A long non-coding RNA is required for targeting centromeric protein A to the human centromere
Figures
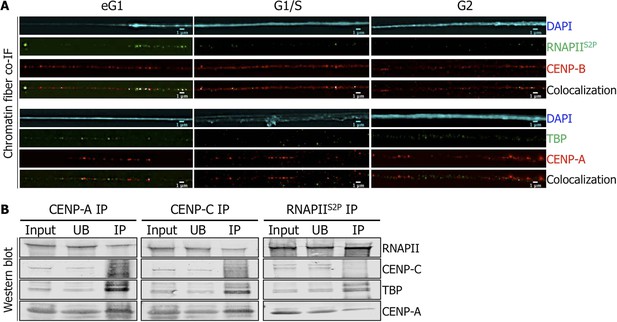
Active RNA Polymerase II (RNAPIIS2P) and TATA-box binding protein (TBP) are associated with centromeric chromatin at early G1 (eG1).
(A) Chromatin fibers prepared from synchronized HeLa cells at eG1, G1/S, and G2 phases were co-stained for RNAPIIS2P and TBP (green) with centromeric proteins CENP-B and CENP-A (red), respectively. The DAPI raw image is shown for a representative chromatin fiber (cyan). Three independent experiments were performed and in each, a minimum of five fibers were analyzed per slide (co-localization on the same chromatin fiber at eG1: between CENP-B and RNAPIIS2P = 10/15; and between CENP-A and TBP = 9/16). Scale bar: 1 μm. (B) CENP-A, CENP-C or RNAPIIS2P were immunoprecipitated, and co-purifying partners were detected on Western blots (1.5% of input and UB, 75% of IP). Co-IF, co-immunofluorescence; IP, immunoprecipitate; UB, unbound.
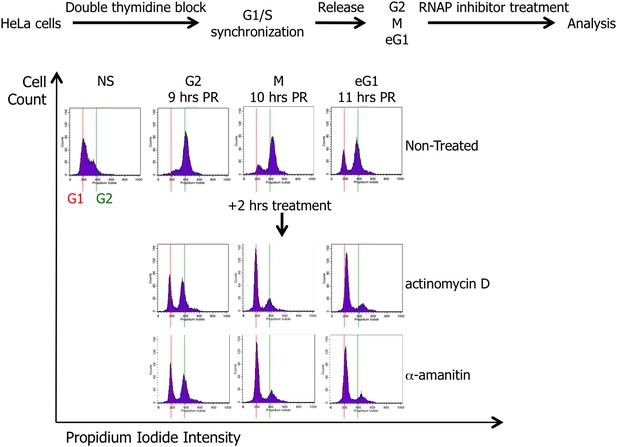
Scheme presenting the strategy for HeLa cell synchronization and treatment with drugs (actinomycin D or α-amanitin) inhibiting either RNA Polymerase I or RNA Polymerase II activity.
HeLa cells were synchronized in G1/S phase by two consecutives treatments of thymidine, and then released until G2 (9 hr), mitosis (M; 10 hr), and early G1 (eG1; 11 hr) cell cycle phases. The synchronization and the potential effect of actinomycin D or α-amanitin treatment (2 hr) were assessed by cell cycle analysis. NS, non-synchronized cells; PR, post-release.
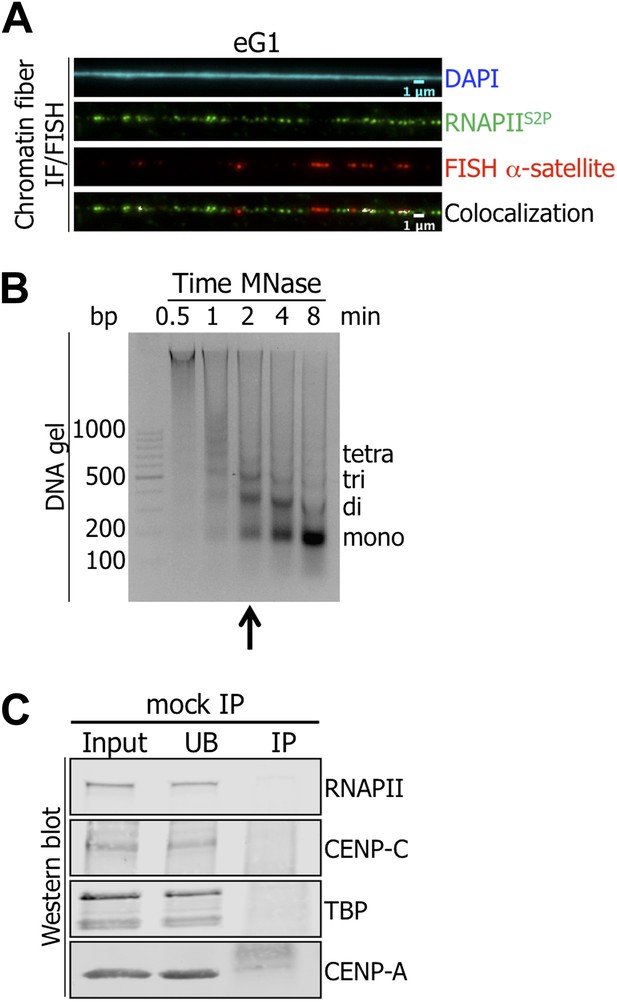
Active RNA Polymerase II (RNAPIIS2P) is associated with centromeric α-satellite sequences on chromatin fibers at early G1 (eG1).
(A) RNAPIIS2P (green) was co-stained with centromeric α-satellite DNA FISH probe (red) on chromatin fibers from eG1-synchronized cells. (cyan, DAPI). Three independent experiments were performed, and a minimum of five fibers were counted per slide (co-localization on the same chromatin fiber: between CENP-B and centromeric α-satellite DNA = 11/15). (B) The progressive accumulation of mono-nucleosomes after chromatin digestion (0.5–8 min) with MNase was analyzed on agarose gel. The arrow indicates the chosen MNase time used for experiments in the manuscript. (C) Chromatin extract was incubated in presence of agarose beads and co-precipitated proteins were analyzed on Western blot (1.5% of input and UB, 75% of IP). FISH, fluorescence in situ hybridization; co-IF, co-immunofluorescence; IP, immunoprecipitate; UB, unbound.
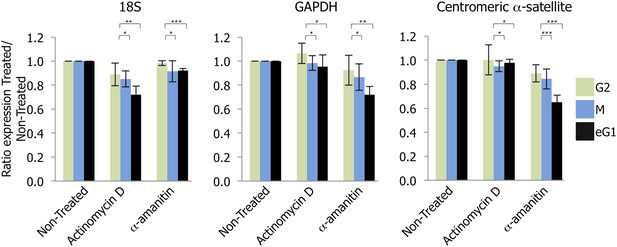
Transcription of centromeres is dependent on RNA Polymerase II (RNAPII) and occurs at early G1 (eG1).
G2 (green), mitotic (M; blue), and eG1 (black) synchronized cells were treated 2 hr with either actinomycin D or α-amanitin, to block RNA Polymerase I or RNAPII, respectively. After RNA purification and retro-transcription, expression levels of control target genes (18S rRNA and GAPDH) and centromeric α-satellite transcripts were assessed by semi-quantitative PCR. The graph represents the average of three biological replicates, the y-axis plots the ratio (±SD) of gene expression after treatment (actinomycin D or α-amanitin) compared to the non-treated condition. p-values indicating statistical significance are presented where appropriate above the histograms. *p > 0.1, **0.001 < p < 0.05, and ***p < 0.001.
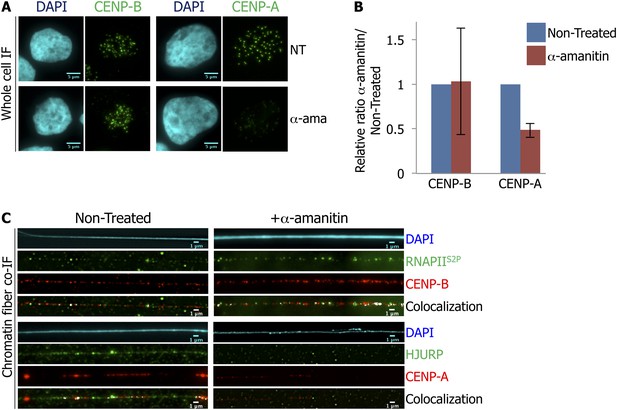
RNA Polymerase II (RNAPII)-dependent transcription is required for the recruitment of CENP-A and its chaperone HJURP onto the centromere at early G1 (eG1).
(A) eG1 synchronized HeLa cells were treated or not (NT) with α-amanitin (α-ama) for 2 hr before staining for centromeric proteins CENP-A or CENP-B (green). The DAPI raw image is shown for a representative cell (cyan). Three independent experiments were performed and in each, a minimum of 30 cells were analyzed per slide. Scale bar: 5 μm. (B) Signal intensity of CENP-A and CENP-B spots from (A) was quantified using ImageJ, and relative ratios of α-amanitin vs non-treated conditions were determined. Means ± SD from three independent experiments is represented on the graph. Quantification values are tabulated in Supplementary file 1. (C) Centromeric proteins CENP-A and CENP-B (red) were co-stained with HJURP (green) and RNAPII phosphorylated on serine 2 (RNAPIIS2P, green), respectively, on centromeric chromatin fibers prepared from eG1 synchronized cells treated or not with α-amanitin for 2 hr (cyan, DAPI). Three independent experiments were performed and in each, a minimum of five chromatin fibers were analyzed per slide (co-localization on the same chromatin fiber after α-amanitin compared to non-treated: between CENP-B and RNAPIIS2P = 9/15 vs 10/15; and between CENP-A and HJURP = 2/15 vs 9/15). Scale bar: 1 μm. Co-IF, co-immunofluorescence.

Inhibition of RNA Polymerase II-dependent transcription at early G1 (eG1) does not affect CENP-A and HJURP protein levels.
(A) eG1-synchronized HeLa cells were treated with α-amanitin for 2 hr before staining for centromeric protein CENP-B (green) and DAPI (cyan). The heterogeneity of the CENP-B signal on a picture is shown with magnification of one cell with high signal (1 and 2) and low signal (3 and 4). Scale bar: 5 μm. (B) eG1-synchronized cells were treated, or not, with α-amanitin (2 hr), before total protein extraction, and the expression levels of CENP-A and HJURP were quantified by Western blot. The graph represents the average of three biological replicates, the y-axis plots the ratio (±SD) of protein level after α-amanitin compared to the non-treated condition for CENP-A and HJURP Western blot, respectively. IF, immunofluorescence.
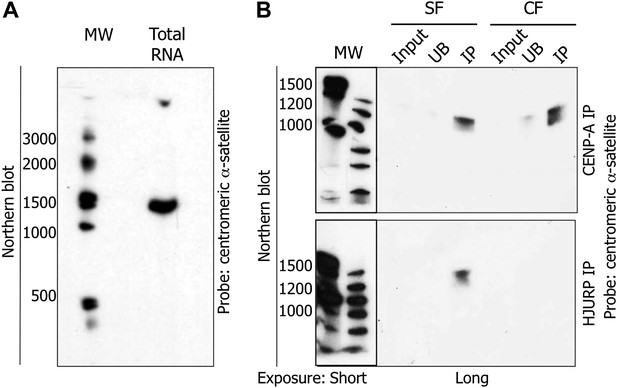
The pre-assembly soluble HJURP/CENP-A complex binds a 1.3 kb centromeric transcript at early G1 (eG1).
(A) Total RNAs from HeLa cells were separated on denaturing gel and visualized by Northern blot with radiolabeled centromeric α-satellite probes. (B) Co-immunoprecipitated RNAs by CENP-A or HJURP were analyzed by Northern blot as in (A). CF, chromatin fraction; IP, immunoprecipitate; MW, molecular weight; SF, soluble fraction; UB, unbound.
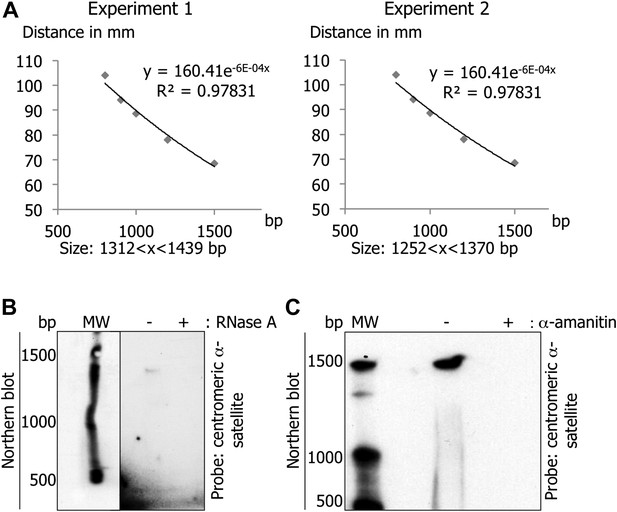
Centromeric transcripts are 1.3 kb in length.
(A) To determine the size of the centromeric α-satellite transcripts, the graph of the distance (in y) between the border of the Northern blot and each band of the molecular weight as a function of the number of bases was made. The distance of the centromeric α-satellite transcript band was analyzed using the standard curve from this graph to deduce its size. (B) Total RNAs treated with RNase A were separated on a denaturing gel, and revealed by Northern blot with radiolabeled centromeric α-satellite probes. (C) eG1-synchronized cells were treated, or not, with α-amanitin (2 hr). RNAs were processed and analyzed on Northern blot as in (B) to examine whether trace DNA contamination could yield the same band as in (A).
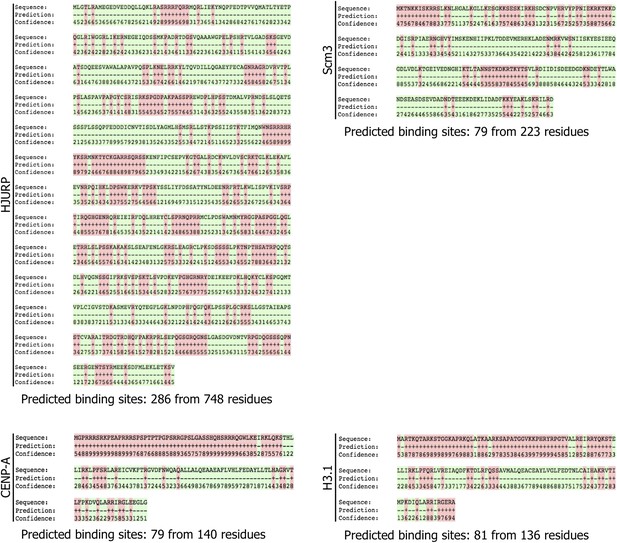
HJURP and CENP-A display potential RNA binding residues.
RNA binding residues in CENP-A, H3, Scm3, and HJURP were predicted by computational analysis using the BindN+ program. Binding residues are labeled with ‘+’ and in red, whereas non-binding residues are labeled with ‘−’ and in green. The confidence of binding potential ranges from low (0) to high (9).
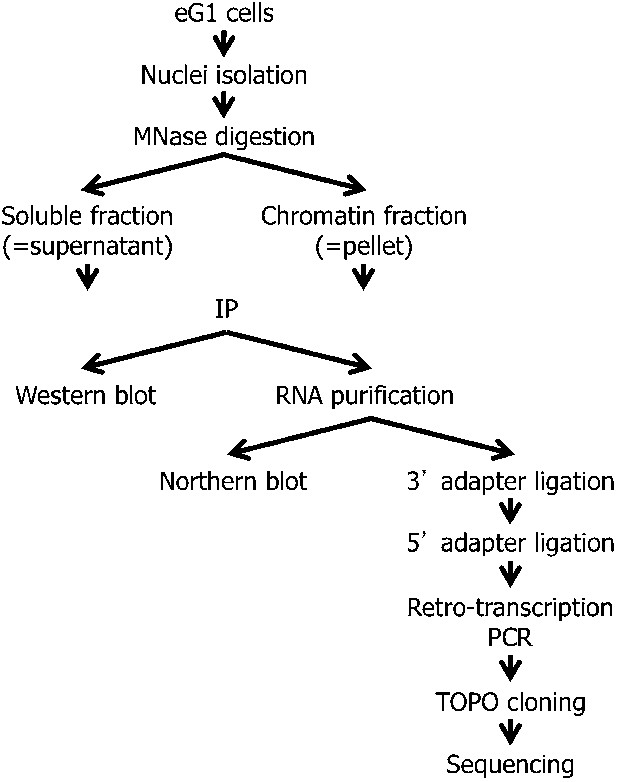
Scheme presenting the strategy for RNA-chromatin immunoprecipitation, RNA purification, and sub-cloning for sequencing.
https://doi.org/10.7554/eLife.03254.012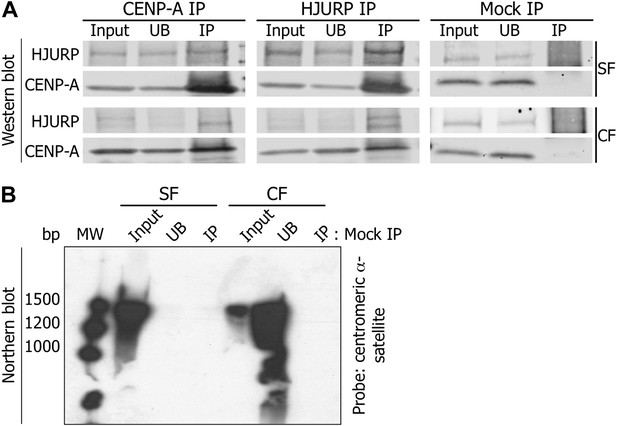
Mock IPs demonstrate specificity of the 1.3 kb RNA binding to CENP-A and HJURP.
(A) CENP-A and HJURP were immunoprecipitated from eG1 cells, separated on SDS-PAGE and revealed on Western blot (1.5% of input and UB, 100% of IP). To exclude non-specific binding of CENP-A or HJURP to the secondary beads, immunoprecipitation was performed with beads in the absence of antibody (mock IP). (B) RNAs immunoprecipitated from the mock IP in (A) were analyzed on Northern blot using radiolabeled centromeric α-satellite probes. CF, chromatin fraction; IP, immunoprecipitate; MW, molecular weight; SF, soluble fraction; UB, unbound.
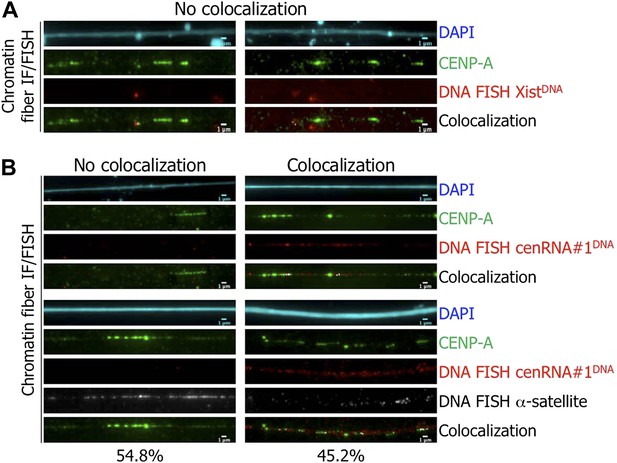
The identified cenRNA#1 transcript associated with CENP-A has a centromeric origin.
(A) CENP-A (green) was co-stained with Xist DNA FISH probe (FISH XistDNA, red) on chromatin fibers (cyan, DAPI). Two independent experiments were performed and in each, a minimum of eight chromatin fibers were analyzed per slide (co-localization on the same chromatin fiber between CENP-A and FISH XistDNA = 0/18). Scale bar: 1 μm. (B) Chromatin fibers were either co-stained by IF/FISH with CENP-A (green) and cenRNA#1DNA (red), or IF/double-FISH with CENP-A (green), cenRNA#1DNA (red) and centromeric α-satellite probes (gray). The DAPI raw image is shown for a representative chromatin fiber (cyan). Two independent experiments were performed and in each, a minimum of eight chromatin fibers were analyzed per slide (co-localization on the same chromatin fiber between CENP-A and cenRNA#1DNA and FISH α-satellite compared to no co-localization = 9/20 vs 11/20). Scale bar: 1 μm. FISH, fluorescence in situ hybridization; IF, immunofluorescence.

Sequence of cenRNA#1.
RNAs immunoprecipitated with CENP-A were retro-transcribed and subcloned. One 675 nucleotide long sequence called cenRNA#1 was obtained by sequencing, and is reported here. The sequence of shRNAcenRNA#1 is underlined.

shRNAcenRNA#1-transfected cells have a cell survival defect.
(A) 6 days post-transfection of shRNAscram or shRNAcenRNA#1, cell morphology was observed by phase contrast microscopy. Scale bar: 100 pixels. (B) Cells were treated as in (A) and stained for β-actin (green) and DAPI (cyan) to reveal alterations in cell morphology. Scale bar: 5 μm. cDNA, complementary DNA; IF, immunofluorescence ; NT, non-treated.
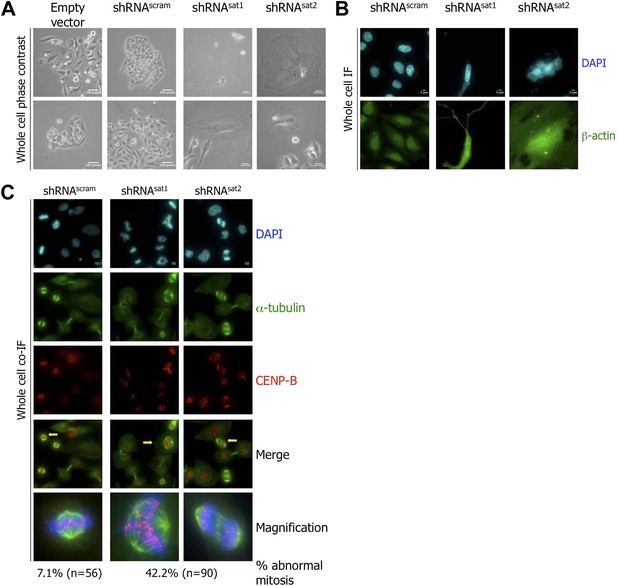
Targeted down-regulation of centromeric α-satellite transcript results in mitotic defect.
(A) Six days post-transfection of empty vector, control scrambled (shRNAscram), or α-satellite shRNA (shRNAsat1, shRNAsat2), cell morphology was observed by phase contrast microscopy. Scale bar: 100 pixels. (B) Cells were treated as in (A) and stained for β-actin (green) and DAPI (cyan) to reveal alterations in cell morphology. Scale bar: 5 μm. (C) Cells were treated as in (A), synchronized in mitosis and stained for α-tubulin (green), CENP-B (red) and DAPI (cyan/blue). Three independent experiments were performed and in each, a minimum of 30 cells were analyzed per slide. Scale bar: 5 μm. IF: immunofluorescence.
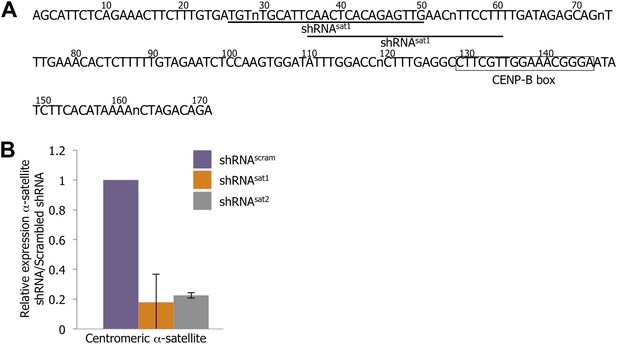
Down-regulation of centromeric RNAs by a targeted shRNA approach.
(A) The centromeric α-satellite consensus sequence was used to design α-satellite shRNA (shRNAsat1 and shRNAsat2) compared to control shRNA containing a scrambled sequence (shRNAscram). Residues of the CENP-B box are marked by a black box, and the sequences of shRNAsat1/2 are underlined. (B) Centromeric α-satellite transcript level after transfection of shRNAscram, shRNAsat1, or shRNAsat2 was measured by qtPCR. The graph represents the average of three biological replicates, with relative expression (±SD) of α-satellite transcript in shRNAsat-transfected cells compared to shRNAscram-transfected cells.
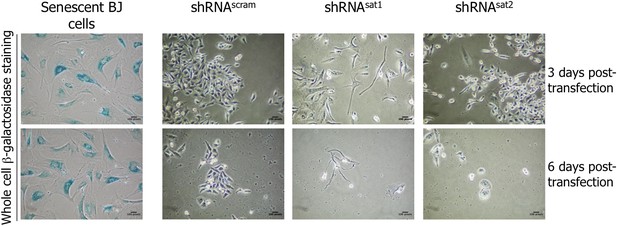
Down-regulation of centromeric transcripts does not induce senescence.
Six days post-transfection of empty vector, control scrambled (shRNAscram) or α-satellite shRNA (shRNAsat1 or shRNAsat2), the potential senescent status of transfected cells was tested by a β-galactosidase assay. Senescent BJ cells were used as positive control (blue).
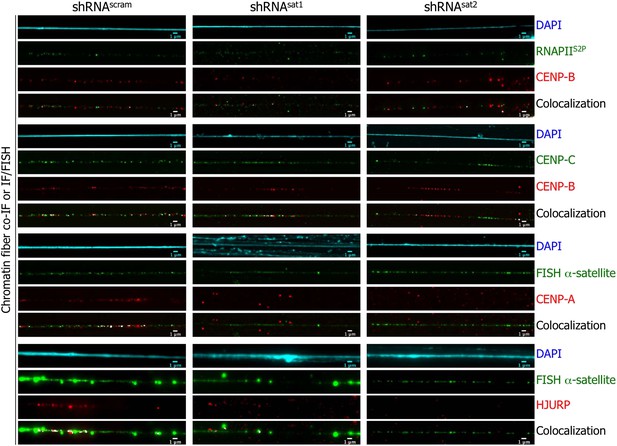
Targeted down-regulation of centromeric α-satellite abrogates HJURP/CENP-A targeting to centromeric chromatin at early G1 (eG1).
Chromatin fibers were prepared from shRNAscram, shRNAsat1, or shRNAsat2-transfected cells synchronized at eG1. To visualize centromeric domains, co-IF was performed for CENP-B (red), CENP-C (green) and RNA Polymerase II (phosphorylated on serine 2, RNAPIIS2P, green), whereas CENP-A (red) and HJURP (red) antibodies were co-stained with a DNA FISH probe against centromeric α-satellite DNA repeats (green). The DAPI raw image is shown for a representative chromatin fiber (cyan). Three independent experiments were performed and in each, five chromatin fibers were analyzed per slide (co-localization on the same chromatin fiber in shRNAsat1 or shRNAsat2-transfected cells: between CENP-B and RNAPIIS2P = 9/15 and 8/15; CENP-B and CENP-C = 14/15 and 13/15; between CENP-A and centromeric α-satellite DNA = 3/15 and 1/15; between HJURP and centromeric α-satellite DNA = 1/15 and 0/15, respectively). Scale bar: 1 μm. FISH, fluorescence in situ hybridization; IF, immunofluorescence.
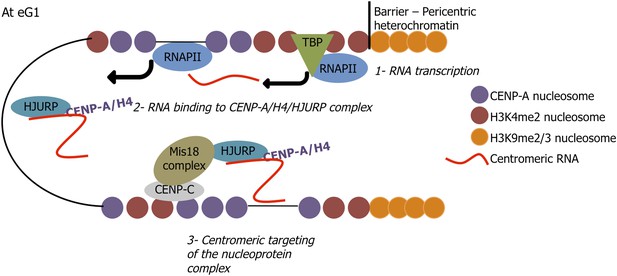
A speculative model proposing a mechanism by which centromeric long non-coding RNAs target soluble HJURP/CENP-A complexes to centromeres at early G1 (eG1).
At mitosis/eG1, Mis18 primes centromeres for active transcription by RNA Polymerase II (RNAPII) (step 1), leading to the synthesis of long non-coding RNAs, which are released into the nucleoplasm at eG1. These centromeric transcripts bind pre-assembly HJURP/CENP-A/H4 in the nucleoplasm (step 2), and specifically target the resulting nucleoprotein complex back to active native centromeres. Thus, a ‘dual lock’ system for active centromeric loading is provided by the Mis 18 complex facilitating eG1 centromeric transcription, and the lncRNA/HJURP/CENP-A complex binding centromeres (step 3), potentially via the formation of RNA–DNA hybrids with centromeric DNA.
Additional files
-
Supplementary file 1
RNA Polymerase II inhibition results in CENP-A loss at centromere at early G1. eG1 synchronized cells were treated 2 hr with or without α-amanitin and stained for centromeric protein CENP-A or CENP-B. After image acquisition, immunofluorescent signals were quantified using ImageJ.
- https://doi.org/10.7554/eLife.03254.022
-
Supplementary file 2
List of antibodies used in this study.
- https://doi.org/10.7554/eLife.03254.023
-
Supplementary file 3
List of primer sequences used in this study.
- https://doi.org/10.7554/eLife.03254.024





