Stapled Golgi cisternae remain in place as cargo passes through the stack
Figures
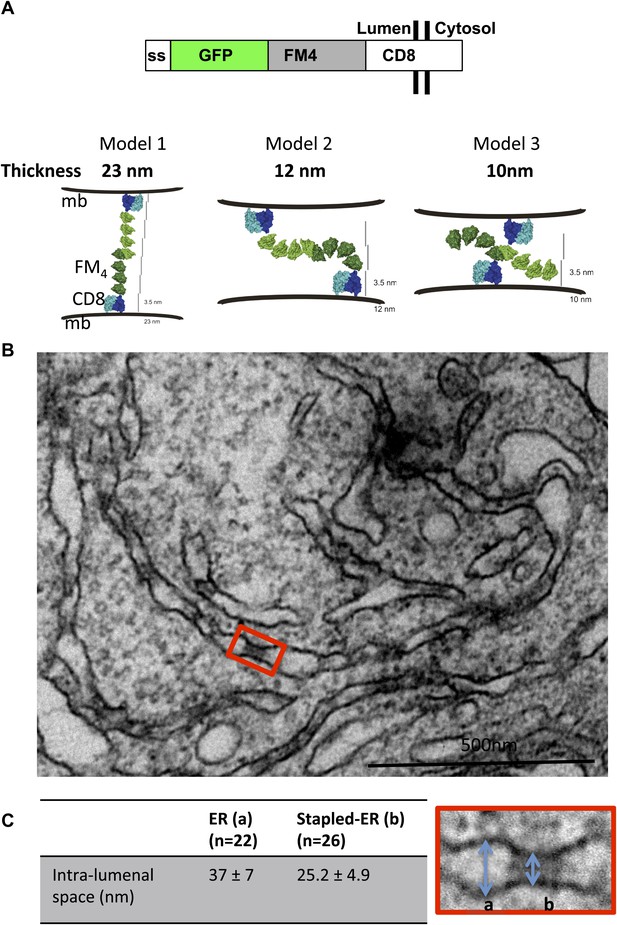
Aggregated-CD8Lumenal as an ER staple.
(A) Domains harbored by CD8lumenal. Predicted models of trans-interacting CD8lumenal preoteins that are expected to form lumenal staples. Predicted intra-lumenal space varies from 10 to 23 nm. (B) Electron-micrograph showing ER of HeLa cells expressing CD8lumenal in the absence of the disaggregating drug. ER-aggregated CD8lumenal, which form ER-staples, appear as electron-dense flat features (red square). (C) Lumenal staples trigger lumenal constriction of ER tubules, consistent with model 1.

Cytosolic staples stack the ER.
HeLa cells expressing CD8cytosolic were incubated without the disaggregating drug. Electron-micrograph showing that cytosolic staples trigger artificial stacking of ER membranes.
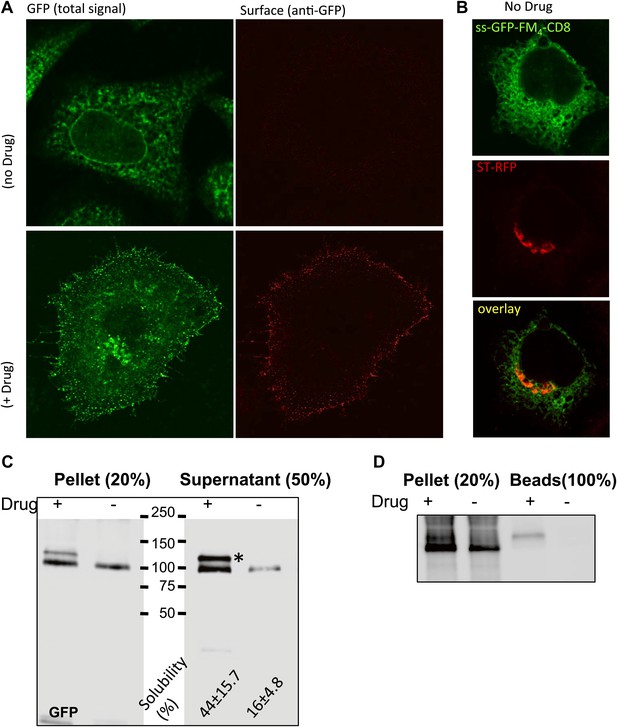
Disaggregated-CD8lumenal as an anterograde cargo.
(A) Confocal micrograph showing HeLa cells expressing CD8lumenal in the presence or in the absence of the disaggregating drug. Without drug, ER-aggregated CD8lumenal remained at the ER (ER staples), whereas with drug (for 4 hr) disaggregated CD8lumenal is transported to the plasma membrane (PM), where it could be detected with an anti-GFP antibody in non-permeabilized cells. (B) Confocal micrograph showing HeLa cells co-expressing CD8lumenal with ST-RFP in the absence of the disaggregating drug. The ER staples do not alter Golgi targeting of ST-RFP. (C) Immunoblot showing that 4 hr treatment of HeLa cells with the disaggregating drug increases TritonX-100 solubility of CD8lumenal. Disaggregated-CD8lumenal shows reduced mobility on SDS-PAGE gel (* upper band). (D) Immunoblot showing that Jacalin, a lectin that binds galactose residues, exclusively precipitates disaggregated CD8lumenal.
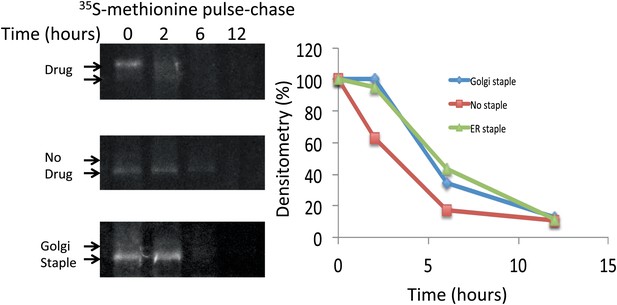
Lifetime of staples and disaggregated CD8lumenal.
HeLa cells expressing CD8lumenal were radiolabeled with 35S methionine for 30 min in the presence or the absence of the disaggregating drug (pulse). Then cells were washed and incubated at 20°C in media containing 3 mM methionine, and harvested at different time-points (chase). For the study of Golgi staples, after the radiolabeling pulse, cells were shifted to 16°C for 1 hr in the presence of the drug, after which the drug was removed and cells were incubated at 20°C. CD8lumenal from the cell extracts were immuno-precipitated using anti-GFP antibody, processed for SDS-PAGE. Lifetime was estimated by measuring the densitometry (using ImageJ) on a film exposed overnight to the gel containing the radioactive samples.
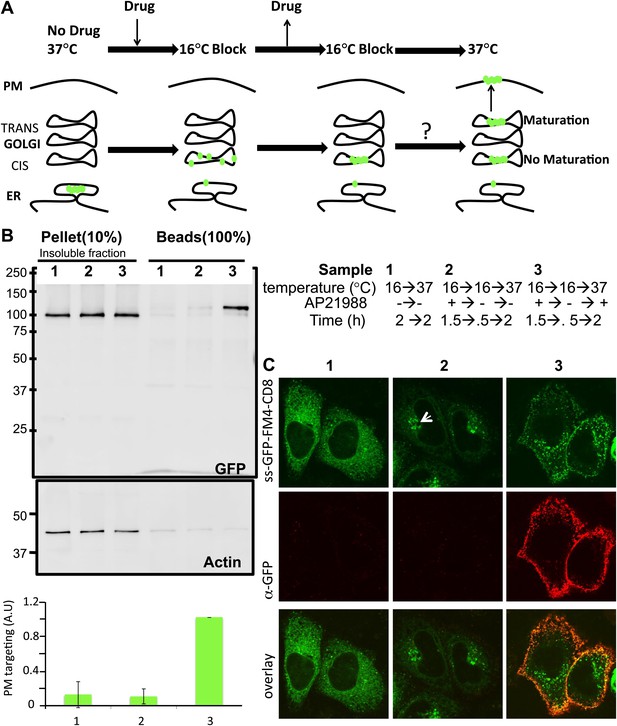
Re-aggregated CD8lumenial as a Golgi staple, and its retention within the Golgi.
(A) General procedure: 16°C temperature block and disaggregation/re-aggregation cycles are combined to form the staples in the cis-Golgi and assess the cisternal progression model. HeLa cells expressing CD8lumenal were incubated for 1.5 hr at 16°C in the presence of the disaggregating drug, and then the drug was removed for 30 min at 16°C to allow re-aggregation in the cis-Golgi. Temperature was shifted to 37°C for 2 hr in the absence (2) or in the presence (3) of the disaggregating drug. As a negative control, cells were incubated at the same temperatures but without any drug at any time (1). (B) Immunoblot, after surface biotinylation, showing that disaggregated CD8lumenal (3) is targeted to the plasma membrane (PM), whereas ER-aggregated-CD8lumenal (1), and Golgi-re-aggregated-CD8lumenal (2), and endogenous actin (lower panel) are not. Graph, normalized quantification of PM targeted CD8lumenal, with (3) set to 1. Data represent the mean of three independent experiments, (C) Confocal micrograph confirming the ER localization of aggregated CD8lumenal, (ER staples, 1), the PM localization of disaggregated CD8lumenal (3), and the Golgi retention of re-aggregated CD8lumenal (Golgi staples, white arrow, 2). All experiments were conducted in the presence of cycloheximide.

Glycosylation profile of CD8lumenal.
HeLa cells expressing CD8lumenal were incubated with the disaggregating drug at 16°C for 2 hr prior to being shifted at 37°C for 2 hr in the presence (1) or absence (2) of the drug. As negative control, cells were incubated at the same temperatures but without drug at any time (3). (A) Immunoblot showing that Jacalin, a lectin that binds Galatose residues, precipitates disaggregated CD8lumenal from cells incubated at 37°C (accordingly to Figure 1D). Helix-Pomatia, a lectin that binds N-Acetyl-Galactosamine, precipitates disaggregated CD8lumenal from cells incubated at 16°C.
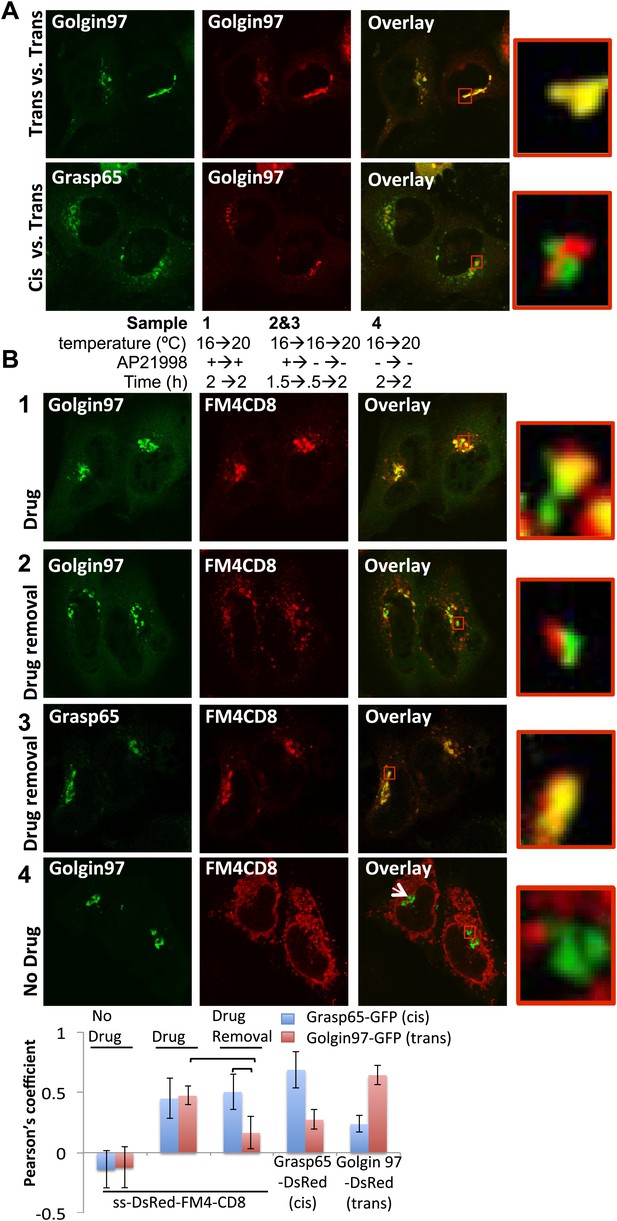
cis-Golgi retention of Golgi staples at the light microscopy level.
(A) Confocal micrograph showing HeLa cells co-expressing DsRed-Golgin97, a trans-Golgi marker, with GFP-Golgin97 (upper panel) or Grasp65-GFP, a cis-Golgi marker (lower panel). Cis- and trans-Golgi are readily distinguishable at the confocal microscopy resolution. Red square, higher magnification. (B) HeLa cells co-expressing DsRed-CD8lumenal with GFP-Golgin97 (1, 2, 4) or Grasp65-GFP (3) were incubated at 16°C for 2 hr with the disaggregating drug, prior to shifting the temperature to 20°C for two additional hours in the presence (1) or in the absence (2, 3) of the disaggregating drug. As a negative control, cells were incubated at the same temperature but without any drug at any time (4). Confocal micrograph showed that Golgi-re-aggregated-CD8lumenal, presumably forming cis-Golgi lumenal staples, showed a better co-localization with Grasp65 (3) than with Golgin97 (2). Disaggregated-CD8lumenal showed a stronger co-localization with Golgin97 than with Grasp65 (1), whereas ER-aggregated-CD8lumenal is segregated from both Golgi markers (4). Graph, Pearson’s coefficient on the entire field of view, illustrating the co-localization between the different markers. The data represent the mean of three experiments in which a total of >20 fields were counted.
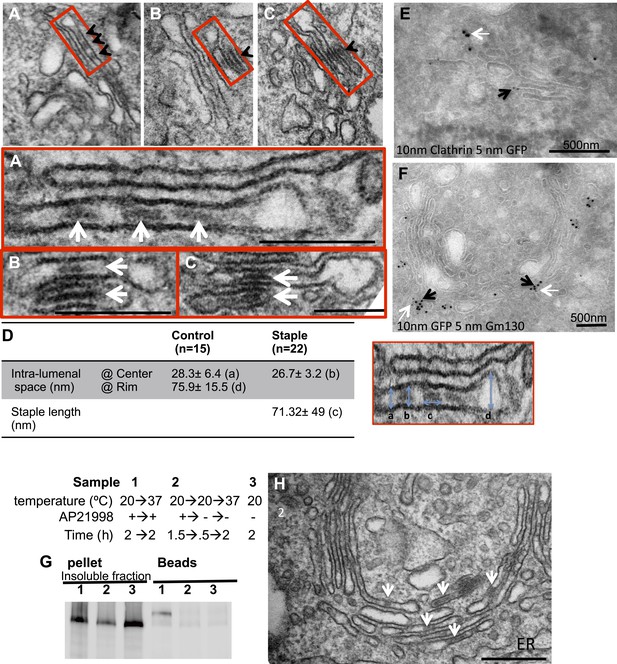
cis-Golgi retention of Golgi staples at the EM level.
HeLa cells expressing CD8lumenal were incubated at 16°C in the presence of the disaggregating drug for 2 hr, and then the drug was removed for 30 min, prior to shifting the temperature to 20°C for two to six additional hours. (A)–(C) Electron-micrograph showing that the staples (white arrows) remain at one face of the Golgi, within the first cistern (A) or in the first two cisternae (B and C) after 2 or 6 hr chase at 20°C, respectively. Red square, higher magnification. Scale bar, 200 nm. (D) Intra-lumenal space of stapled Golgi cisternae. Staples that form flat features are localized at the center of the cisternae. Their topological arrangements prevent them from localizing at the rim of the cisternae. (E) Electron-micrograph showing clathrin (10 nm gold particle), which label the trans-Golgi, and the staples (5 nm gold particles) are at two opposite faces of the Golgi. (F) Gm130 (5 nm gold particles), a cis Golgi marker, is at the same face as the staples (10 nm gold particles). (G and H) HeLa cells expressing CD8lumenal were incubated at 20°C (instead of 16°C) in the presence of the disaggregating drug for 2 hr, and then the drug was removed for 30 min, prior to being incubated at 20°C for two additional hours. (G) Immunoblot, after surface biotinylation, showing that the staples formed in the medial/trans Golgi do not reach the PM. (H) Electron micrograph showing that the staples formed at 20°C are actually retained throughout the Golgi and not only at the TGN face.

Staples within the Golgi.
Similar to Figure 5 Hela cells expressing CD8lumenal were incubated at 16°C in the presence of the disaggregating drug for 2 hr, and then the drug was removed for 30 min, prior to shifting the temperature to 20°C for six additional hours. Electron micrographs show the staple at one face of the Golgi (arrow), which is opposite the swollen face of the Golgi (typical of trans Golgi face at 20°C).
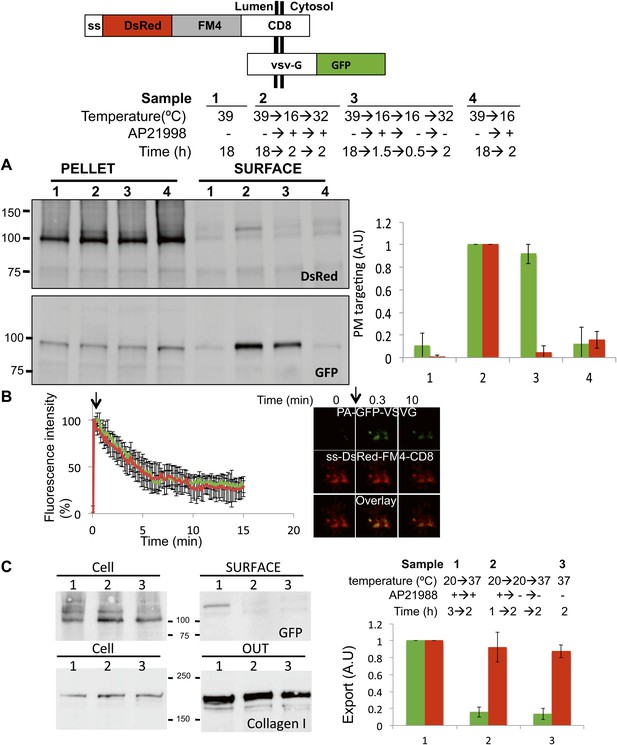
Unaltered anterograde transport within short and long-term stapled-Golgi.
HeLa cells co-expressing DsRed-CD8lumenal with VSVG-GFP were incubated overnight at 39°C in the absence of the disaggregating drug (1) prior to shifting the temperature to 16°C for 2 hr in the presence of the disaggregating drug (4). Then the temperature was shifted to 32°C in the presence (2) or in the absence (3) of the drug. (A) Immunoblot, after surface biotinylation, showing that VSVG-GFP is targeted to the PM regardless of the re-aggregation status of CD8lumenal within the Golgi. Graph, normalized quantification of VSVG-GFP (green bar) and DsRed-CD8lumenal (red bar). Data represent the mean of three independent experiments. (B) HeLa cells co-expressing VSVG-PAGFP and DsRed-CD8lumenal were incubated at 20°C in the presence of the disaggregating drug for 1 hr, prior to removing the drug for 4 hr to form staples throughout the Golgi. VSVG-PAGFP was photo-activated (arrow) within the stapled Golgi and VSVG-PAGFP fluorescent signal was measured over time at 32°C. HeLa cells co-expressing VSVG-PAGFP with ST-RFP were used as a control. Confocal micrographs illustrating the release of VSVG-PAGFP from long-term stapled-Golgi. Graph, normalized fluorescence quantification of VSVG-PAGFP over the time in stapled Golgi (green) and control Golgi (red). Data represent the mean of at least three different experiments. (C) Saos-2 cells expressing GFP-CD8lumenal were incubated at 20°C in the presence (1, 2) or in the absence (3) of the disaggregating drug. When required Golgi staples were formed for 2 hr at 20°C (2), prior to shifting the temperature to 37°C in the presence of ascorbate to allow collagen-I release. Fresh media was added at the beginning of the chase. Immunoblot, after surface biotinylation and TCA precipitation of the media, showing that collagen-I is secreted whether or not staples are pre-positioned in the Golgi (2) or ER (3). Cell fractions that are used as loading control correspond to the Triton X-100 soluble fraction after sonication (10% input lysate). Graph, normalized export of CD8lumenal (green bar) and Collagen-I (red bar). Data represent the mean of two independent experiments.

Golgi staples do not inhibit the rate of secretion of Collagen-I and MMP2.
Saos-2 cells expressing GFP-CD8lumenal were incubated at 20°C for 1 hr in the presence of the disaggregating drug. When required Golgi staples were formed for 2 hr (2), prior to shifting the temperature to 37°C in the presence of ascorbate to allow collagen-I release. The media of each sample (one per time point) was harvested at the indicated time and subjected to TCA precipitation. Cells corresponding to the 2 hr time point were submitted to surface biotinylation. Cell fractions that are used as loading control correspond to the Triton X-100 soluble fraction after sonication (10% lysate). Immunoblots show the rate of transport of MMP2 and collagen-I (measured by densitometry, see graph), and the status (Glycan-induced gel mobility reduction (*) and PM targeting) of GFP-CD8lumenal.
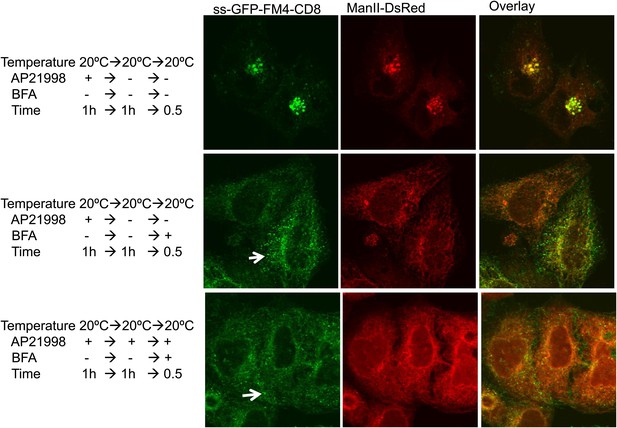
Golgi staples do not prevent redistribution of Golgi membranes within ER mediated by BFA.
HeLa cells co-expressing ManII-DsRed and GFP-CD8lumenal were incubated at 20°C in the presence of the disaggregating drug for 1 hr. Then, when required, the drug was removed to promote staples formation within the Golgi (upper panel). BFA (10 μg/ml) was added for 30 min, then cells were fixed and prepared for confocal microscopy. Confocal micrographs show redistribution of CD8lumenal and ManII-DsRed under BFA treatment regardless of the aggregation status of CD8lumenal. White arrows, residual staples.
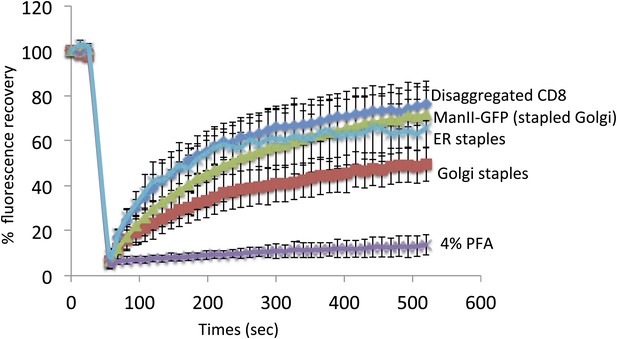
Staples are laterally mobile and do not perturb lateral diffusion of Golgi resident enzymes.
HeLa cells expressing GFP-CD8lumenal were incubated in the absence of the disaggregating to keep the protein aggregated within the ER. FRAP was performed at 20°C (ER staples). As a negative control, cells were fixed 15 min with 4% PFA prior to performing FRAP. For Golgi localization of CD8lumenal, the disaggregating drug was added for 2 hr at 20°C to load the disaggregated cargo within the Golgi. FRAP was performed at 20°C in the presence of the drug (Disaggregated CD8). To monitor the lateral diffusion of the Golgi staples, drug was removed for 30 min prior to monitoring the fluorescence recovery of the Golgi staples at 20°C in the absence of the disaggregating drug (Golgi staples). Finally, ManII-GFP was co-expressed with DsRed-CD8lumenal, staples were positioned within the Golgi as explained above, and FRAP of ManII-GFP was performed at 20°C in the absence of the disaggregating drug (ManII-GFP [Stapled Golgi]). ROI used for FRAP analysis corresponded approximately to 1/3 of the Golgi. Graph shows the fluorescence intensity signal overtime. The data for each condition represents the mean of two or three independent experiments.
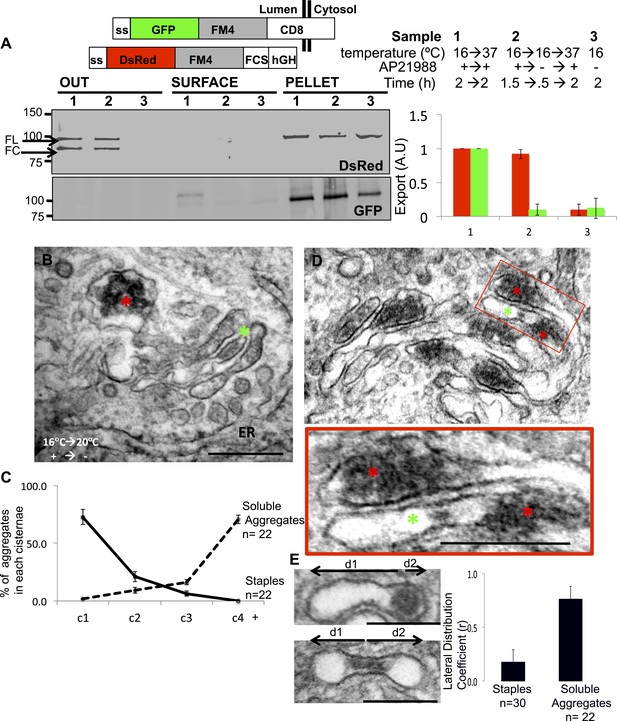
Unaltered transport of soluble aggregates within stapled Golgi.
HeLa cells co-expressing GFP-CD8lumenal and DsRed-hGH, both harboring the FM-aggregation domains, were incubated at 16°C for 2 hr in the presence of the disaggregating drug (3, negative control) prior to shifting the temperature to 37°C in the presence (1) or in the absence of the drug (2). (A) Immunoblot, after surface biotinylation and TCA precipitation of the media, showing that DsRed-hGH is secreted regardless of its own re-aggregation status, or the re-aggregation status of CD8lumenal. Prior to being secreted disaggregated and re-aggregated DsRed-hGH are cleaved by furin. FL, full-length isoform of DsRed-hGH. FC, furin-cleaved isoform of DsRed-hGH. Graph, normalized quantification of PM targeted CD8lumenal (green bar) and normalized secreted DsRed-hGH (red bar). Data represent the mean of three independent experiments. (B) Cells were incubated at 16°C for 2 hr in the presence of the disaggregating drug, then the temperature was shifted to 20°C for two additional hours in the absence of the drug. Electron micrograph showing Golgi staples (green star) that remains at the cis-Golgi, which is adjacent to ER membranes, whereas the soluble aggregates (red star) is localized to the opposite face of the Golgi. (C) Graph, distribution of staples and soluble aggregates within the Golgi stack: cells expressing CD8lumenal or hGH were subjected to the re-aggregation procedure at 16°C, prior to being incubated at 20°C for 2 hr and prepared for classical EM. The distribution of the aggregates (soluble and staples) within the Golgi stack is shown as a percentage of the total in Golgi areas. (D) Electron micrograph showing segregation between Golgi lumenal staple (green star) and soluble aggregates (red star). Golgi staples form one flat feature in the center of one cisterna whereas the soluble aggregates form spherical aggregates that are localized at the rims of the cisternae throughout the Golgi. (E) HeLa cells expressing hGH or CD8lumenal were subjected to the re-aggregation procedure at 16°C for 2 hr prior to being processed for conventional EM. The upper panel shows the distribution of the soluble aggregates at the rim of the cistern, the lower panel illustrates the central positioning of the staple. Graph, lateral distribution coefficient (r) of staples and soluble aggregates within Golgi cisternae. The distances that separate the middle of the aggregates from each end of the cisterna (d1 and d2) were measured, and (r) was calculated as follow r = (d1 − d2)/(d1 + d2) with d1 ≥ d2. As a result, (r) → 0 for object at the center, whereas (r) → 1 for object at the rim.

Co-immunoprecipitation of ER-staples and ER-soluble aggregates.
HeLa cells co-expressing GFP-CD8lumenal or GFP-hGH, with DsRed-hGH, both harboring the FM-aggregation domains, were incubated overnight in the presence of the disaggregating drug (1) or in the absence of the disaggregating drug to allow aggregation within the ER (2). Cells were incubated for 2 hr with the disaggregating drug at 20°C (3) or 16°C (4) prior to removing the drug to trigger re-aggregation over 1 hr. Cell extracts were incubated with an anti-GFP antibody and Protein-A agarose beads. After elution, samples were subjected to SDS-PAGE. Immunoblots showing that hetero-interactions are only detected within the ER after longtime incubation, whereas homo-interactions are detected even within the Golgi at 16°C or 20°C.

Segregation of staples from soluble aggregates within the Golgi at the light level.
HeLa cells co-expressing GFP-CD8lumenal and DsRed-hGH, both harboring the FM-aggregation domains, were incubated at 20°C for 2 hr in the presence of the disaggregating drug prior to shifting the temperature to 37°C in the absence of the drug. (A) Confocal micrograph showing the localization of both aggregated proteins within the Golgi. Graph, intensity profile showing the lack of co-localization.






