Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1
Figures

Angpt1 was expressed by megakaryocytes and hematopoietic stem/progenitor cells in the bone marrow.
(A–C) Immunostaining of femur sections from Col1a1*2.3-GFP mice with anti-Angpt1 antibody showed that Angpt1 was not detectably expressed by bone lining Col1a1*2.3-GFP+ osteoblasts. Nuclei were stained with DAPI (blue). (n = 3 mice from 3 independent experiments). (D–I) Representative femur sections from wild-type mice showed that anti-Angpt1 antibody stained CD41+ megakaryocytes (arrows, D–F) and c-kit+ hematopoietic progenitors (HPCs) (arrows, G–I) throughout the bone marrow. * in F indicates trabecular bone—note the lack of Angpt1 staining in bone-lining cells (n = 3 mice from 3 independent experiments). (J–O) Images of femur sections from Angpt1GFP mice showed that GFP was expressed by CD41+ megakaryocytes (arrows, J–L) and c-kit+ HPCs (arrows, M–O) (n = 3 mice from 3 independent experiments). (P–Y) Flow cytometric analysis of non-enzymatically dissociated Angpt1GFP bone marrow cells (which contains hematopoietic but few stromal cells) showed that GFP was rarely expressed by whole bone marrow (WBM) cells (P) or c-kit− cells (Q) but was expressed by most c-kit+ cells (R), CD150+CD48−LSK hematopoietic stem cells (HSCs) (S), CD150−CD48−LSK multipotent progenitor cells (MPPs) (T), CD48+LSK HPC cells (U), Flt3+IL7Rα+Lineage−Sca1lowc-kitlow common lymphoid progenitors (CLPs) (V), CD34+FcγR−Lineage−Sca1−c-kit+ common myeloid progenitor cells (CMPs) (W) and CD34+FcγR+Lineage−Sca1−c-kit+ granulocyte/macrophage progenitors (GMPs) (X). CD34−FcγR−Lineage−Sca1−c-kit+ megakaryocytic/erythroid progenitors (MEPs) expressed little GFP (Y). Data represent mean ± s.d. from 4 mice from 4 independent experiments.
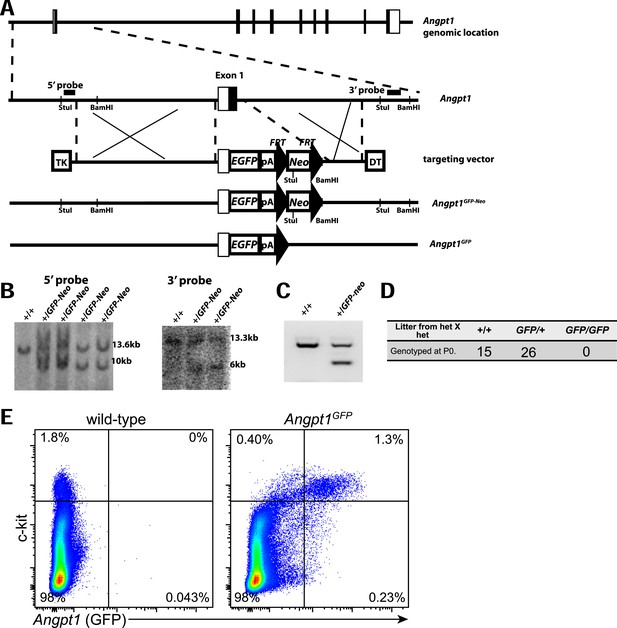
Generation of Angpt1GFP knock-in mice.
(A) Targeting strategy to generate the Angpt1GFP knock-in allele. A BAC clone containing the Angpt1 genomic region was used to generate the targeting vector by recombineering. The knock-in allele resulted in the replacement of the first exon of Angpt1 by GFP, in-frame with the ATG start codon of Angpt1. (B) The targeting vector was electroporated into Bruce4 ES cells. Correctly targeted clones were identified by Southern blotting with probes indicated in panel A. These ES cells were used to generate chimeric mice by blastomere injection. Chimeric mice were then bred with C57BL/6-Tyrc-2J to obtain germline transmission. These mice were bred with Flpe mice (Rodriguez et al., 2000) to remove the Neo cassette. (C) PCR genotyping demonstrated germline transmission of the Angpt1GFP-Neo allele. (D) No live Angpt1GFP/GFP pups were born from Angpt1GFP × Angpt1GFP matings, as would be expected for Angpt1 deficient mice, indicating that Angpt1GFP is a strong loss-of-function allele. (E) In mechanically dissociated bone marrow from Angpt1GFP mice (which contains hematopoietic but not stromal cells) 85% of GFP+ cells were c-kit+ and 76% of c-kit+ cells were GFP+ (n = 4 mice from 4 independent experiments).
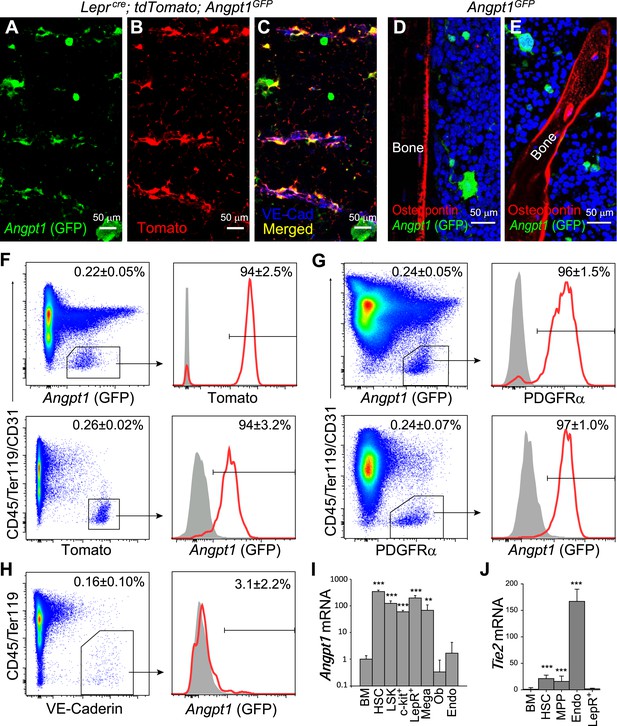
Angpt1 was expressed by Leptin Receptor+ (LepR+) perivascular stromal cells but not endothelial cells or osteoblasts in bone marrow.
(A–C) Representative femur sections showed that LepR+ perivascular stromal cells (Tomato+) expressed GFP in Leprcre; tdTomato; Angpt1GFP mice. Endothelial cells were stained with anti-VE-cadherin antibody (blue) (n = 3 mice from 3 independent experiments). Note that Angpt1 expression in LepR+ cells is much easier to see in sections from GFP mice than in antibody stained sections. (D and E) Representative femur sections from Angpt1GFP mice showed that GFP was not detectably expressed by Osteopontin+ (red) osteoblasts in the diaphyseal (D) or metaphyseal (E) regions (n = 3 mice from 3 independent experiments). (F) In the bone marrow stroma from Leprcre; tdTomato; Angpt1GFP mice, nearly all LepR+ cells were positive for GFP, and vice versa. Data represent mean ± s.d. from 4 mice from 3 independent experiments. (G) In the bone marrow stroma from Angpt1GFP mice, nearly all PDGFRα+ cells were positive for GFP, and vice versa. Data represent mean ± s.d. from 4 mice in 3 experiments. (H) Bone marrow CD45−Ter119−VE-cadherin+ endothelial cells did not express detectable GFP (n = 3 mice from 3 independent experiments). (I and J) Angpt1 (I) and Tie2 (J) transcript expression levels by qRT-PCR of unfractionated bone marrow cells, HSCs, LSK cells, c-kit+ cells, EYFP+ cells from Leprcre; loxp-EYFP mice, CD41+ megakaryocytes, Col1a1*2.3-GFP+ osteoblasts, VE-cadherin+ bone marrow endothelial cells. All data represent mean ± s.d. from 3–8 mice/genotype in 3 independent experiments. Two-tailed Student's t-tests were used to assess statistical significance relative to unfractionated bone marrow cells (*p < 0.05, **p < 0.01, ***p < 0.001).
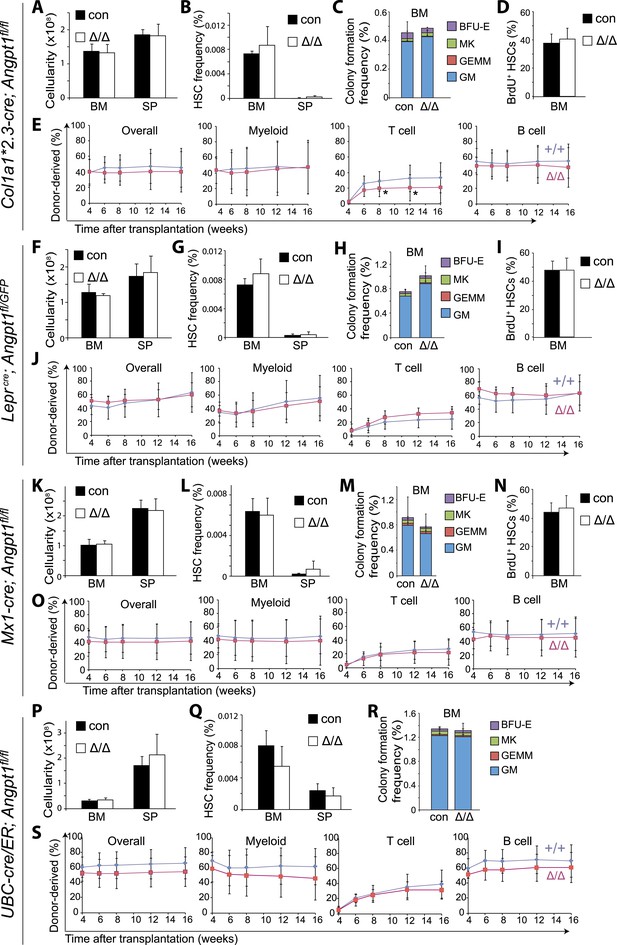
Angpt1 was dispensable for HSC maintenance and hematopoiesis.
(A–E) Deletion of Angpt1 from osteoblasts using Col1a1*2.3-cre did not significantly affect bone marrow or spleen cellularity (A, n = 3 mice/genotype from 3 independent experiments), HSC frequency (B, n = 3 mice/genotype from 3 independent experiments), colony-forming progenitor frequency in bone marrow (C, n = 3 mice/genotype 3 independent experiments), incorporation of a 10-day pulse of BrdU by HSCs (D, n = 3 pairs of male mice and 3 pairs of female mice/genotype), or reconstituting capacity of bone marrow cells in a competitive reconstitution assay (E, n = 14–15 recipient mice/genotype from 3 independent experiments). (F–J) Leprcre; Angpt1GFP/fl mice had normal bone marrow and spleen cellularity (F, n = 4 mice/genotype from 4 independent experiments), HSC frequency in bone marrow and spleen (G, n = 5–6 mice/genotype from 5 independent experiments), colony-forming cell frequency in bone marrow (H, n = 3 mice/genotype from 3 independent experiments), BrdU incorporation into HSCs (I, n = 3 pairs of male mice and 3 pairs of female mice/genotype), and reconstituting capacity upon transplantation into irradiated mice (J, n = 13 recipient mice/genotype from 3 independent experiments). Angpt1fl/fl and Angpt1GFP/fl mice (lacking Cre) were indistinguishable and were therefore pooled together as controls. (K–O) Mx1-cre; Angpt1fl/fl mice had normal bone marrow and spleen cellularity (A, n = 3 mice/genotype), HSC frequency in bone marrow and spleen (K, n = 3 mice/genotype), colony-forming cell frequency in bone marrow (L, n = 6 mice/genotype from 4 independent experiments), BrdU incorporation into HSCs (M, n = 3 pairs of male mice and 3 pairs of female mice/genotype), and reconstituting capacity upon transplantation into irradiated mice (N, n = 10–14 recipient mice/genotype from 3 independent experiments). (P–S) Global deletion of Angpt1 in adult mice using UBC-Cre/ER (2–5 months after tamoxifen treatment) did not significantly affect cellularity in the bone marrow or spleen (P, n = 9–11 mice/genotype from 7 independent experiments), HSC frequency in the bone marrow (Q, n = 9–11 mice/genotype from 7 independent experiments), colony-forming progenitor frequency in bone marrow (R, n = 5 mice/genotype from 3 independent experiments), or reconstituting capacity of bone marrow cells upon transplantation into irradiated mice (S, n = 13–14 recipient mice/genotype from 3 independent experiments). Two-tailed Student's t-tests were used to assess statistical significance. See Figure 3—figure supplement 2 for data on recombination efficiency.
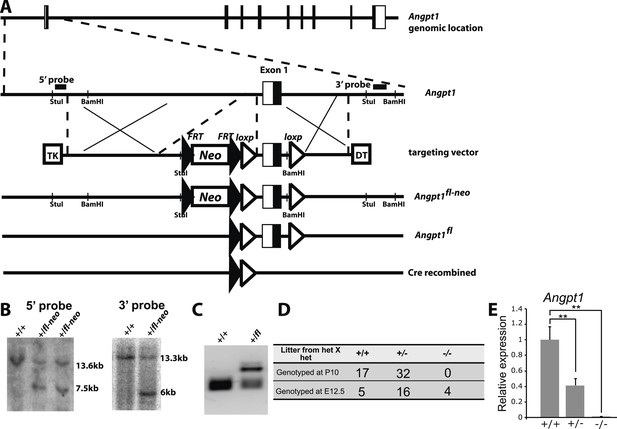
Generation of Angpt1fl mice.
(A) Targeting strategy for the generation of the Angpt1fl allele. A BAC clone containing the Angpt1 genomic region was used to generate the targeting vector by recombineering. An Frt-Neo-Frt-loxp cassette was inserted 5′ of exon1 and a loxp site was inserted 3′ of exon1. Sequence conservation among species was examined to avoid disrupting conserved intronic regulatory elements. Upon Cre mediated recombination, exon1 containing the translational start codon was excised, leading to the loss of the 5′ UTR and the first 99 amino acids of Angpt1. Linearized targeting vector was electroporated into Bruce4 ES cells. (B) Southern blotting identified correctly targeted ES clones using the probes indicated in panel E. These ES cells were injected into blastomeres to generate chimeric mice. Chimeric mice were bred with C57BL/6-Tyrc-2J mice to obtain germline transmission. These mice were then bred with Flpe mice to remove the Neo cassette. (C) PCR genotyping confirmed germline transmission of the Angpt1fl allele. (D) A predicted null allele of Angpt1 (Angpt1−) was generated by mating Angpt1fl/+ mice with CMV-cre mice. No live Angpt1−/− mice were born from Angpt1−/+ × Angpt1−/+ matings. Consistent with the reported E12.5 lethal phenotype of Angpt1 null mice (Suri et al., 1996; Jeansson et al., 2011), Angpt1−/− mice were found dead at E12.5. (E) qRT-PCR analysis confirmed the absence of Angpt1 mRNA in cells from Angpt1−/− fetal livers (n = 3–5 mice/genotype). Data represent mean ± s.d. Two-tailed Student's t-tests were used to assess statistical significance. *p < 0.05, **p < 0.01, ***p < 0.001.
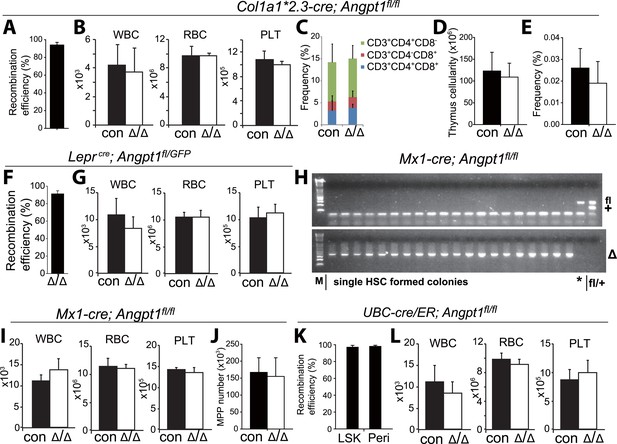
Deletion of Angpt1 did not significantly affect blood cell counts.
(A, F, K) Col1a1*2.3-cre, Leprcre and UBC-Cre/ER efficiently recombined Angpt1fl alleles in Col2.3-GFP+ osteoblasts from Col1a1*2.3-cre; Ang1fl/fl; Col2.3-GFP mice (A), LepR+ cells from Leprcre; Angpt1fl/GFP mice (F) and LSK cells and LepR+ cells from UBC-cre/ER; Ang1fl/fl mice (K). The recombination efficiency of Angpt1fl was measured by real-time PCR analysis of genomic DNA from flow cytometrically isolated cells. The amplification of the recombined allele in Col1a1*2.3-cre; Ang1fl/fl; Col2.3-GFP cells or UBC-cre/ER; Ang1fl/fl cells was compared to the amplification of the same product from Angpt1fl/fl cells. An unrelated genomic locus was amplified in parallel to normalize DNA content. The amplification of the recombined allele in Leprcre; Angpt1fl/GFP cells was compared to the amplification of the same product from Angpt1−/+ cells (germline heterozygous for the recombined allele) (F; n = 3 mice/genotype from 3 independent experiments). (H) Genotyping of hematopoietic colonies formed by individual HSCs from Mx1-cre; Angpt1fl/fl mice showed efficient recombination of the Angpt1fl allele. Overall, 65 of 66 colonies examined (>98%) exhibited complete recombination of the Angpt1fl allele (*, a single clone that was not recombined). (B, G, I, L) Normal white blood cell, red blood cell, and platelet counts in young adult Col1a1*2.3-cre; Angpt1fl/fl mice (A; n = 3 mice/genotype from 3 independent experiments), Leprcre; Angpt1fl/fl or Leprcre; Angpt1fl/GFP mice (C, n = 6–7 from 5 independent experiments), Mx1-cre; Angpt1fl/fl mice (E, n = 3 mice/genotype from 3 independent experiments) and UBC-cre/ER; Angpt1fl/fl mice 2–5 months after tamoxifen treatment (F, n = 7–8 mice/genotype from 6 independent experiments). (C–E) Col1a1*2.3-cre; Ang1fl/fl mice had normal frequencies of CD4+ and/or CD8+ T cells in the thymus (C), thymus cellularity (D) and CLP frequency in the bone marrow (E) (n = 3 mice from 3 independent experiments).
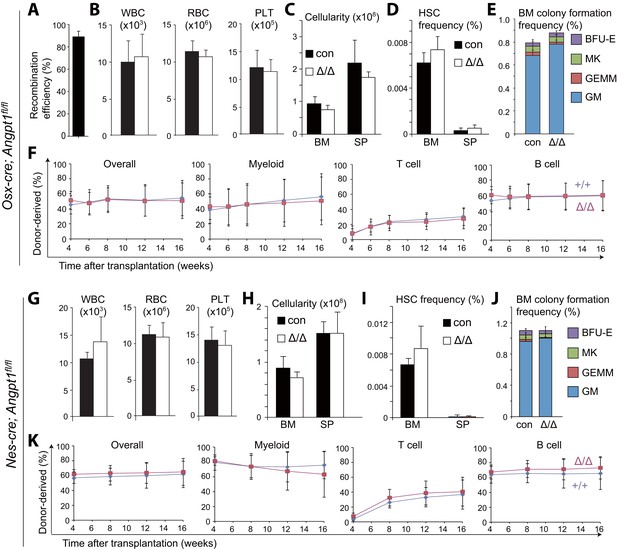
Angpt1 from osteoblast progenitors or Nestin-Cre-expressing cells is dispensable for HSC maintenance and hematopoiesis.
(A) Osx-Cre recombined 93 ± 3.0% of Angpt1fl alleles in CD105+PDGFRα+CD45−Ter119−CD31− osteoprogenitors from Osx-cre; Angpt1fl/fl mice. Recombination efficiency was measured as described in Figure 3—figure supplement 2A (n = 3 mice from 3 independent experiments). (B–F) Osx-cre; Angpt1fl/fl mice had normal blood cell counts (B, n = 6 mice/genotype from 3 independent experiments), bone marrow and spleen cellularity (C, n = 6–7 mice/genotype from 6 independent experiments), HSC frequency in bone marrow and spleen (D, n = 4 mice/genotype from 4 independent experiments), colony-forming cell frequency in bone marrow (E, n = 5 mice/genotype from 5 independent experiments), and reconstituting capacity upon transplantation into irradiated mice (F, n = 23–24 recipient mice/genotype from 5 independent experiments). (G–K) Young adult Nestin-cre; Angpt1fl/fl mice had normal white blood cell counts, red blood cell counts, and platelet counts (G, n = 4 mice/genotype from 3 independent experiments), bone marrow and spleen cellularity (G, n = 4 mice/genotype from 3 independent experiments), HSC frequency (I, n = 4 mice/genotype from 3 independent experiments), colony-forming progenitor frequency in the bone marrow (J, n = 3 mice/genotype), and competitive reconstituting capacity upon transplantation into irradiated mice (K, n = 9–10 recipient mice/genotype from 2 independent experiments). Two-tailed Student's t-tests were used to assess statistical significance.
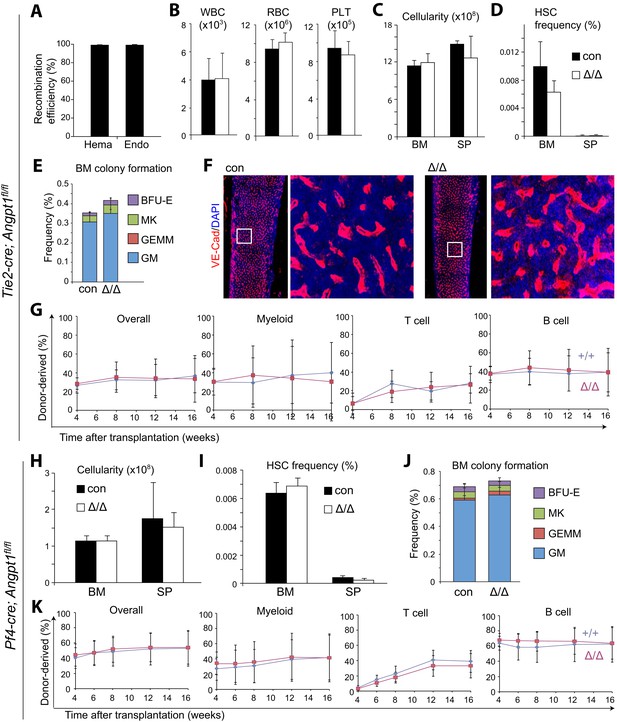
Angpt1 from endothelial cells or megakaryocytes is dispensable for HSC maintenance and hematopoiesis.
(A) Tie2-Cre recombined 97 ± 0.4% of Angpt1fl alleles in CD45+/Ter119+ hematopoietic cells and 97 ± 0.6% in VE-Cadherin+ endothelial cells from Tie2-cre; Angpt1fl/fl mice (measured as described in Figure 3—figure supplement 2A; n = 3 mice from 3 independent experiments). (B–G) Tie2-cre; Angpt1fl/fl mice had normal blood counts (B, n = 3–6 from 3 independent experiments), bone marrow and spleen cellularity (C, n = 5–10 mice/genotype from 4 independent experiments), HSC frequency in bone marrow and spleen (D, n = 5–10 mice/genotype from 4 independent experiments), colony-forming cell frequency in bone marrow (E, n = 3 mice/genotype from 3 independent experiments), vascular density and morphology (F, n = 3 mice/genotype from 3 independent experiments) and reconstituting capacity upon transplantation into irradiated mice (F, n = 8 recipient mice/genotype from 2 independent experiments). All data represent mean ± s.d. Two-tailed Student's t-tests were used to assess statistical significance. (H–K) Pf4-cre; Angpt1fl/fl mice had normal bone marrow and spleen cellularity (H, n = 5 mice/genotype from 4 independent experiments), HSC frequency in bone marrow and spleen (I, n = 5 mice/genotype from 4 independent experiments), colony-forming cell frequency in bone marrow (J, n = 5 mice/genotype from 5 independent experiments) and reconstituting capacity upon transplantation into irradiated mice (K, n = 14–15 recipient mice/genotype from 3 independent experiments).
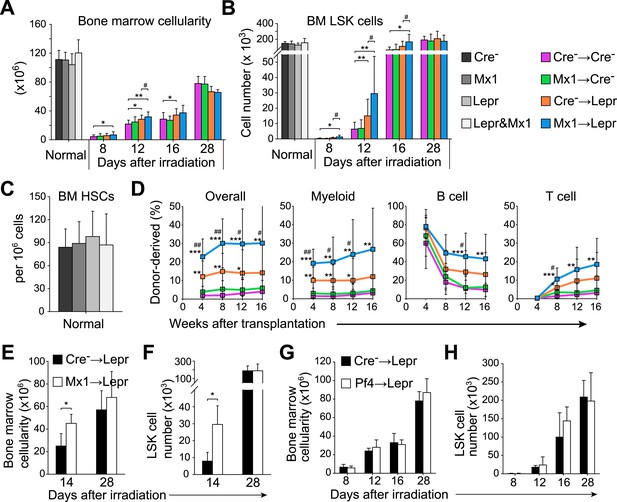
Angpt1 deficiency in hematopoietic stem/progenitor cells and LepR+ stromal cells accelerated the recovery of HSCs and hematopoiesis after irradiation.
One million bone marrow cells from Angpt1fl/fl (Cre−) or Mx1-cre; Angpt1fl/fl (Mx1) mice were transplanted into irradiated Angpt1fl/GFP or Angpt1GFP (Cre−) or Leprcre; Angpt1fl/GFP (Lepr) mice (all panels reflect mean ± s.d. from 6–11 mice/genotype/time point from 5 independent experiments). Bone marrow cellularity (A) and LSK cell numbers (B) were analyzed at the indicated time points after irradiation and transplantation, always in two femurs and two tibias per mouse. (C) Mx1-cre; Leprcre; Angpt1fl/GFP mice had a normal frequency of CD150+CD48−LSK HSCs in the bone marrow as compared to control (Angpt1fl/fl or Angpt1fl/GFP), Mx1-cre; Angpt1fl/fl, or Leprcre; Angpt1fl/GFP mice. (n = 6 mice/genotype from 5 independent experiments). (D) Competitive long-term multilineage reconstitution assay in which 1.5 × 106 donor bone marrow cells from the indicated primary recipient mice 12 days after irradiation were transplanted along with 3 × 105 recipient bone marrow cells into irradiated secondary recipient mice. The recipient cells were previously-transplanted compromised bone marrow cells. (n = 11–15 recipient mice/genotype from 3 independent experiments) Two-tailed Student's t-tests were used to assess statistical significance (* or #p < 0.05, ** or ##p < 0.01, *** or ###p < 0.001). * indicates statistical significance relative to Cre− control cells. # indicates statistical significance relative to Mx1 cells. (E and F) 4000 LSK cells from Angpt1fl/fl (Cre−) or Mx1-cre; Angpt1fl/fl (Mx1) mice were transplanted into irradiated Leprcre; Angpt1fl/GFP (Lepr) mice. Bone marrow cellularity (E) and LSK cell number in the bone marrow (F) were analyzed 14 and 28 days after irradiation and bone marrow transplantation. Data represent mean ± s.d. from 4 mice/genotype/time point from 3 independent experiments. Two-tailed Student's t-tests were used to assess statistical significance (*p < 0.05). (G and H) One million bone marrow cells from Angpt1fl/fl (Cre−) or Pf4-cre; Angpt1fl/fl (Pf4) mice were transplanted into irradiated Leprcre; Angpt1fl/GFP (Lepr) mice. Bone marrow cellularity (G) and LSK cell number in the bone marrow (H) were analyzed at 8, 12, 16, and 28 days after irradiation and transplantation. Data represent mean ± s.d. from 4 mice/genotype/time point from 3 independent experiments. Two-tailed Student's t-tests were used to assess statistical significance.
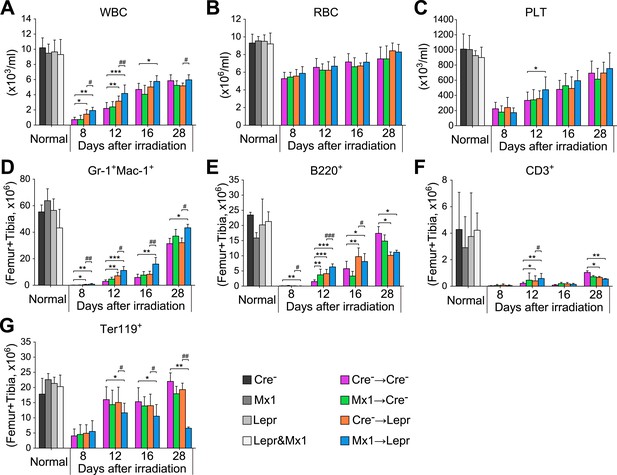
Angpt1 deletion accelerated hematopoietic recovery after irradiation.
One million of bone marrow cells from control (Cre-) or Mx1-cre; Angpt1fl/fl (Mx1) mice were transplanted into lethally irradiated control (Cre-) or Leprcre; Angpt1fl/GFP (Lepr) mice. White blood count (A), red blood count (B), platelet count (C), Gr-1+Mac1+ myeloid cell number (D), B220+ B cell number (E), CD3+ T cell number (F) and Ter119+ erythroid cell number (G) from two femurs and two tibias. Data represent mean ± s.d. from 6–11 mice/genotype/time point from 5 independent experiments. Two-tailed Student's t-tests were used to assess statistical significance (* or #p < 0.05, ** or ##p < 0.01, *** or ###p < 0.001).
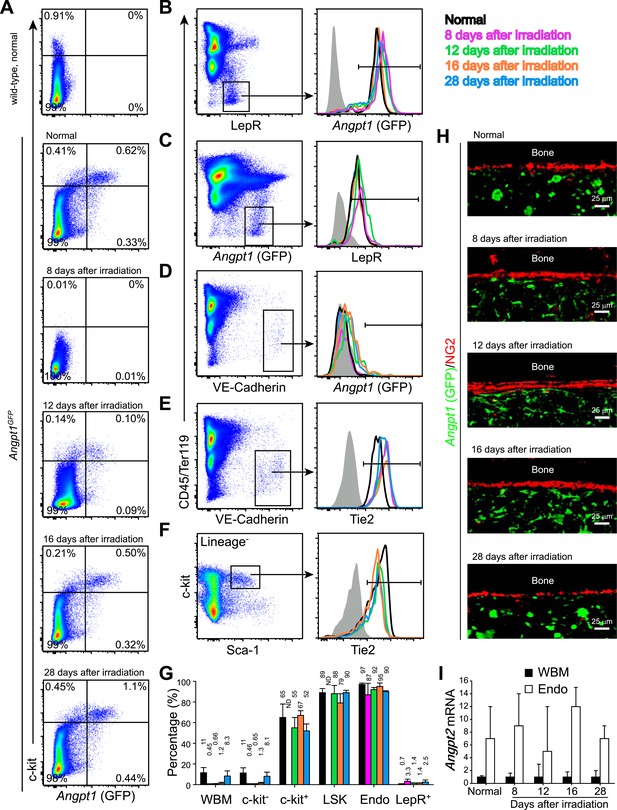
Angpt1, Tie2, and Angpt2 expression patterns were similar in adult bone marrow before and after irradiation.
One million of WBMs from Angpt1GFP mice were transplanted into lethally irradiated Angpt1GFP mice, which were then analyzed at 8, 12, 16 and 28 days after irradiation and transplantation (n = 3 donor and 3 recipient mice from 3 independent experiments). (A) Flow cytometric analysis of mechanically dissociated bone marrow cells (containing hematopoietic cells but not stromal cells) showed that most GFP+ bone marrow cells were c-kit+ before and after irradiation. (B and C) Flow cytometric analysis of enzymatically dissociated bone marrow showed that nearly all LepR+ stromal cells were GFP+, and vice versa, before and after irradiation. (D) VE-Cadherin+ endothelial cells from enzymatically dissociated bone marrow did not express GFP before or after irradiation. (E and F) VE-Cadherin+ endothelial cells (E) and LSK cells (F) uniformly expressed Tie2 before and after irradiation. Day 8 after irradiation was not included in (F) because of few LSK cells. (G) Percentage of Tie2+ cells among WBMs, c-kit− hematopoietic cells, c-kit+ hematopoietic cells, LSK cells, VE-Cadherin+CD45−Ter119− endothelial cells (Endo) and LepR+ perivascular stromal cells (Lepr+) before and after irradiation. (H) Representative femur sections showing that GFP was not detectably expressed by NG2+ osteoblasts before or after irradiation. (I) Angpt2 transcript expression levels by qRT-PCR of unfractionated bone marrow cells and VE-cadherin+ bone marrow endothelial cells before and after irradiation.
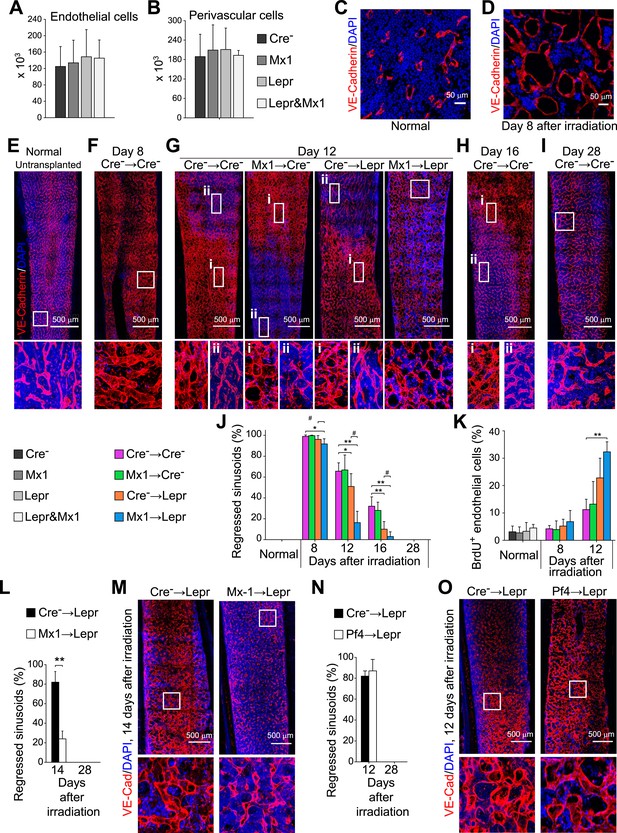
Angpt1 deficiency in hematopoietic stem/progenitor cells and LepR+ stromal cells increased endothelial cell proliferation and accelerated the recovery of vascular morphology after irradiation.
(A and B) Deletion of Angpt1 from hematopoietic cells (Mx1), LepR+ stromal cells (Lepr), or both (Lepr and Mx1) did not significantly affect the number of VE-cadherin+ endothelial cells (A) or LepR+ perivascular stromal cells (B) in the bone marrow of normal young adult mice. Cell number in enzymatically dissociated bone marrow cells was determined in 2 pairs of femurs and tibias per mouse (n = 3 mice/genotype from 3 independent experiments). (C and D) Representative images showing normal (C) and regressed (D) sinusoids in transverse femur sections. Regressed sinusoids were distinguished from non-regressed sinusoids by being dilated and having few hematopoietic cells around them. (E–K) One million bone marrow cells from Angpt1fl/fl (Cre−) or Mx1-cre; Angpt1fl/fl (Mx1) mice were transplanted into irradiated Angpt1fl/GFP or Angpt1GFP (Cre−) or Leprcre; Angpt1fl/GFP (Lepr) mice. Three-dimensional reconstructions of 50 μm thick sections of femoral bone marrow stained with anti-VE-cadherin antibody revealed the regression and regeneration of blood vessels after irradiation. Representative images for control (Cre−) mice were taken at steady state (E), 8 days (F), 12 days (G), 16 days (H) and 28 days (I) after irradiation and transplantation. Representative images for Mx1 → Cre−, Cre− → Lepr and Mx1 → Lepr mice were taken 12 days after irradiation (G). (J) The percentage of regressed sinusoids in sections through the bone marrow. Data represent mean ± s.d. from 5–6 mice/genotype/time point from 4 independent experiments. (K) Incorporation of a 24-hr pulse of BrdU into VE-cadherin+ endothelial cells (mean ± s.d. from 3–4 mice/genotype/time from 3 experiments). Two-tailed Student's t-tests were used to assess statistical significance (* or #p < 0.05; ** or ##p < 0.01; *** or ###p < 0.001). (L and M) 4000 LSK cells from Angpt1fl/fl (Cre−) or Mx1-cre; Angpt1fl/fl (Mx1) mice were transplanted into irradiated Leprcre; Angpt1fl/GFP (Lepr) mice. Vascular morphology (M) and the percentage of regressed sinusoids (L) were analyzed at the indicated time points. (N and O) One million bone marrow cells from Angpt1fl/fl (Cre−) or Pf4-cre; Angpt1fl/fl (Pf4) mice were transplanted into irradiated Leprcre; Angpt1fl/GFP (Lepr) mice. Vascular morphology (O) and the percentage of regressed sinusoids (N) were analyzed at the indicated time points. Two-tailed Student's t-tests were used to assess statistical significance (*p < 0.05).
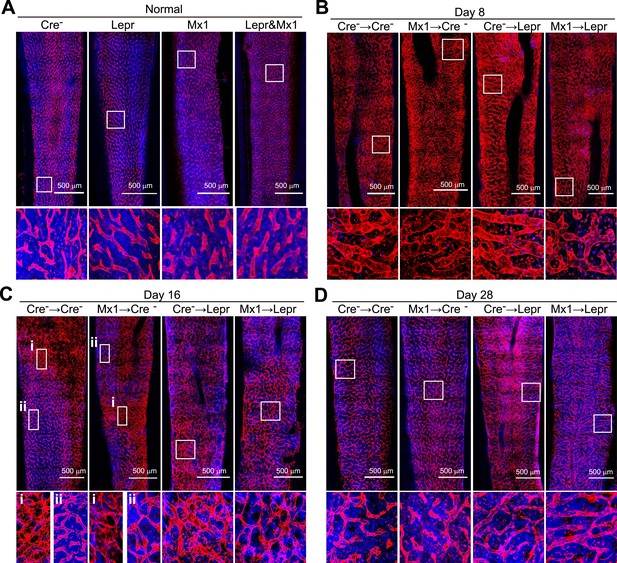
Angpt1 deficiency accelerated the recovery of vascular morphology after irradiation.
Representative confocal images of VE-cadherin (red) and DAPI (blue) stained bone marrow sections from control (Cre-) or Mx1-cre; Angpt1fl/fl (Mx1) mice under normal conditions (A), or at 8 (B), 16 (C), or 28 (D) day after transplantation of bone marrow from control (Cre-) or Leprcre; Angpt1fl/GFP (Lepr) mice (n = 3–4 mice/genotype/time point in 4 independent experiments).
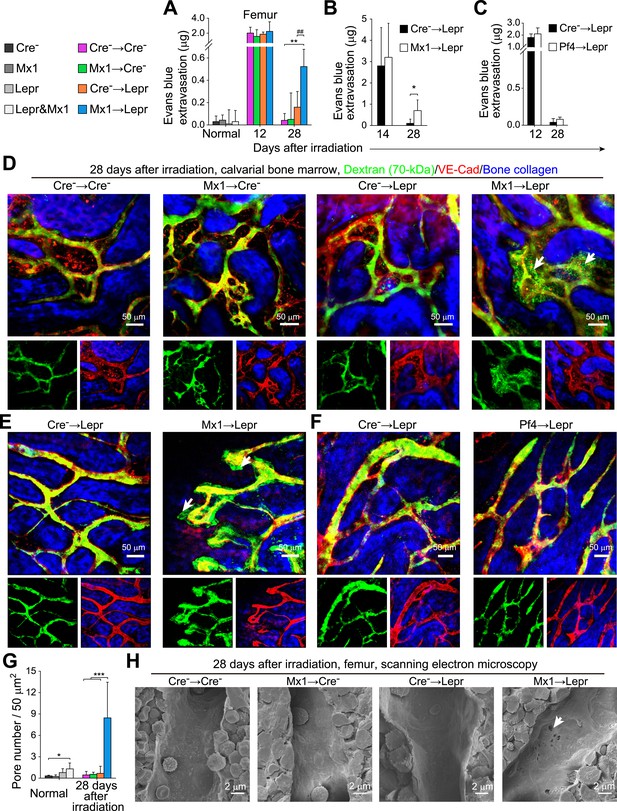
Angpt1 deficiency in hematopoietic stem/progenitor cells and LepR+ stromal cells increases the leakiness of regenerated blood vessels.
One million bone marrow cells from Angpt1fl/fl (Cre−) or Mx1-cre; Angpt1fl/fl (Mx1) mice were transplanted into lethally irradiated Angpt1GFP/fl or Angpt1GFP (Cre−) or Leprcre; Angpt1GFP/fl (Lepr) mice (A, D, H) (n = 4 mice/genotype/time point from 3 independent experiments). 4000 LSK cells Angpt1fl/fl (Cre−) or Mx1-cre; Angpt1fl/fl (Mx1) mice were transplanted into Leprcre; Angpt1GFP/fl mice (Lepr) (B and E) (n = 4 mice/genotype/time point from 3 independent experiments). One million bone marrow cells from Angpt1fl/fl (Cre−) or Pf4-cre; Angpt1fl/fl (Pf4) were transplanted into Leprcre; Angpt1GFP/fl (Lepr) mice (C and F) (n = 4 mice/genotype/time point from 3 independent experiments). (A–C) Extravasation of intravenously injected Evans blue into femoral bone marrow at the indicated time points after irradiation and bone marrow transplantation. (D–F) Live imaging of calvarial bone marrow at 28 days after irradiation and bone marrow transplantation to assess dextran-FITC extravasation (arrows). The mice were injected with dextran-FITC and anti-VE-cadherin antibody before microscopy. (G) Quantification of the number of pores larger than 100 nm in diameter per 50 µm2 of sinusoidal endothelium (n = 3–5 mice/genotype from 3 independent experiments). Two-tailed Student's t-tests were used to assess statistical significance (* or #, p < 0.05; ** or ##, p < 0.01; *** or ###, p < 0.001). (H) Scanning electron microscopy of bone marrow sinusoids from Cre− → Cre−, Mx1 → Cre−, Cre− → Lepr, and Mx1 → Lepr mice at 28 days after irradiation. Arrows indicate pores greater than 100 nm in diameter in sinusoidal endothelium 28 days after irradiation.
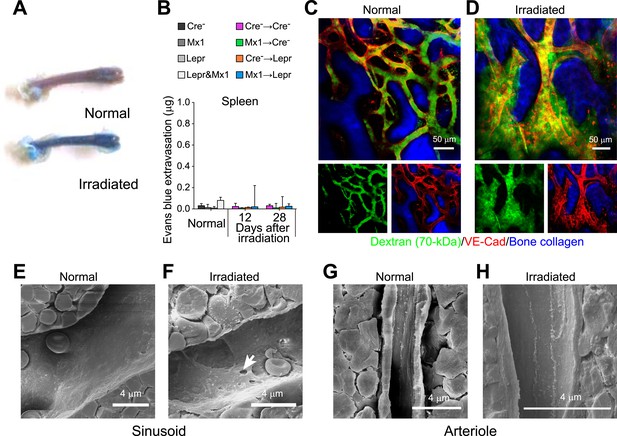
Angpt1 deficiency led to the persistence of pores and leakiness in blood vessels after irradiation.
(A) Femoral bone marrow was heavily infiltrated by intravenously injected Evans blue that extravasated 12 days after irradiation. (B) Lack of extravasation of intravenously injected Evans blue into spleen from Cre− → Cre−, Mx1 → Cre−, Cre− → Lepr, and Mx1 → Lepr mice (n = 4 mice/genotype/time point from 3 independent experiments). (C and D) Dextran-FITC was confined within VE-cadherin+ blood vessels in the calvarial bone marrow in normal adult mice (B) but extravasated out of vessels 12 days after irradiation (C) (n = 3–4 mice from 3 independent experiments). (E–H) Scanning electron microscopy of sinusoidal (D and E) and arterial (F and G) blood vessels in normal adult bone marrow (E and G) or 15 days after irradiation (F and H). We only detected pores in sinusoidal endothelium (n = 3–6 mice from 3 independent experiments).
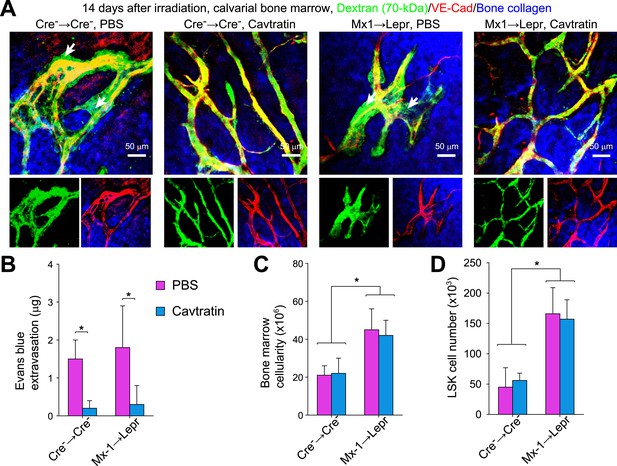
Vascular leakage does not promote hematopoietic regeneration in Angpt1 mutant mice.
One million whole WBMs from control and Mx1-cre; Angpt1fl/fl mice were transplanted into irradiated control and Leprcre; Angpt1GFP/fl mice, respectively. Cavtratin was administered into control (Cre− → Cre−), and mutant (Mx1 → Lepr) recipients from 7 to 13 days after irradiation and transplantation. 14 days after irradiation mice were analyzed for Dextran-FITC extravasation in calvarial bone marrow (A, n = 3 mice/genotype from 3 independent experiments), Evans blue extravasation in femoral bone marrow (B, n = 3 mice/genotype from 3 independent experiments), bone marrow cellularity, and LSK cell number in the bone marrow (C and D, n = 4 mice/genotype from 3 independent experiments). Cell numbers reflect two femurs and two tibias. Two-tailed Student's t-tests were used to assess statistical significance (*p < 0.05).





