Lymph node stromal cells constrain immunity via MHC class II self-antigen presentation
Figures
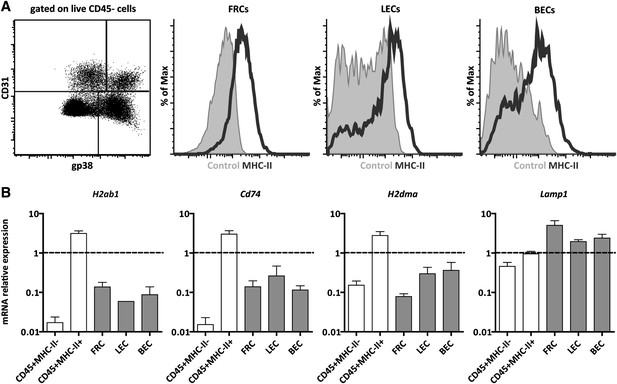
Lymph node stromal cells express MHC-II in the steady-state.
(A) MHC-II expression on lymph node stromal cells was assessed by flow cytometry. Fibroblastic reticular cells (FRCs) were identified as CD45−gp38+CD31− cells; lymphatic endothelial cells (LECs) as CD45−gp38+CD31+ cells; and blood endothelial cells (BECs) as CD45−gp38−CD31+ cells. Filled histograms represent control staining, whereas open histograms represent MHC-II expression. Representative example of five independent experiments performed. (B) mRNA expression of MHC-II (H2-Ab1) and MHC-II-related genes, CD74, H2-M, and LAMP-1, was determined on wild-type FACS-sorted stromal cells by real-time PCR. Total pLN cells (arbitrarily set at one) and FACS-sorted CD45+MHC-II- and CD45+MHC-II+ cells were used as controls. The data represent mean ± SEM; n = 4.
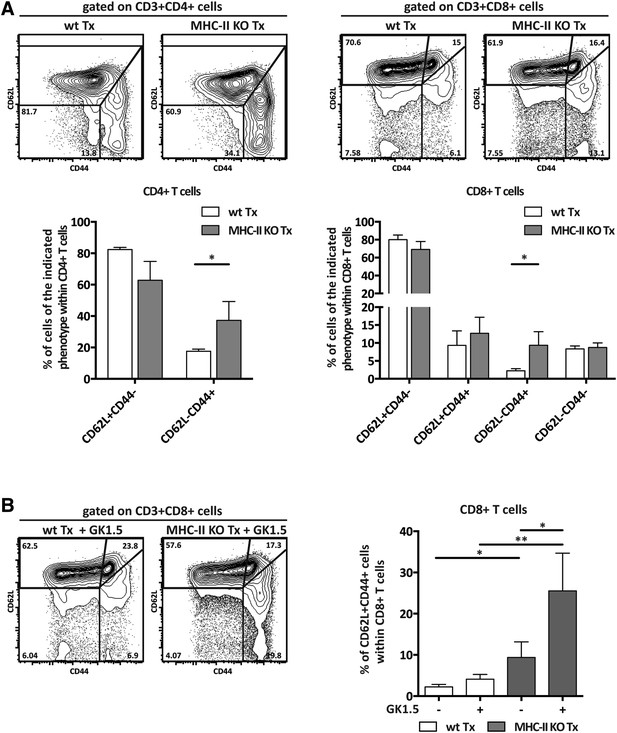
MHC-II+ lymph node stromal cells regulate T cell activation.
Wild-type mice were transplanted with either wild-type (wt Tx) or MHC-II KO (MHC-II KO Tx) lymph nodes. After 4 weeks, host-derived CD4+ and CD8+ T cells present within the transplants were characterized by flow cytometry (A). In (B), transplanted animals were depleted of CD4+ T cells by administration of the antibody GK1.5. For easier comparison, the data of Figure 2A regarding CD62L−CD44+CD8+ T cells are duplicated here. Representative contour plots are shown; the numbers in the plots indicate the frequency of cells within the drawn gates. The data represent mean ± SEM; n = 4; *p ≤ 0.05, **p ≤ 0.01.
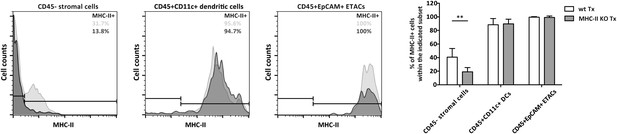
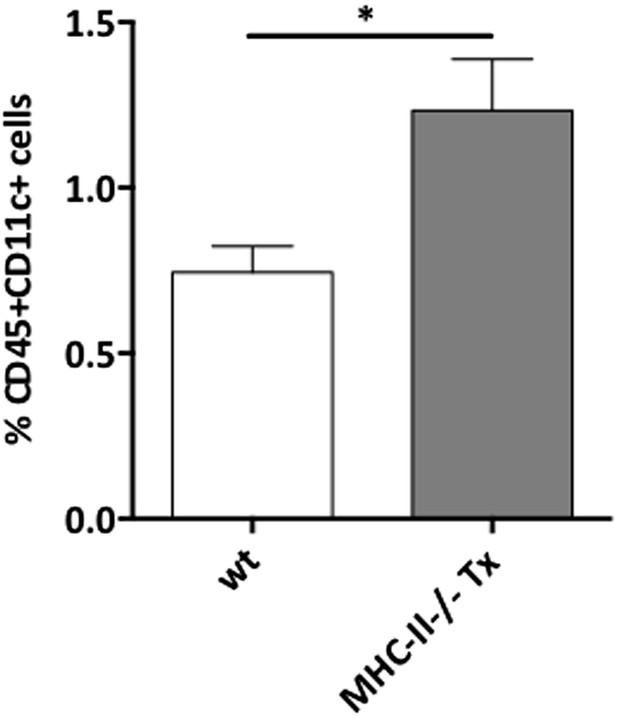
Stromal cell MHC-II expression in MHC-II KO lymph node transplants.
Wild-type mice were transplanted with either wild-type (wt Tx) or MHC-II KO (MHC-II KO Tx) lymph nodes. After 4 weeks, transplants were dissected and MHC-II expression on CD45− stromal cells, CD45+CD11c+ dendritic cells, and CD45+EpCAM+ extra-thymic AIRE-expressing like cells (ETACs) was determined by flow cytometry. Pale histograms represent MHC-II expression in wild-type transplants; whereas, dark histograms represent MHC-II expression in MHC-II KO transplants. Following the same color scheme, the numbers in the histograms represent the percentage of MHC-II+ cells. The graph on the right summarizes the data of seven transplants per group. The data represent mean ± SEM; **p ≤ 0.01.
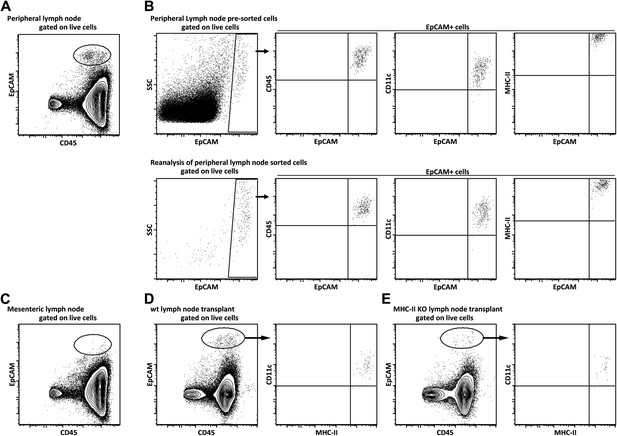
Lymph node EPCAM+ cells phenotypically represent a dendritic cell subset that is still present in MHC-II KO lymph node transplants as MHC-II expressing cells.
Lymph node EpCAM+ cells were characterized by flow cytometry. Single cell suspensions of peripheral (A, B), mesenteric (C), and wild-type (D) or MHC-II KO (E) transplanted lymph nodes obtained by enzymatic digestion were stained for EpCAM and CD45 (A, C), or EpCAM, CD45, MHC-II, and CD11c (B, D, E). In (B), EpCAM+ cells were sorted and reanalyzed by flow cytometry.
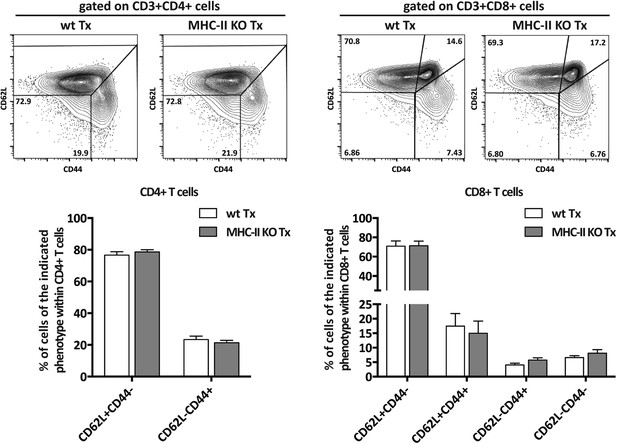
In normopenic conditions, T cell activation is restricted to MHC-II KO lymph node transplants.
Wild-type mice were transplanted with either wild-type (wt Tx) or MHC-II KO (MHC-II KO Tx) lymph nodes. After 4 weeks, host-derived CD4+ and CD8+ T cells present within the hosts' endogenous lymph nodes were characterized by flow cytometry. Representative contour plots are shown; the numbers in the plots indicate the frequency of cells within the drawn gates. The data represent mean ± SEM; n = 4 for CD4+ T cell analysis and n = 8 for CD8+ T cell analysis.
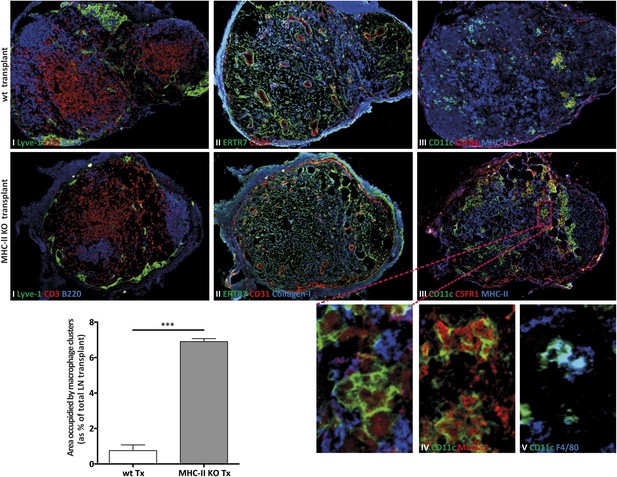
MHC-II KO lymph node transplants are rejected in wild-type recipients.
Wild-type mice were transplanted with either wild-type (wt Tx) or MHC-II KO (MHC-II KO Tx) lymph nodes. After 4 weeks, the transplants were collected and analyzed by immunofluorescence. (A) Tissue sections were stained for (I) Lyve-1 (green), CD3 (red), and B220 (blue); (II) ERTR7 (green), CD31 (red), and Collagen type I (blue); (III) CD11c (green), CSFR1 (red), and MHC-II (blue); (IV) CD11c (green) and Moma2 (red); and (V) CD11c (green) and F4/80 (blue). Small figures are high magnifications of the area highlighted. In (B), the area occupied by CSFR1+CD11c+ clusters was quantified and presented as the % of the area of the lymph node transplants. The data represent mean ± SEM; n = 4; ***p ≤ 0.001.
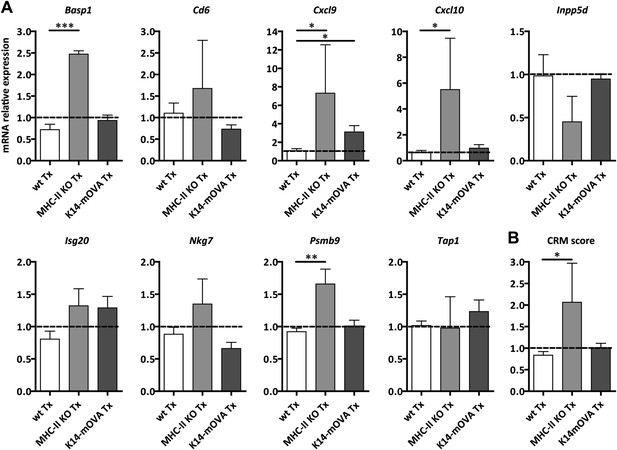
MHC-II KO lymph node transplants exhibit increased common rejection module (CRM) score.
Wild-type mice were transplanted with wild-type (wt Tx), MHC-II KO (MHC-II KO Tx), or K14-mOVA (K14-mOVA Tx) lymph nodes. After 4 weeks, the transplants were collected and analyzed by real-time PCR. (A) Abundance of transcripts belonging to the CRM. Lck and runx3 transcripts were not detected in all the samples. Gene expression in non-transplanted endogenous lymph nodes was arbitrarily set at 1 and is shown as a dotted line in each graph. (B) Graph showing the CRM score. Data represent mean ± SEM; n = 4 for wild-type non-transplanted lymph nodes, n = 5 for wild-type transplants, n = 2 for MHC-II KO transplants and n = 3 for K14-mOVA transplants. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
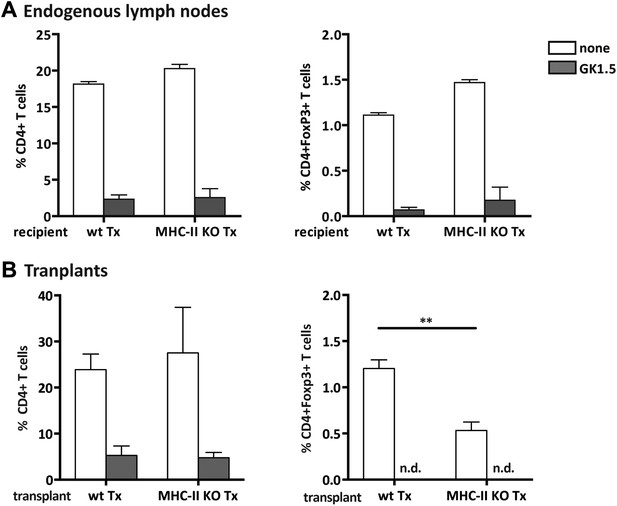
Efficient CD4+ T cell depletion in donor and recipient tissues.
Wild-type mice were transplanted with either wild-type (wt Tx) or MHC-II KO (MHC-II KO Tx) lymph nodes and depleted of CD4+ cells by administration of the anti-CD4 antibody GK1.5. Graphs represent the frequency of host-derived CD4+ T cells and CD4+Foxp3+ T cells in the endogenous lymph nodes (A) and in the transplants (B), 4 weeks after the transplantation procedure, among total lymph node cells. The extremely low number of CD4+ T cells present in GK1.5-treated animals precluded the analysis of transplant infiltrating CD4+Foxp3+ T cells. Data represent mean ± SEM; n = 6 for endogenous lymph nodes and n = 3 for transplants. **p ≤ 0.01.
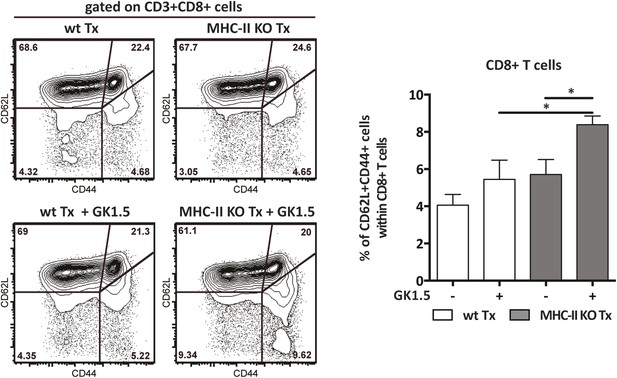
CD4+ T cells prevent the systemic spreading of MHC-II-deficient stromal cell-mediated CD8+ T cell activation.
Wild-type mice were transplanted with either wild-type (wt Tx) or MHC-II KO (MHC-II KO Tx) lymph nodes and depleted of CD4+ cells by administration of the anti-CD4 antibody GK1.5. Counter plots show the cytometric characterization of host-derived CD8+ T cells present within the recipient's endogenous lymph nodes. Numbers in plots indicate the frequency of cells within the drawn gates. The graph depicts the percentage of activated CD62L−CD44+ cells within CD8+ T cells. Data represent mean ± SEM; n = 8; *p ≤ 0.05.
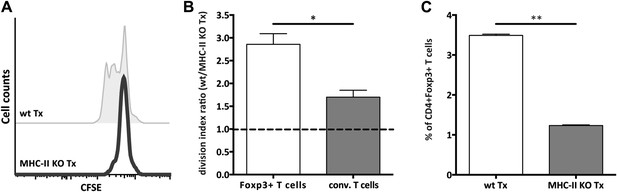
MHC-II+ lymph node stromal cells support Treg homeostatic proliferation.
Rag2-deficient mice transplanted with either wild-type (wt Tx) or MHC-II KO (MHC-II KO Tx) lymph nodes were injected with 107 CFSE-labeled wild-type lymphocytes. 48 hr later, mice were sacrificed and the transferred cells within the lymph node transplants analyzed by flow cytometry. The CFSE profile of transferred Foxp3+CD4+ T cells is shown in (A). In (B), the ratio between the division indexes of wild-type (B6) and MHC-II KO lymph node transplant recovered CD4+Foxp3+ Tregs and CD4+Foxp3− conventional T cells is shown. The frequency of CD4+Foxp3+ Tregs recovered from wild-type and MHC-II KO lymph node transplant is shown in (C). The data represent mean ± SEM; n = 2 independent experiments with 2–3 animals per group; *p ≤ 0.05, **p ≤ 0.01.
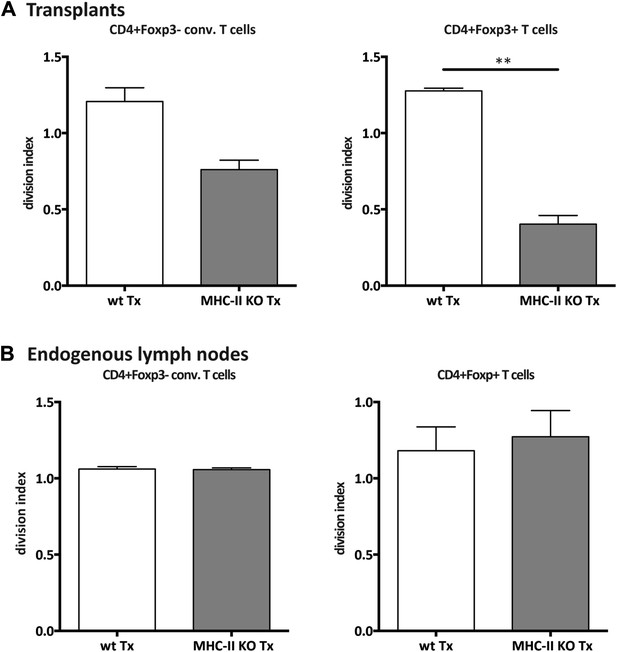
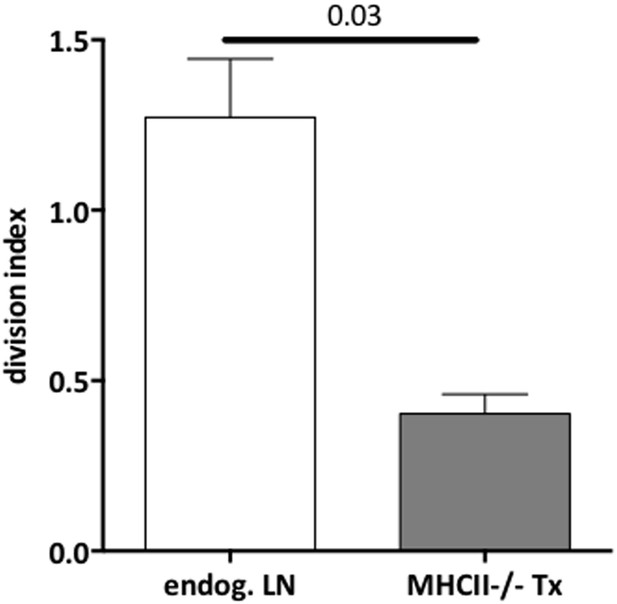
Reduced CD4+ T cell proliferation in the absence of lymph node stromal cell MHC-II expression.
Rag2-deficient mice transplanted with either wild-type (wt Tx) or MHC-II KO (MHC-II KO Tx) lymph nodes were injected with 107 CFSE-labeled wild-type lymphocytes. 48 hr later, mice were sacrificed and the transferred cells within the lymph node transplants analyzed by flow cytometry. The division index of CD4+Foxp3− conventional T cells and CD4+Foxp3+ Tregs in the transplanted lymph nodes (A) and in the endogenous lymph nodes of transplant recipients (B) is shown. The data represent mean ± SEM; n = 2 independent experiments with 2–3 animals per group; *p ≤ 0.05, **p ≤ 0.01.
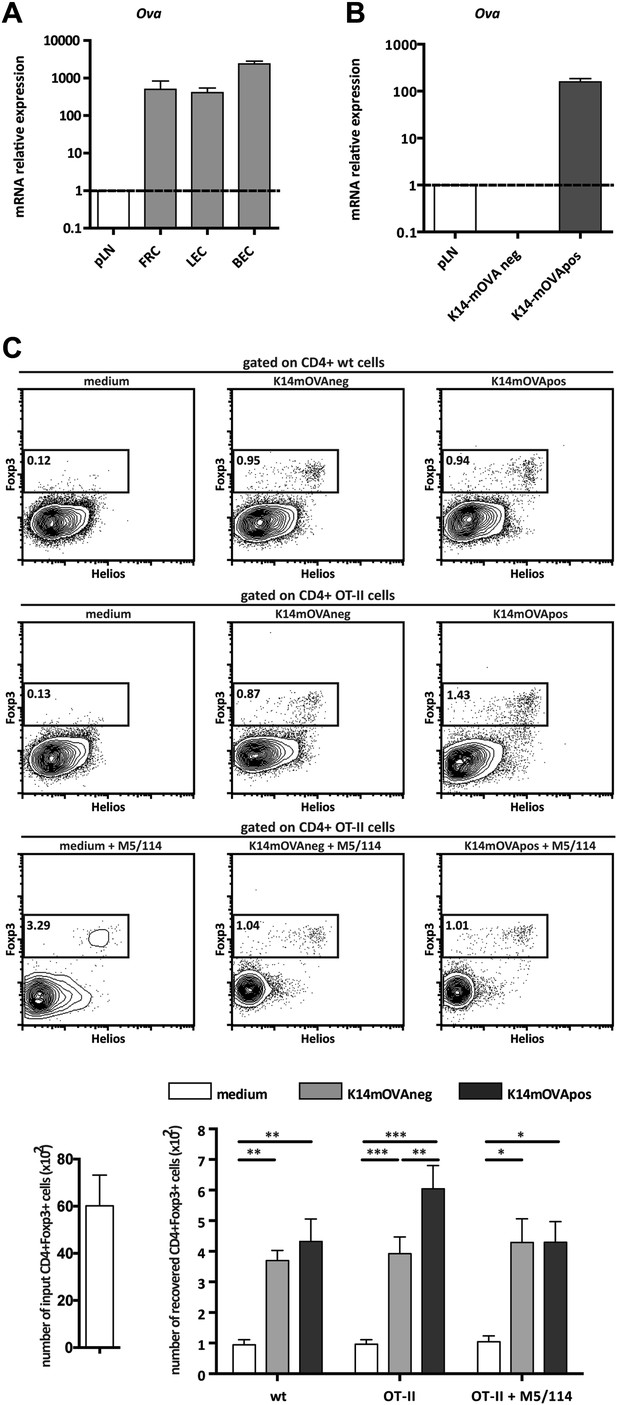
Endogenous OVA presentation by lymph node stromal cells promotes Treg maintenance in vitro.
OVA mRNA expression in primary FACS-sorted stromal cells (A; n = 2) and in vitro-generated stromal cell lines derived from K14-mOVA mice (B; n = 5) was determined by real-time PCR. Peripheral lymph nodes (pLN) from K14-mOVA mice were used as controls. (C) MACS-sorted CD4+ wild-type or OT-II transgenic cells were cultured together with in vitro-generated stromal cell lines of K14-mOVA origin in the absence or presence of the MHC-II blocking antibody M5/114. After 72 hr of co-culture, OT-II cells were characterized by flow cytometry. Representative counterplots are shown; the numbers in the plots represent the frequency of CD4+Fox3+ T cells. Graphs depict the number of CD4+Fox3+ T cells in the beginning and at the end of culture. Data represent mean ± SEM; n = 3 for wild-type CD4+ T cells; n = 8 for OT-II cells; and n = 3 for OT-II cells + M5/114. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
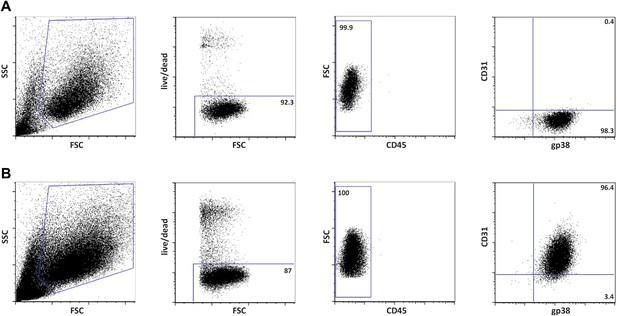
Phenotypically K14-mOVAneg and K14-mOVApos cells represent fibroblastic reticular cells (FRCs) and lymphatic endothelial cells (LECs), respectively.
Flow cytometrical characterization of the cell lines, K14-mOVAneg (A) and K14-mOVApos (B), derived by long-term culture of primary lymph node stromal cells of K14-mOVA origin.
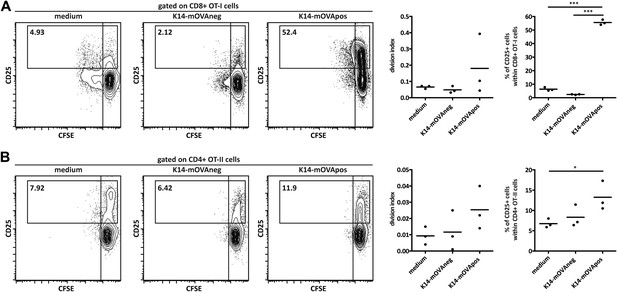
K14-mOVA stromal cells present endogenous OVA-derived peptides in vitro.
MACS-sorted CD8+ OT-I (A) and CD4+ OT-II (B) transgenic cells were cultured in the absence or presence of in vitro-generated stromal cell lines of K14-mOVA origin. Cell division and CD25 expression were determined after 72 hr. Left panels: representative contour plots; numbers in plots indicate the percentages of cells within the drawn gates. Right panels: summary of all experiments. n = 3, each dot represents one individual observation. *p ≤ 0.05, ***p ≤ 0.001.
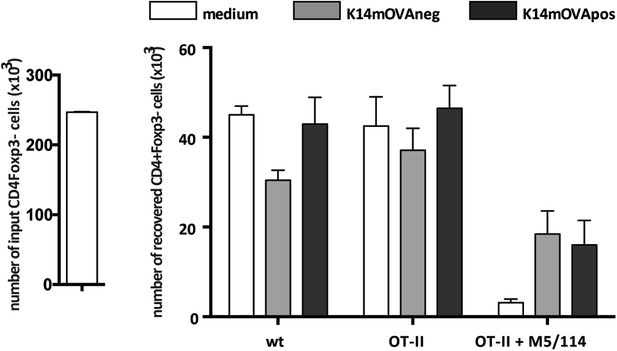
Endogenous OVA presentation by lymph node stromal cells does not affect CD4+Foxp3− conventional T cells.
MACS-sorted CD4+ wild-type or OT-II transgenic cells were cultured together with in vitro-generated stromal cell lines of K14-mOVA origin in the absence or presence of the MHC-II blocking antibody M5/114. After 72 hr of co-culture, OT-II cells were characterized by flow cytometry. The number of CD4+Fox3− conventional T cells in the beginning and at the end of culture is shown. Data represent mean ± SEM. n = 3 for wild-type CD4+ T cells; n = 8 for OT-II cells; and n = 3 for OT-II cells + M5/114.
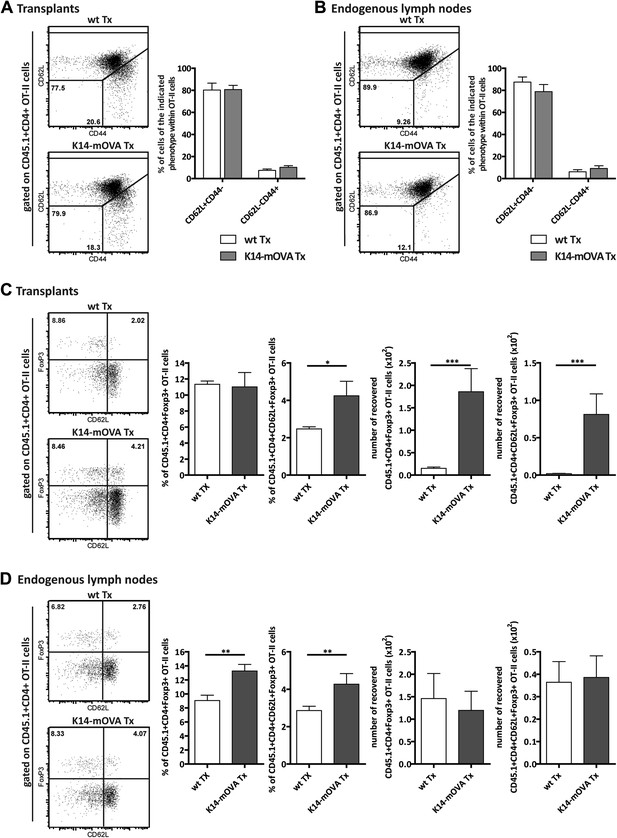
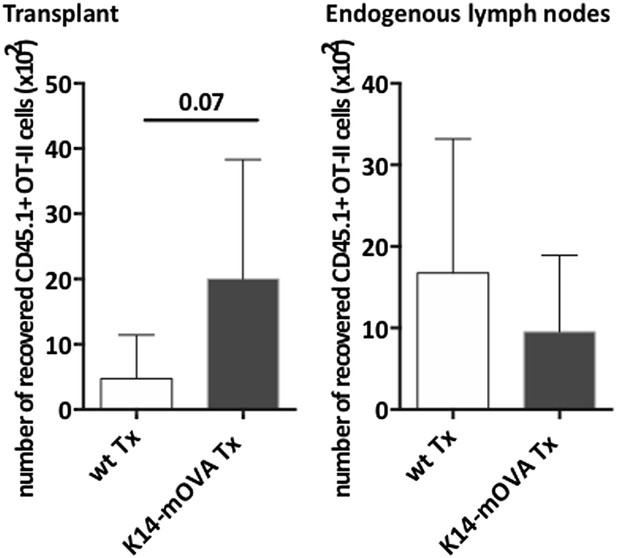
Endogenous OVA presentation by lymph node stromal cells promotes Treg maintenance in vivo.
Wild-type mice transplanted with either wild-type (wt Tx) or K14-mOVA transgenic (K14-mOVA Tx) lymph nodes were injected with 107 CD45.1+ OT-II cells. 3 days after the transfer, mice were sacrificed and the transferred OT-II cells in both the transplanted lymph nodes (A, C) and the endogenous lymph nodes (B, D) analyzed by flow cytometry. Naïve and activated OT-II T cells were defined as CD62L+CD44- and CD62L−CD44+ cells, respectively (A, B); naïve-like OT-II Tregs as CD4+CD62L+Foxp3+ cells (C, D). Representative contour plots of the analysis performed are shown on the left; the numbers in the plots indicate the frequency of cells within the drawn gates. The graphs shown on the right represent the mean ± SEM of 2 independent experiments; n = 6 and n = 8 for wild-type and K14-mOVA lymph node transplanted animals, respectively; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
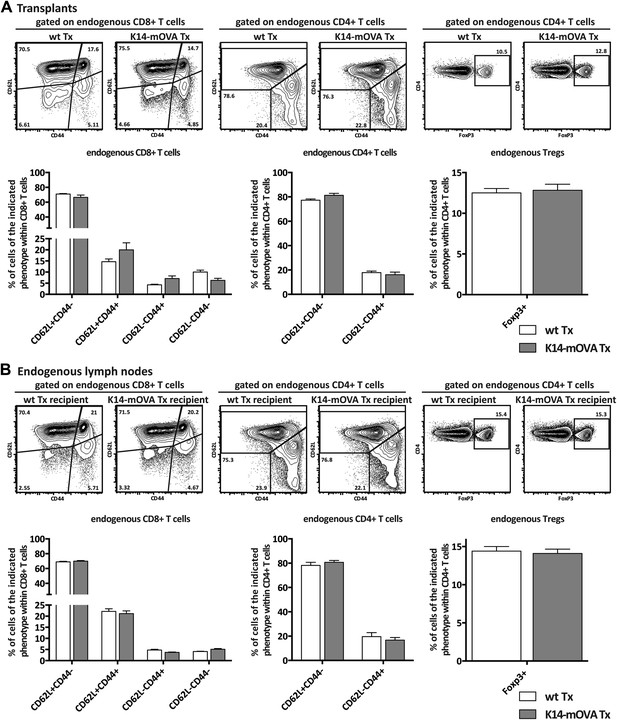
Endogenous CD8+ and CD4+ T cells are not affected by the transplantation of K14-mOVA transgenic lymph nodes.
Wild-type recipient mice were transplanted with either wild-type (wt Tx) or K14-mOVA transgenic (K14-mOVA Tx) lymph nodes. 4 weeks later, host-derived lymphocytes in transplanted lymph nodes (A) as well as in endogenous lymph nodes (B) were analyzed by flow cytometry. Naïve, effector, and memory CD8+ and CD4+ T cells were defined by the pattern of expression of CD44 and CD62L. Tregs were defined as CD4+FoxP3+ T cells. Representative contour plots of the analysis performed are shown on top; the numbers in the plots indicate the frequency of cells within the drawn gates. Summarizing graphs are shown on the bottom. Data depict mean ± SEM and represent two independent experiments; n = 6 and n = 8 for wild-type and K14-mOVA lymph node transplanted animals, respectively.
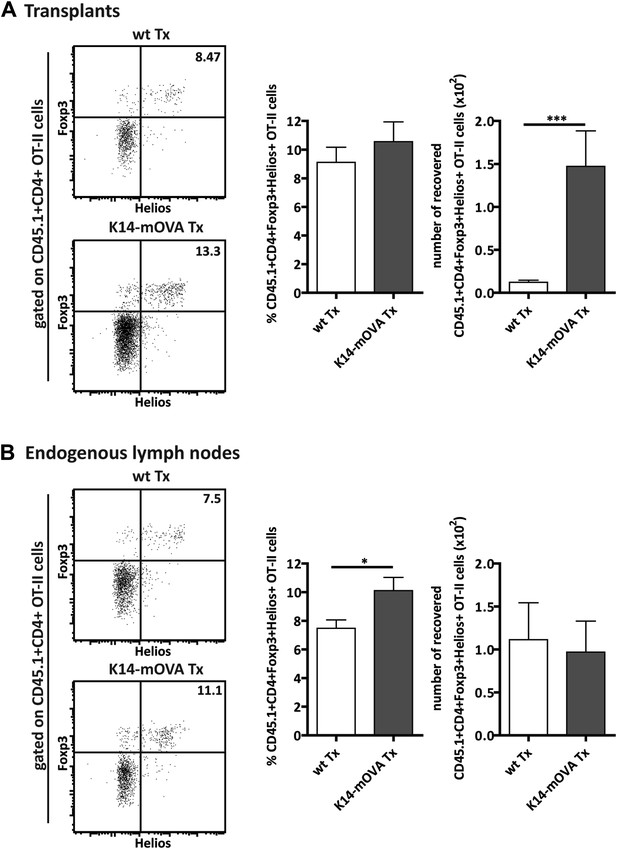
Expansion of CD4+FoxP3+Helios+ OT-II Tregs in mice transplanted with K14-mOVA lymph nodes.
Wild-type recipient mice were transplanted with either wild-type (wt Tx) or K14-mOVA transgenic (K14-mOVA Tx) lymph nodes. 4 weeks later, 107 CD45.1+ OT-II cells were intravenously injected. 3 days after the transfer, OT-II cells in both transplanted lymph nodes (A) as well as in endogenous lymph nodes (B) were analyzed by flow cytometry. Left panels: dot plots depict the expression of FoxP3 and Helios on CD45.1+CD4+ OT-II cells; the numbers on the dot plots indicate the frequency of FoxP3+Helios+ cells. Right panels: graphs show the frequency and the number of recovered CD45.1+CD4+FoxP3+Helios+ OT-II cells and represent the mean ± SEM of 2 independent experiments; n = 6 and n = 8 for wild-type and K14-OVA lymph node transplanted animals, respectively; *p ≤ 0.05, ***p ≤ 0.001.
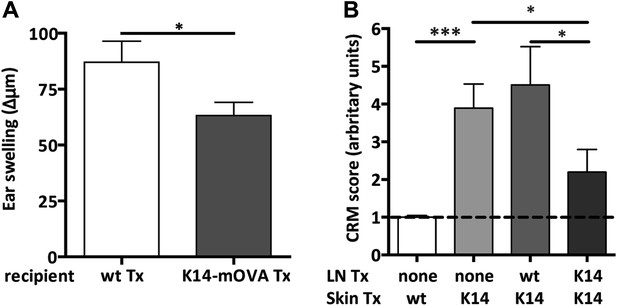
Endogenous OVA presentation by lymph node stromal cells constrains immune reactivity.
(A) Wild-type mice transplanted with either wild-type (wt Tx) or K14-mOVA transgenic (K14-mOVA Tx) lymph nodes were immunized with OVA in incomplete Freund's adjuvant (IFA) in the tail base and re-challenged with OVA alone in both ears. In vivo delayed-type hypersensitivity (DTH) responses were determined by ear swelling. The data represent mean ± SEM; n = 5 mice per group; *p ≤ 0.05. (B) Wild-type mice either left untreated or transplanted with wild-type or K14-mOVA transgenic lymph nodes, at week -4, were transplanted with either wild-type or K14-mOVA skin on day 0. 4 weeks after skin transplantation, skin grafts were isolated and mRNA transcripts belonging to the common rejection module (CRM) analyzed by real-time PCR. The aggregate CRM score is shown. n = 6 mice per group; *p < 0.05, ***p ≤ 0.001.
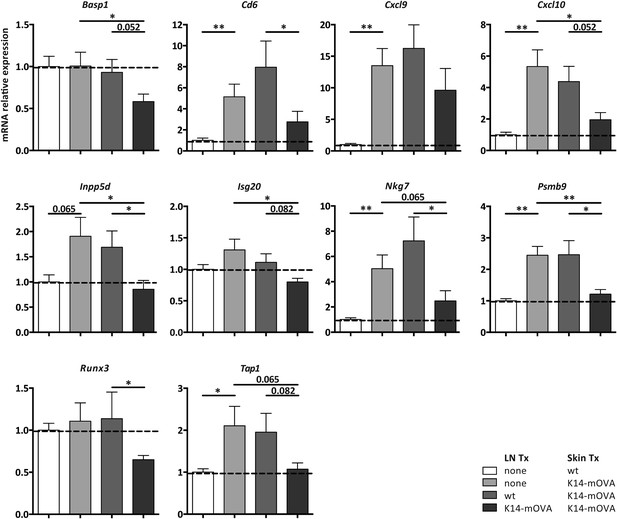
Self-antigen-presenting stromal cells constraint immune reactivity in a dual transplantation system.
Wild-type mice either left untreated or transplanted with wild-type or K14-mOVA transgenic lymph nodes, at week -4, were transplanted with either wild-type or K14-mOVA skin on day 0. 4 weeks after skin transplantation, skin grafts were isolated and mRNA transcripts belonging to the common rejection module (CRM) analyzed by real-time PCR. n = 6 mice per group; *p ≤ 0.05, ***p ≤ 0.001.