Reciprocal and dynamic polarization of planar cell polarity core components and myosin
Figures
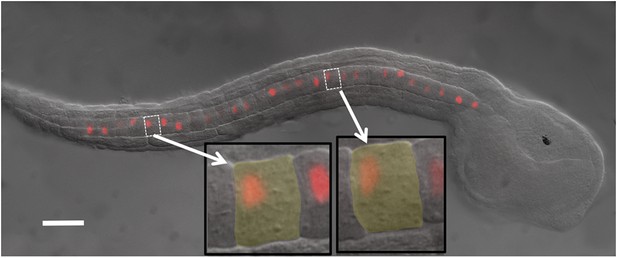
Ciona intestinalis late-tailbud embryo (stage 23) expressing an electroporated Histone 2A/Red Fluorescent Protein (H2A-RFP) in the notochord.
Insets show two cells to illustrate the polarization of the nuclei to the posterior of the cells. Scale bar is 50 μm.
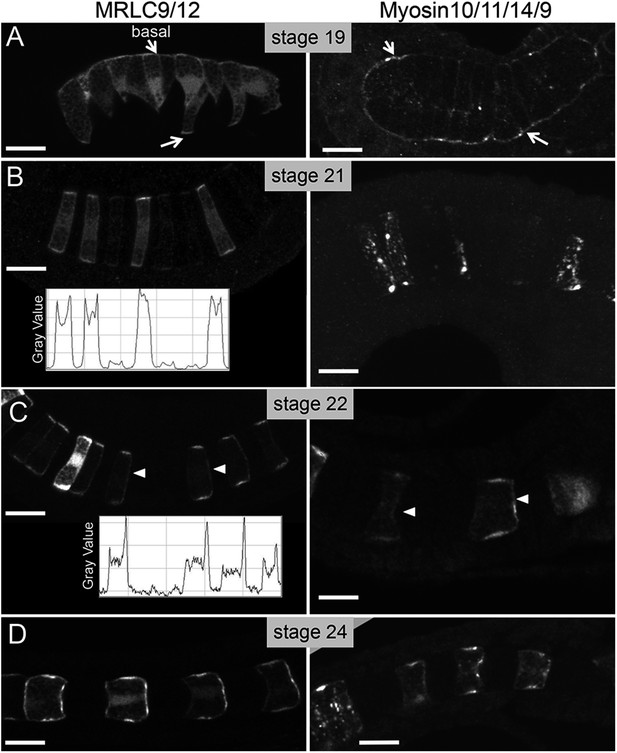
Time course for the subcellular localization of myc-tagged myosin10/11/14/9-tagged myosin regulatory light chain (MRLC) and Venus-tagged myosin regulatory light chain (MRLC9/12-Venus) at the stages indicated.
Arrows in A indicate contacts of cells with the basement membrane, and arrowheads in C indicate anteriorly localized protein. Densitometry readings across the center of four cells demonstrate the transition in MRLC9/12 localization from stage 21 to stage 22 (charts in B and C). (D) At stage 24, the anterior localization of both proteins persists. Anterior is to the right in all panels. Scale bars are 10 μm.
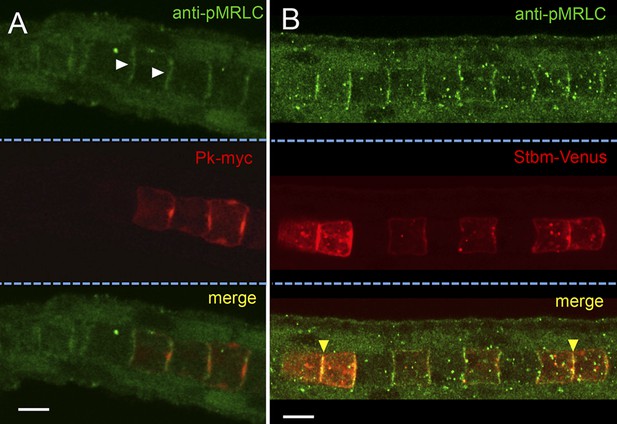
Subcellular localization of endogenous phospho-Myosin Regulatory Light Chain (pMRLC) and electroporated myc-tagged Prickle (Pk-myc) (A), and Venus-tagged strabismus (Stbm-Venus) (B).
White arrowheads in A indicate anti-pMRLC staining, while yellow arrowheads in B indicate punctae of strong pMRLC and Stbm-Venus colocalization. Scale bars are 10 μm.
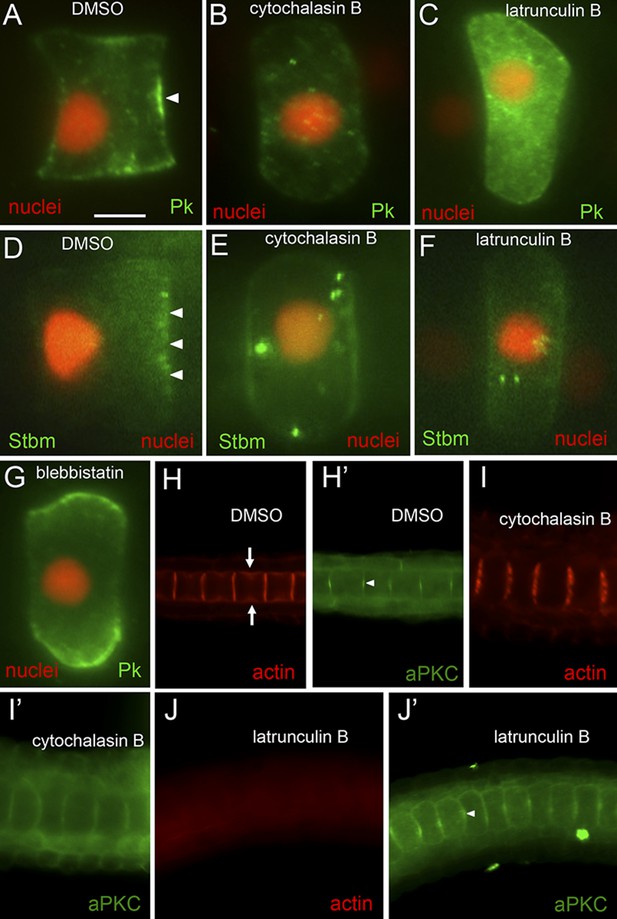
Loss of planar cell polarity protein localization following actin/myosin disruptions.
(A–G) Single notochord cells from embryos expressing H2A-RFP (nuclear RFP), and either a Pk-myc or Stbm-Venus fusion protein (as indicated). Arrowheads indicate anteriorly localized reporter protein. Embryos were treated with either DMSO (vehicle), cytochalasin B, latrunculin B, or blebbistatin (also as indicated). (H, I, and J) Cortical actin staining of notochord cells with phalloidin in embryos treated with vehicle only (DMSO), cytochalasin B, or latrunculin B, as indicated. Arrows in H indicate the equatorial contractile ring of actin. (H′, I′, and J′) Co-staining of samples for the cortical protein atypical protein kinase C (aPKC). Arrowhead in H′ indicates concentrated staining at center of cell cortex. Scale bar in A is 5 μm.
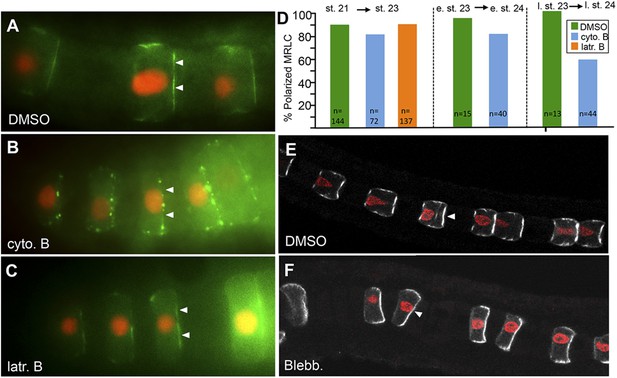
MRLC9/12 localization is resistant to cytochalasin B and blebbistatin.
(A) Control, vehicle-treated embryo shows polarization of MRLC9/12 to the anterior membrane of notochord cells. (B and C) MRLC9/12 polarization is resistant to cytochalasin B and latrunculin B treatments. MRLC9/12-Venus is labeled green, and H2A-RFP (nuclei) is labeled red. (D) Percentage of notochord cells showing polarized MRLC9/12 for three treatment regimes. The number of cells scored is indicated at the bottom of the columns. e. st., early stage; l. st., late stage. (E and F) Persistence of MRLC9/12-Venus polarization (white) in embryos treated with blebbistatin or vehicle (DMSO) from stage 21 to stage 23. Arrowheads indicate anteriorly localized proteins.
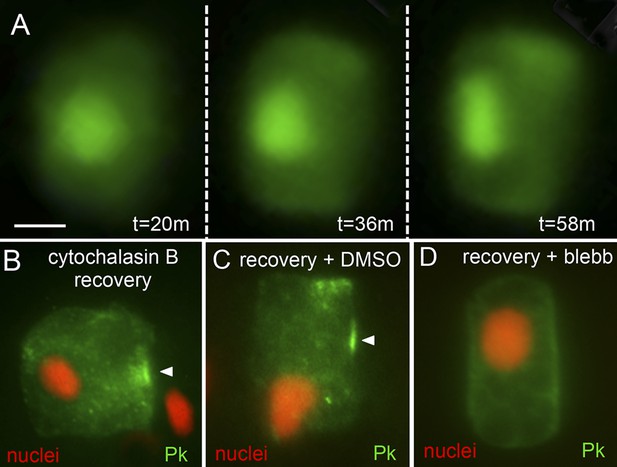
Recovery of polarity following cytochalasin B treatment.
(A) Time lapse of nuclear repolarization following removal of embryos from cytochalasin B. Times are from end of cytochalasin B treatment. (B) Recovery of Pk-myc polarization following cytochalasin B treatment and wash out. Anterior is to the right in all panels. (C and D) Blebbistatin blocks recovery from cytochalsin B treatment. Following removal of embryos from cytochalsin B, embryos were either treated with vehicle (DMSO) (C) or 10 μm blebbistatin (D). Arrowheads indicate anterior localized Pk. Scale bar in A is 5 μm.
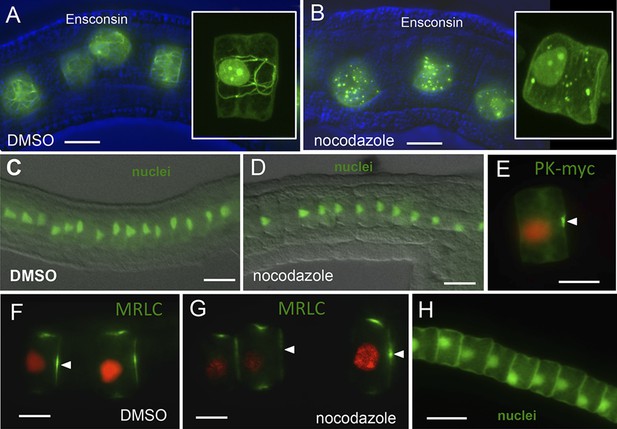
Notochord A/P polarity does not require microtubular network.
(A) Visualization of the notochord microtubule network with GFP-tagged ensconsin, and B, disruption of the network with 10 μm nocodazole. (C–E) Nocodazole treatment from stage 21 disrupts neither nuclear (D) nor Pk-myc (E) polarity. Embryos/cells are shown at stage 23. Similarly, nocodazole treatment at stage 21 does not disrupt MRLC9/12 polarity (control, F; treated, G). (H) Cell nuclei properly repolarized in the presence of nocodazole following cytochalasin treatment. Arrowheads indicate anterior localized MRLC9/12. Scale bars are 10 μm.
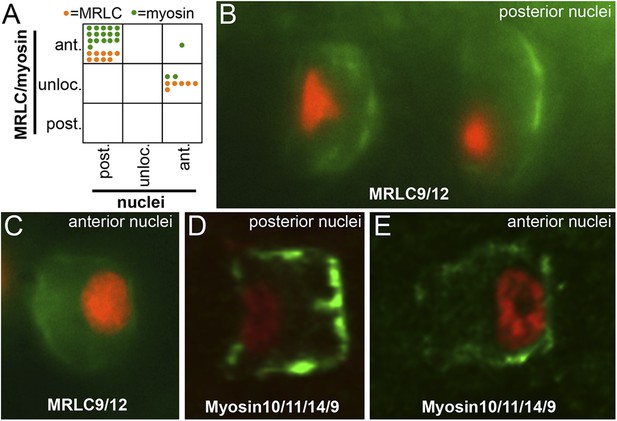
Myosin10/11/14/9 and MRLC9/12 localization in homozygous aim embryos.
(A) Distribution of nuclear and Myosin10/11/14/9 (green) or MRLC9/12 (orange) localization phenotypes (ant., anterior; post., posterior; unloc., unlocalized) in aimless embryos. Each dot represents a single scored cell. (B and C) MRLC9/12-Venus (green) localization in cells with posterior and anterior nuclei (red), respectively. (D and E) Myosin10/11/14/9-myc (green) localization in cells with posterior and anterior nuclei (red), respectively. Anterior is to the right in all panels.
Videos
Detachment of nuclei during cytochalasin B treatment.
The video represents 30 min in real time, beginning approximately 10 min after the addition of cytochalasin B.
Movement of nuclei to the posterior poles of notochord cells following removal in cytochalasin B.
The video represents 28 min in real time, beginning approximately 10 min after removal from cytochalasin B.





