Isl1+ Nkx2.5−cells in the PA2—directly linked to the Isl1+ Nkx2.5+ OT of the growing heart—that migrated to the heart and became heart cells. The Isl1+ Nkx2.5− CPCs continued to expand in vivo, ex vivo, and in vitro without cardiac differentiation in the PA2, suggesting that the CPCs may serve as a renewable cell source for the developing heart. This cell renewal system may provide a parsimonious and efficient way to quickly generate a large number of cells used to build the heart during embryogenesis, which can be advantageous over local proliferation of differentiated cardiac cells. Further characterization of the CPCs will be necessary to provide quantitative information on their cellular contribution to the developing heart. Recent studies suggested that subsets of head and cardiac muscles share their progenitors (Lescroart et al., 2010). While the progenitor has not been identified, it will be interesting to investigate if this progenitor is derived from Isl1+ Nkx2.5− cells.
PAs are transient, segmented bulges that appear on the craniolateral side of developing embryos (Grevellec and Tucker, 2010). Each PA is composed of a mesodermal core and neural crest cell–derived mesenchymal cells that are surrounded by ectoderm outside and endoderm inside. At E8.5, the PA1—positioned most cranially among PAs—is structurally distinct, while the PA2 becomes noticeable after E9.0. Mesp1-derived Isl1+ Nkx2.5− cells proliferate in PA2s and initiate expression of Nkx2.5+ soon after exiting the arch. This suggests that PA2s function as a microenvironment to maintain the CPCs in an undifferentiated and expanding state and likely contain cells secreting paracrine factors that control the CPC number and fate. In fact, numerous signaling molecules are secreted from PA endoderm, ectoderm, and mesenchyme including Wnts, bone morphogenetic proteins, sonic hedgehog, and fibroblast growth factors (Rochais et al., 2009), and dysregulation of these signals is often associated with OT/RV defects (Frank et al., 2002; Stottmann et al., 2004; Washington Smoak et al., 2005; Kwon et al., 2007). Thus, it will be important to identify the extrinsic factors and cell types that provide key signals for the CPC maintenance and PA2 development.
Although the heart phenotype (hypoplastic OT/RV) caused by Numb/Numbl DKO may not result entirely from CPC depletion in the PA2, our findings together with published literatures suggest that the CPCs in the PA2 might be a major source of cells contributing to the OT/RV. For instance, the SHF—giving rise to the entire OT/RV—is located in PAs (Kelly et al., 2001; Rochais et al., 2009) and we found that proliferating cells are present predominantly in the PA1 and PA2 and rarely detected in the rest of the PAs. Our ex-vivo culture further showed that PA2 cells robustly differentiate into cardiac cells, whereas PA1 cells appear to give rise to myotubes without cells expressing Nkx2.5. It is also worth noting that nearly all, if not all, embryos showing severely hypoplastic OT/RV exhibit hypoplastic PA2s (Srivastava et al., 1997; Gottlieb et al., 2002; Cai et al., 2003; Kwon et al., 2007, 2009).
Numb and Numbl are highly conserved proteins that participate in cell fate determination by mediating asymmetric division, endocytosis and recycling of specific proteins, ubiquitination and cell migration (Santolini et al., 2000; Cayouette and Raff, 2002). Classic studies of Drosophila demonstrated Numb's spatio-temporal segregation to one pole of the mitotic cell as the primary mechanism by which cell fate is determined in single organ precursors (Uemura et al., 1989). In mammals, Numb and Numbl are required for the self-renewal of neural progenitors to maintain their number during development; while in the other settings they promote a neuronal fate by neural progenitor specification (Petersen et al., 2002, 2004). Similarly, Numb and Numbl were required for the renewal of CPCs during cardiogenesis, suggesting a conserved role in progenitor maintenance. It is unlikely that disruption of the yolk sac or angiogenesis contributed to the restriction of cardiac growth because there was no discernable difference in the phenotype of embryos or yolk sacs at E8.5. Furthermore, the results from somatic mosaicism demonstrated that Numb/Numbl DKO CPCs were unable to proliferate in normal PA2 environment, suggesting a cell-autonomous role of Numb and Numbl. Curiously, the deletion of Numb and Numbl in CPCs appears to affect the growth of neighboring PA2 cells as well. This suggests that Numb and Numbl may also influence CPC renewal and expansion by regulating PA2 development in a non-cell autonomous manner.
Numb and Numbl may affect CPC renewal and expansion via Notch, a conserved transmembrane receptor, given that Numb and Notch are mutually antagonistic (Schweisguth, 2004). In fact, Notch1 deficiency causes CPC expansion in the OT (Kwon et al., 2009). The expansion of CPCs is at least in part mediated by accumulation of the Wnt signaling effector β-catenin that are negatively regulated by membrane Notch (Kwon et al., 2011). Membrane Notch appears to require Numb and Numbl for the negative regulation of β-catenin (Kwon et al., 2011; Andersen et al., 2012), suggesting Numb and Numbl may be essential regulators of Notch and Wnt signaling during CPC development. With our current study, it will be necessary to re-examine the roles of Notch and Wnt signals at the level of renewing CPCs in the PA2.
Materials and methods
Mouse genetics and ES cell culture
Request a detailed protocolNumb/Numbl DKO mouse embryos were generated by mating Mesp1Cre; Numbflox/+; Numbl−/+ with Numbflox/flox; Numbl−/+; Ai9 mice. Embryos were harvested from E7.0–10.0 and genotyped as described (Saga et al., 1999; Petersen et al., 2002; Madisen et al., 2010). Nkx2.5GFP mice were used for ex-vivo culture (Biben et al., 2000). For ES cell work, ESMesp1−Cre; Ai9 cells (this work), ESMesp1−Cre; Numb flox/flox or flox/+; Numbl−/+ or −/−; Ai9 cells (this work), ESIsl1−Cre; Ai9 cells (Uosaki et al., 2012), ESNkx2.5−GFP cells (Hsiao et al., 2008), and ESIsl1−Cre; Ai9; Myh6−GFP cells (this work) were used. ESMesp1−Cre; Ai9, ESMesp1−Cre; Numb flox/flox or flox/+; Numbl−/+ or −/−; Ai9 or ESIsl1−Cre; Ai9; Myh6−GFP were derived from mice harboring Mesp1Cre; Ai9, Mesp1Cre; Numbflox/flox or flox/+; Numbl+/− or −/−; Ai9 or Isl1Cre; Ai9; Myh6-GFP, respectively. ES cells were maintained on gelatin-coated dishes in maintenance medium (Glasgow minimum essential medium with 10% fetal bovine serum and 1000 U/ml ESGRO (Millipore, Billerica, MA), Glutamax (Life Technologies/Thermo Fisher Scientific K.K., Waltham, MA), sodium pyruvate, MEM non-essential amino acids) and CPCs were induced with activin A, BMP4, and VEGF (R&D Systems, Minneapolis, MN) (Kattman et al., 2011; Cheng et al., 2013).
Ex-vivo culture and PA2 co-culture
Request a detailed protocolFor explant culture, PA1s or PA2s were carefully dissected from embryos at E9.0–9.5. Absence of contamination from the adjacent outflow tract was confirmed by absence of Nkx2.5+ cells. The explants were cultured in standard serum free medium supplemented with ascorbic acid at 37°C in 5% CO2. For 3D cultures, the PA explants were cultured in Matrigel. For PA2 cell co-culture, PA2s were isolated from E9.0–9.5 Isl1Cre; Ai9 embryos and cultured in gelatin-coated plate with PA media (DMEM: F-12, 7.5% FBS, 1X penicillin-streptomycin, 1X Glutamax). RFP− cells from PA's with significant outgrowth were isolated and further passaged. The PA2-derived or control (embryonic heart) cells were plated at a density of 10,000–50,000 cells/cm2 in multi-well plates and ES cell-derived CPCs were plated on top of the PA2 cells at a density of ∼10,000 cells/cm2 in SFD medium.
Generation of Mesp1 lineage-specific somatic cells
Request a detailed protocolFor Numb/Numbl DKO ES cell derivation, 3- to 4-week-old Numbflox/flox; Numbl−/+; Ai9 female mice were super-ovulated and mated with Mesp1Cre; Numbflox/+; Numbl−/+ males. Blastocysts were flushed at E3.5 and cultured to establish mouse ES cell lines (Ying et al., 2008). Numb/Numbl DKO ES cell lines were identified by genotyping and karyotyped to select suitable lines for the production of chimera. Numb/Numbl DKO ES cells were injected into the UBI-GFP/BL6 or wildtype blastocysts to generate chimera, which were transferred to E0.5–1.5 pseudo-pregnant recipient mothers. Chimeric embryos were harvested and analyzed at E7.5, 9.0, 14.0.
siRNA, constructs, and transfection
Request a detailed protocolFor Numb and Numbl knockdown experiments, Numb/Numbl ON-TARGETplus SMARTpool siRNA (L-046935/L-046983) or scrambled siRNA (Dharmacon/Thermo Fisher Scientific K.K.) was used at 100 nM for cell transfection (Kwon et al., 2011). For Numb overexpression, full-length Numb cDNA was cloned into CTV vector (Xiao et al., 2007) and used to increase Numb levels by transfecting in Cre-expressing cells. Cells were transfected with Lipofectamine LTX or Lipofectamine 2000 (Life Technologies) in single-cell suspensions.
EdU labeling, immunohistochemistry, and microscopy
Request a detailed protocolFor EdU pulse-tracing experiments, 10 mM EdU was injected at 0.075 mg per gram bodyweight intraperitoneally to pregnant mice at E9.0. Embryos were harvested at 2, 4, 8, and 32 hr post EdU injection. We used the Click-it EdU kit (Life Technologies) for EdU detection followed by immunostaining with primary and secondary antibodies. For the dual injection experiment, EdU and BrdU (300 μl, 10 mM each) were sequentially injected at a 4-hr interval. Embryos were harvested 4 hr post BrdU injection (8 hr of EdU), fixed, and sectioned (12 μm thickness). Antigen retrieval was performed with microwave for 20 min in 10 mM EDTA solution. The section was epimerized with 0.2% PBS Triton X for 15 min and stained with the Click-it EdU kit. The resulting sections were washed and incubated with anti BrdU antibody in incubation buffer (Roche BrdU Labeling and Detection Kit I). Anti-Mouse Ig-Fluorescein was used as secondary antibody. For microscopy, embryos were fixed in 4% paraformaldehyde overnight and then 30% sucrose, and then embedded in OCT, sectioned and stained using standard protocols. Antibodies used were: goat α-Islet1 (1:200; R&D), goat α-PECAM (1:200; R&D), mouse α-Islet1 (1:200; Developmental Studies Hybridoma Bank, Iowa City, IA), rabbit α-MEF2c (1:200; Cell Signaling Technology, Danvers, MA), rabbit α-RFP (1:400; Clontech Laboratories, Inc., Mountain View, CA), rabbit α-Numb (pre-absorbed, 1: 500; Abcam, Cambridge, MA or from Dr. Zhong), goat or rabbit anti-β1 integrin (1:400; R&D or 1:1000; Abcam), mouse α-sarcomeric Actinin (1:500; Sigma-Aldrich, St. Louis, MO), goat α-Nkx2.5 (1:20; Santa Cruz Biotechnology, Dallas, Texas), mouse α-PH3 (1:500; Abcam), rabbit α-GFP (1:400; Abcam), goat α-GFP (1:200; R&D), rabbit α-caspase 3 (Abcam). Alexa Fluor secondary antibodies (Life Technologies) were used for secondary detection and confocal images acquired with a Zeiss LSM 510 Meta confocal microscope using Zen acquisition software.
Flow cytometry and time-lapse imaging
Request a detailed protocolFor flow cytometry, cells were dissociated and analyzed with Accuri C6 Flowcytometer (BD Biosciences, San Jose, CA) and FlowJo software (TreeStar, Ashland, OR). For intracellular-flow cytometry, cells were stained with indicated antibodies after dissociation as previously described (Uosaki et al., 2011). For FACS, dissociated cells were resuspended in PBS containing 0.1% FBS, 20 mM Hepes and 1 mM EDTA and sorted with FACSAria II (BD Biosciences) and SH800 sorter (Sony Biotechnology, Japan). Time-lapse imaging was done with a BZ9000 All-in-One Fluorescence microscope (Keyence, Japan).
Ca2+ transient measurement and analysis
Request a detailed protocolPA2 explants were incubated with 3 µM Fura-2 AM (Invitrogen, Molecular Probes, Carlsbad CA) for 20 min at 37°C. After washing and de-esterification for 20 min the explants were placed in an imaging chamber and electrical field stimulated at 1 Hz, 37°C, pH 7.4 with Ca2+. The change in intracellular Ca2+ was measured with an inverted fluorescence microscope (TE2000, Nikon, Japan) and Myocam (IonOptix, Milton, MA) by Fura-2 AM fluorescence intensity ratio at 340 nm and 380 nm.
Statistical analyses
Request a detailed protocolDifferences between groups were examined for statistical significance using the paired Student's t test. A p-value <0.05 was regarded as significant. Error bars indicate standard error of the mean.
References
-
Non-canonical Notch signaling: emerging role and mechanismTrends in Cell Biology 22:257–265.https://doi.org/10.1016/j.tcb.2012.02.003
-
Building the mammalian heart from two sources of myocardial cellsNature Reviews Genetics 6:826–835.https://doi.org/10.1038/nrg1710
-
Fibronectin mediates mesendodermal cell fate decisionsDevelopment 140:2587–2596.https://doi.org/10.1242/dev.089052
-
Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesisDevelopment 120:1251–1263.
-
An Fgf8 mouse mutant phenocopies human 22q11 deletion syndromeDevelopment 129:4591–4603.
-
The pharyngeal pouches and clefts: development, evolution, structure and derivativesSeminars in Cell & Developmental Biology 21:325–332.https://doi.org/10.1016/j.semcdb.2010.01.022
-
The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesodermDevelopmental Cell 1:435–440.
-
Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitorsProceedings of the National Academy of Sciences of the United States of America 104:10894–10899.https://doi.org/10.1073/pnas.0704044104
-
Notch post-translationally regulates beta-catenin protein in stem and progenitor cellsNature Cell Biology 13:1244–1251.https://doi.org/10.1038/ncb2313
-
Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendantsDevelopment 119:969.
-
Signaling pathways controlling second heart field developmentCirculation Research 104:933–942.https://doi.org/10.1161/CIRCRESAHA.109.194464
-
Asymmetric cell divisionCurrent Opinion in Cell Biology 16:195–205.https://doi.org/10.1016/j.ceb.2004.02.010
-
MesP1: a novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulationDevelopment 122:2769–2778.
-
Mesp1 expression is the earliest sign of cardiovascular developmentTrends in Cardiovascular Medicine 10:345–352.https://doi.org/10.1016/S1050-1738(01)00069-X
-
MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tubeDevelopment 126:3437–3447.
-
Numb is an endocytic proteinThe Journal of Cell Biology 151:1345–1352.https://doi.org/10.1083/jcb.151.6.1345
-
Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivoCellular Immunology 214:110–122.https://doi.org/10.1006/cimm.2001.1895
-
Regulation of notch signaling activityCurrent Biology: CB 14:R129–R138.https://doi.org/10.1016/j.cub.2004.01.023
-
Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3'UTR-ires-Cre allele of the homeobox gene Nkx2-5The International Journal of Developmental Biology 46:431–439.
-
The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart developmentDevelopment 126:1269–1280.
-
Early cardiac development: a view from stem cells to embryosCardiovascular Research 96:352–362.https://doi.org/10.1093/cvr/cvs270
-
Sonic hedgehog is required for cardiac outflow tract and neural crest cell developmentDevelopmental Biology 283:357–372.
-
Mouse numb is an essential gene involved in cortical neurogenesisProceedings of the National Academy of Sciences of the United States of America 97:6844–6849.https://doi.org/10.1073/pnas.97.12.6844
-
Differential expression of mammalian Numb, Numblike and Notch1 suggests distinct roles during mouse cortical neurogenesisDevelopment 124:1887–1897.
Article and author information
Author details
Funding
Magic That Matters Fund
- Chulan Kwon
National Heart, Lung, and Blood Institute (R01HL111198)
- Chulan Kwon
National Heart, Lung, and Blood Institute (R00HL092234)
- Chulan Kwon
Maryland Stem Cell Research Fund (2012-MSCRFE-0127-00)
- Chulan Kwon
Lundbeck foundation
- Peter Andersen
Fondation Leducq
- David A Kass
Max Kade Foundation
- Peter P Rainer
Muscular Dystrophy Association
- David A Kass
National Institutes of Health (HL:107153)
- David A Kass
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank YN Jan (UCSF) for generously providing Numbflox and Numbl knockout mice. The authors thank Kwon and Kass laboratory members for helpful discussions, the Johns Hopkins University Transgenic Core (A Lawler and C Hawkins) for technical assistance, and L Sprague for help with time-lapse experiments. CK was supported by the Magic That Matters Fund, grants from NHLBI/NIH (R01HL111198, R00HL092234), and MSCRF. PA was supported by the Lundbeck foundation (Denmark). HU was supported by Japan Society for the Promotion of Science. DAK was supported by Fondation Leducq, Muscular Dystrophy Association, and HL:107153, and PPR was supported by the Max Kade Foundation.
Ethics
Animal experimentation: All the animals were treated humanely according to AALAC and NIH guidelines (Johns Hopkins Medical Institutions are fully accredited by the AALAC) and the current study was conducted under the animal protocol (MO10M444) approved by the Animal Care and Use Committee at Johns Hopkins University.
Version history
- Received: December 27, 2013
- Accepted: April 1, 2014
- Accepted Manuscript published: April 24, 2014 (version 1)
- Version of Record published: May 6, 2014 (version 2)
Copyright
© 2014, Shenje et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,331
- views
-
- 180
- downloads
-
- 33
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Developmental Biology
- Structural Biology and Molecular Biophysics
A crucial event in sexual reproduction is when haploid sperm and egg fuse to form a new diploid organism at fertilization. In mammals, direct interaction between egg JUNO and sperm IZUMO1 mediates gamete membrane adhesion, yet their role in fusion remains enigmatic. We used AlphaFold to predict the structure of other extracellular proteins essential for fertilization to determine if they could form a complex that may mediate fusion. We first identified TMEM81, whose gene is expressed by mouse and human spermatids, as a protein having structural homologies with both IZUMO1 and another sperm molecule essential for gamete fusion, SPACA6. Using a set of proteins known to be important for fertilization and TMEM81, we then systematically searched for predicted binary interactions using an unguided approach and identified a pentameric complex involving sperm IZUMO1, SPACA6, TMEM81 and egg JUNO, CD9. This complex is structurally consistent with both the expected topology on opposing gamete membranes and the location of predicted N-glycans not modeled by AlphaFold-Multimer, suggesting that its components could organize into a synapse-like assembly at the point of fusion. Finally, the structural modeling approach described here could be more generally useful to gain insights into transient protein complexes difficult to detect experimentally.
-
- Developmental Biology
Inhibitory G alpha (GNAI or Gαi) proteins are critical for the polarized morphogenesis of sensory hair cells and for hearing. The extent and nature of their actual contributions remains unclear, however, as previous studies did not investigate all GNAI proteins and included non-physiological approaches. Pertussis toxin can downregulate functionally redundant GNAI1, GNAI2, GNAI3, and GNAO proteins, but may also induce unrelated defects. Here, we directly and systematically determine the role(s) of each individual GNAI protein in mouse auditory hair cells. GNAI2 and GNAI3 are similarly polarized at the hair cell apex with their binding partner G protein signaling modulator 2 (GPSM2), whereas GNAI1 and GNAO are not detected. In Gnai3 mutants, GNAI2 progressively fails to fully occupy the sub-cellular compartments where GNAI3 is missing. In contrast, GNAI3 can fully compensate for the loss of GNAI2 and is essential for hair bundle morphogenesis and auditory function. Simultaneous inactivation of Gnai2 and Gnai3 recapitulates for the first time two distinct types of defects only observed so far with pertussis toxin: (1) a delay or failure of the basal body to migrate off-center in prospective hair cells, and (2) a reversal in the orientation of some hair cell types. We conclude that GNAI proteins are critical for hair cells to break planar symmetry and to orient properly before GNAI2/3 regulate hair bundle morphogenesis with GPSM2.





