Structure of cellular ESCRT-III spirals and their relationship to HIV budding
Figures
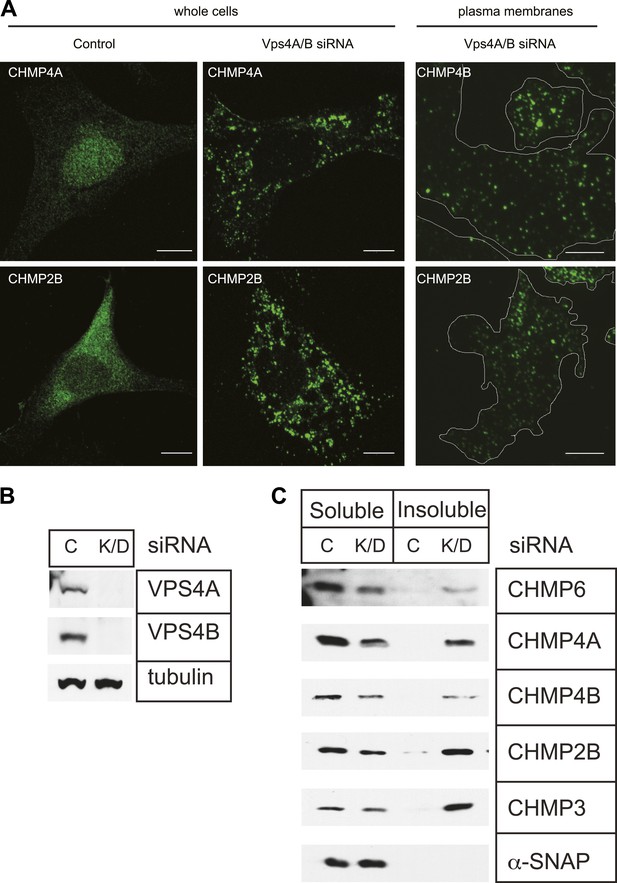
Effects of depleting Vps4 on ESCRT-III.
(A) Localization of indicated ESCRT-III protein in HeLa cells untreated or treated with Vps4A & Vps4B specific siRNA for ∼60 hr. Left and middle panels show maximum intensity projections from confocal z-series through the cells. Right panel shows immunostaining of unroofed plasma membranes from HEK293T cells treated with Vps4A & Vps4B siRNA. Scale bars represent 10 μm. (B) Representative immunoblots comparing lysates from cells untreated or treated with siRNA targeting Vps4A & Vps4B. (C) Immunoblots of detergent soluble (left) and insoluble (right) material from control or Vps4A & Vps4B siRNA-treated HeLa cells.
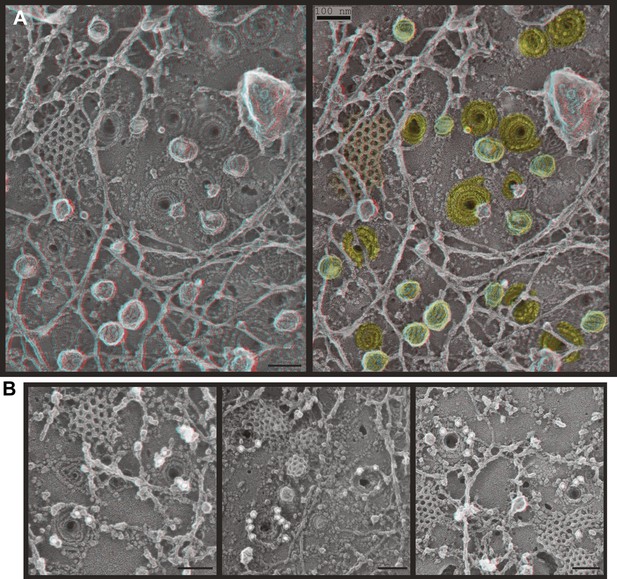
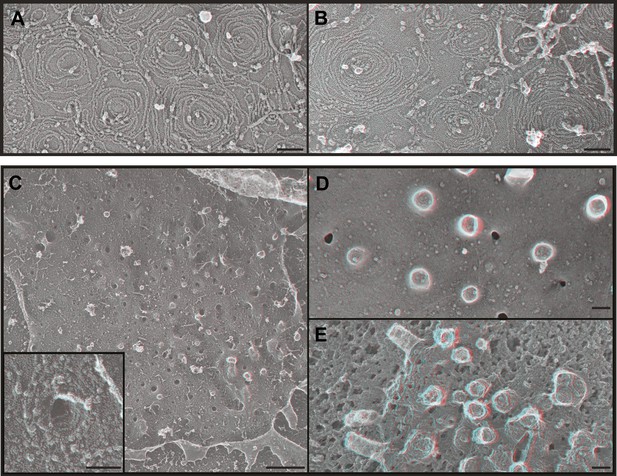
ESCRT-III filaments form conical spirals on the plasma membrane of cells depleted of Vps4A & Vps4B.
(A) Survey view of the cytoplasmic surface of the plasma membrane from a HeLa cell unroofed ∼60 hr after transfection with siRNA targeting Vps4A & Vps4B. Pseudocoloring shows clathrin (orange), caveolae (green), and ESCRT-III spirals (yellow). Similar filament spirals were seen in siRNA treated HEK293T, U2OS, and MCF-7 cells (not shown). (B) Immunodecoration of ESCRT-III proteins in spirals on Vps4-depleted HeLa cell plasma membranes. Antibodies recognizing CHMP6 (left), CHMP2B (middle), and CHMP1B (right) detected with 18 nm gold that appears white in these contrast reversed EM images. Use view glasses for 3D structure in both panels (left eye = red). Scale bars represent 100 nm.
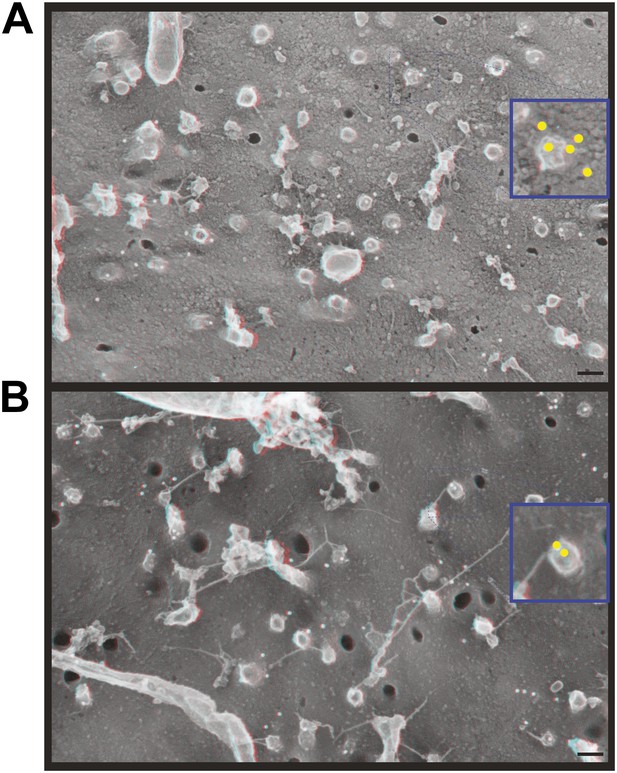
Immunodecoration of ESCRT-III proteins beneath protrusions from the cell surface.
Fortuitous breaks in HeLa cells depleted of Vps4A & B subjected to immunogold labeling allowed antibody staining inside broken cells. (A) Immunodecoration of CHMP1B, (B) immunodecoration of CHMP2B. 18 nm gold particles appear white in these contrast reversed deep-etch EM images. Insets show enlarged images (blue edges) with gold particles marked yellow. Use view glasses for 3D structure. Scale bars represent 100 nm for main panels and 50 nm for insets.
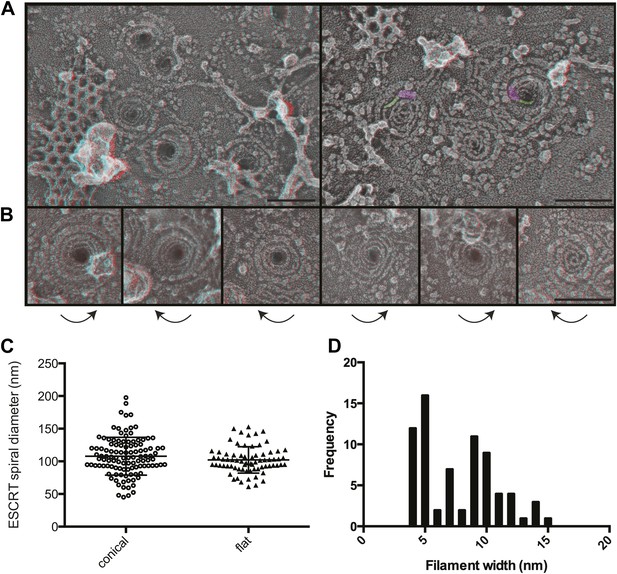
Structural characteristics of endogenous ESCRT-III spirals.
(A) Survey views of filaments and spirals on plasma membranes of HeLa cells depleted of Vps4A & Vps4B show range of shapes, filament diameter, and direction of spiraling. Examples of abrupt changes in filament diameter are highlighted in color (thicker filaments in magenta and thinner filaments in green) in right panel. Use view glasses for 3D structure (left eye = red). Scale bar represents 100 nm. (B) Views of individual spirals with direction of spiral from perimeter towards center as shown. Each box corresponds to 185 nm. (C) Outer diameter of spirals defined as conical or flat based on appearance in 3D (n = 184, 61% conical 108 ± 29 nm; 39% flat 102 ± 20 nm). (D) Distribution of filament widths measured at three points per spiral.
ESCRT-III polymer structure in transfected cells.
Anaglyphs of plasma membranes from COS-7 cells expressing (A) untagged CHMP4A, (B) CHMP4A(α1–α5), and (C–E) CHMP4A(α1–α5) and full-length CHMP2A together. (C) Cytoplasmic surface of the plasma membrane from an unroofed cell with high magnification shown in the inset, (D) corresponding top of a whole cell, and (E) top of a whole cell extracted with Triton X-100 and saponin after fixation to expose the underlying protein scaffold. Use view glasses for 3D structure (left eye = red). Scale bars represent 100 nm except in C where the scale bar on the survey view corresponds to 500 nm.
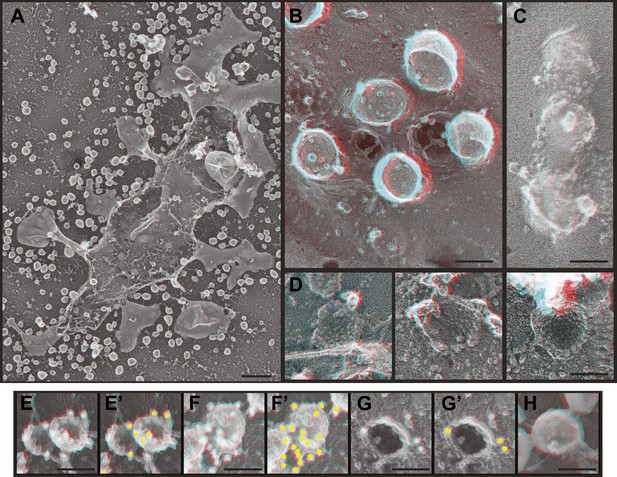
Deep-etch EM of HIV-1 VLP budding.
(A) Low magnification view of an unroofed HIV-1 Gag-transfected HEK293T cell surrounded by VLPs. (B) Top view of whole cell budding VLPs. (C) View of unroofed plasma membrane showing bumps corresponding to VLPs trapped underneath the membrane. (D) Views of unroofed plasma membrane showing Gag assemblies exposed on the cytoplasmic surface of the plasma membrane. (E–H) Immunodecoration of Gag on detergent extracted plasma membranes (E and E′), detergent extracted VLPs (F and F′), intact unroofed plasma membranes (G and G′) and intact VLPs (H). (E′, F′ and G′ are same as E, F and G but show gold in yellow). Use view glasses for 3D structure (left eye = red). Scale bars represent (A) 500 nm, (B–H) 100 nm.
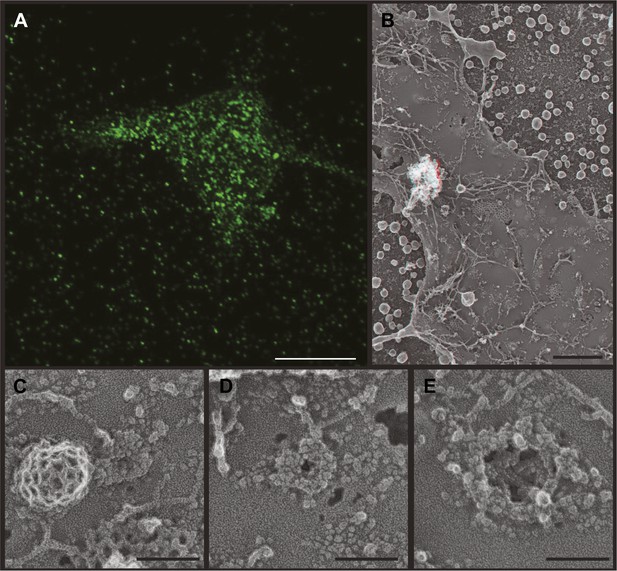
VLP formation by HIV-1 Gag-GFP.
(A) Fluorescence microscopy of a live Gag-GFP expressing HEK293T cell showing released VLPs captured around the single cell. Scale bar represents 10 μm. (B) Low magnification view of an unroofed Gag-GFP transfected HEK293T cell surrounded by VLPs. Use view glasses for 3D structure (left eye = red). Scale bar represents 500 nm. (C–E) High magnification views of developing Gag assemblies on the plasma membrane of unroofed cells. Small Gag-GFP assemblies (C) resemble those formed by untagged Gag, while larger assemblies (E) are discontinuous and do not form solid spherical assemblies. Scale bars in C–E represent 100 nm.
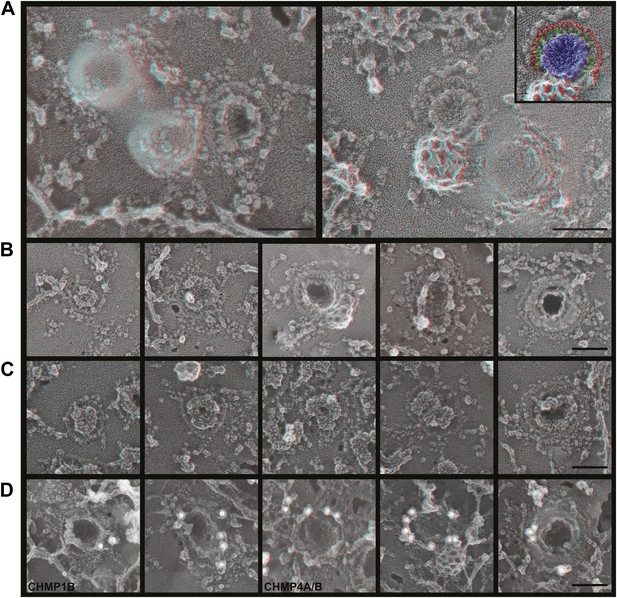
ESCRT-III filaments surround Gag assemblies in cells depleted of Vps4.
(A) HEK293T cells treated with Vps4A & Vps4B siRNA accumulate unique filament-encircled Gag assemblies. Inset is pseudo-colored to show the central Gag assembly (blue), surrounding filament (red), and perpendicular ‘struts’ between them (green). Each field also shows the occasionally seen subplasmalemmal VLP bump to provide a sense of scale. (B) Individual views of Gag assemblies, (C) Gag-GFP assemblies, and (D) Gag assemblies immunodecorated with indicated gold conjugated antibodies. Scale bars represent 100 nm. Each box in B–D is a 320 nm square. Use view glasses for 3D structure (left eye = red).
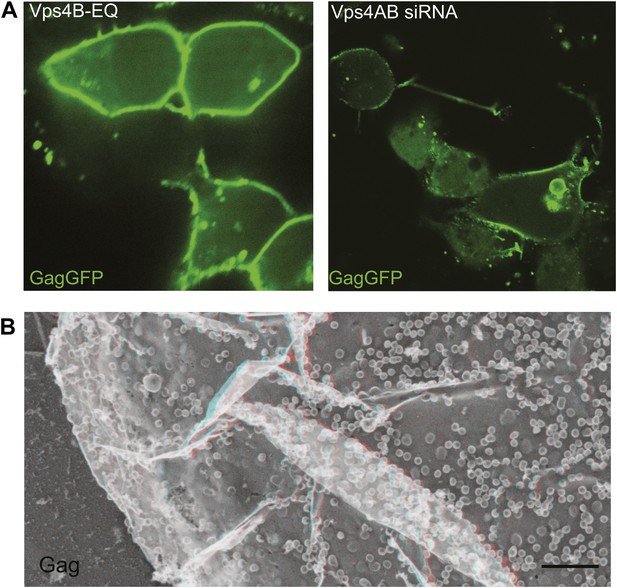
VLP release is impaired by inactivating or depleting Vps4.
(A) Confocal image of HEK293T cells expressing Vps4B-E228Q or Vps4A & Vps4B siRNA and transfected with Gag-GFP. Note that Gag accumulates on the plasma membrane instead of in released VLPs (compare with Figure 5—figure supplement 1A). (B) Deep-etch EM of an unbroken Vps4-depleted cell expressing Gag showing unbudded VLPs accumulating on the cell surface.
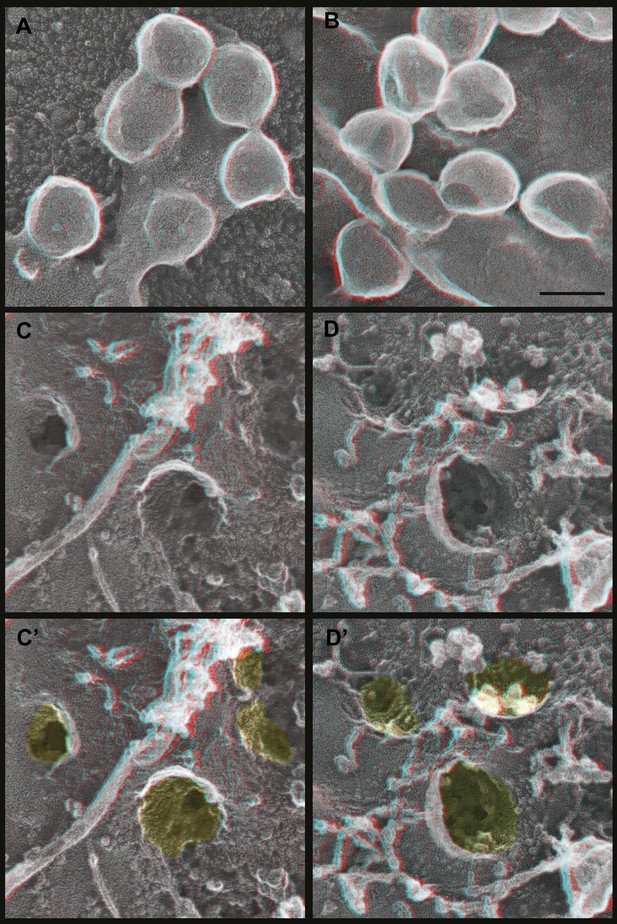
Deep-etch EM of Gag budding without its ESCRT-recruiting p6 domain.
(A) Top surface of HEK293T cell expressing GagΔp6 showing blocked VLP budding. (B) Top surface of Vps4-depleted HEK293T cell expressing GagΔp6 showing similar blocked VLP budding. (C) Unroofed plasma membrane corresponding to A. (D) Unroofed plasma membrane corresponding to B. Note the invaginated Gag assemblies with no surrounding ESCRT-III ring (C and D). C′ and D′ are identical to C and D but with Gag assemblies colored yellow for clarity. Scale bar represents 100 nm. Use view glasses for 3D structure (left eye = red).
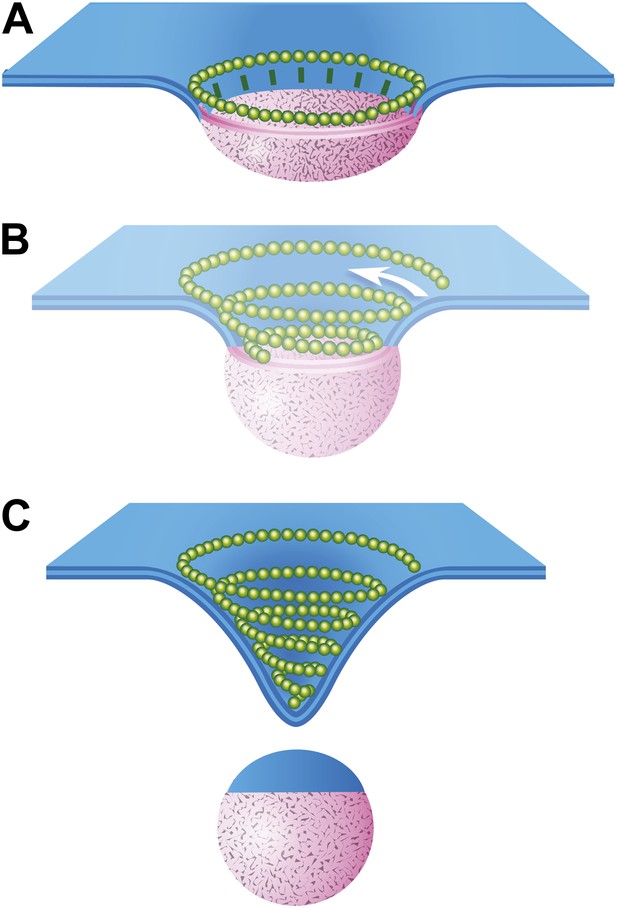
Speculative model describing ESCRT-III and Vps4 function in vesicle biogenesis and release.
(A) Cargo - loosely defined to include either HIV-1 Gag or material destined for incorporation into ILVs–is concentrated in the circular domain shown in pink. After reaching some threshold, cargo recruits and/or activates factors to initiate ESCRT-III assembly. These are represented here by green ‘struts’ perpendicular to the cargo perimeter. Once nucleated, the ESCRT-III filament extends to surround and confine cargo. In the absence of Vps4, this intermediate accumulates. (B) When present, we propose a new role for Vps4 in which it is engaged to break connection(s) between ESCRT-III and cargo, thereby allowing the ESCRT-III spiral to grow into its preferred spiral shape. ESCRT-III recruits new membrane into the neck as it grows, shown by the addition of blue membrane to the budding vesicle. (C) A fully assembled ESCRT-III spiral narrows the membrane neck, ultimately driving vesicle release.





